Abstract
A combination of extrinsic (passive) and intrinsic (active) forces move lymph against a hydrostatic pressure gradient in most regions of the body. The effectiveness of the lymph pump system impacts not only interstitial fluid balance but other aspects of overall homeostasis. This review focuses on the mechanisms that regulate the intrinsic, active contractions of collecting lymphatic vessels in relation to their ability to actively transport lymph. Lymph propulsion requires not only robust contractions of lymphatic muscle cells, but contraction waves that are synchronized over the length of a lymphangion as well as properly functioning intraluminal valves. Normal lymphatic pump function is determined by the intrinsic properties of lymphatic muscle and the regulation of pumping by lymphatic preload, afterload, spontaneous contraction rate, contractility and neural influences. Lymphatic contractile dysfunction, barrier dysfunction and valve defects are common themes among pathologies that directly involve the lymphatic system, such as inherited and acquired forms of lymphoedema, and pathologies that indirectly involve the lymphatic system, such as inflammation, obesity and metabolic syndrome, and inflammatory bowel disease.
Keywords: lymphatic, lymphedema, muscle contraction
Abbreviations
- AMP
contraction amplitude
- AP
action potential
- ApoE−/−
apolipoprotein E knockout
- Cx
connexin
- db/db
leptin receptor knockout
- EC
endothelial cell
- EDD
end‐diastolic diameter
- EF
ejection fraction
- eNOS
endothelial nitric oxide synthase
- ESD
end‐systolic diameter
- ESV
end‐systolic volume
- FPF
fractional pump flow
- FREQ
contraction frequency
- GFP
green fluorescent protein
- iNOS
inducible nitric oxide synthase
- KATP
ATP‐sensitive potassium channel
- LEC
lymphatic endothelial cell
- LMC
lymphatic muscle cell
- MLCK
myosin light chain kinase
- MLCP
myosin light chain phosphatase
- NO
nitric oxide
- NIRF
near infrared fluorescence
- ∆P
hydrostatic pressure gradient
- Pin
inflow pipette pressure
- PL
luminal hydrostatic pressure
- Pout
outflow pipette pressure
- ROS
reactive oxygen species
- VIP
vasoactive intestinal peptide
- VSM
vascular smooth muscle
- WT
wild‐type
Introduction
An extensive network of lymphatic vessels runs in parallel to the blood vascular system, composed of initial lymphatic capillaries that serve an absorptive role, collecting vessels that transport lymph, and lymph nodes/lymphoid organs that facilitate immune responses. Lymphatic vessels or lymphatic‐like structures with fluid and/or immune cell transport function(s) have been identified in almost every organ, including the brain and eye (Schroedl et al. 2014; Aspelund et al. 2015; Louveau et al. 2015). Ultimately, lymphatic collecting vessels coalesce into central lymphatic ducts that return lymph to the venous circulation at the confluence of the great veins in the neck. In humans, 8–12 litres of fluid and protein per day that otherwise would accumulate in extravascular compartments are returned to the blood through the lymphatic system (Renkin, 1986; Wiig & Swartz, 2012).
A combination of extrinsic and intrinsic forces move lymph against a hydrostatic pressure gradient in most regions of the body. At rest, approximately 1/3 of lymph transport in the human lower extremities results from compression by skeletal muscle contractions (extrinsic pump) and 2/3 to active pumping (intrinsic pump) of the collecting vessel network (Engeset et al. 1977). The robust contractions of lymphatic muscle cells are the driving force for active lymph propulsion against adverse pressure gradients (Zweifach & Prather, 1975), which can be particularly large in dependent extremities (Olszewski & Engeset, 1980). Backflow within the lymphatic network is minimized by a system of one‐way valves (Davis et al. 2011).
Overload of the intrinsic lymphatic pump or failure of lymphatic valves leads to, and/or results from, chronic lymphoedema (Olszewski, 2002). Observations in the limbs of patients with lymphoedema suggest failure or weakening of the active lymphatic contractions, chronic distension of the collecting vessels and incompetence of the valves (Olszewski et al. 1968; Olszewski, 2002). Current therapies for lymphoedema are palliative in nature, promoting passive lymph transport through rigorous and daily deep tissue massage. The limitations in lymphoedema treatments are, in large measure, due to our lack of understanding of the molecular and mechanical properties of lymphatic muscle cells (LMCs).
The focus of this review is the intrinsic, contractile properties of collecting lymphatic vessels in relation to their ability to actively transport lymph. The effectiveness of the lymph pump system impacts not only interstitial fluid balance but other aspects of overall homeostasis such as fat absorption (Dixon, 2010), reverse cholesterol transport (Martel et al. 2013) and immune cell trafficking (Angeli et al. 2004; Lim et al. 2009; Cromer et al. 2015; Chakraborty et al. 2015 b).
Normal lymphatic pumping
Active lymph transport by collecting lymphatic vessels depends critically on a combination of factors that combine to produce propulsive and centripetal movement of lymph. Lymph propulsion requires not only robust, spontaneous contractions of LMCs, but contraction waves that are coordinated over the length of a lymphangion, which is the segment of a collecting lymphatic vessel containing two intraluminal valves comprising the elementary pumping unit. In conjunction, unidirectional valves in the vessel lumen must operate normally to minimize backflow. For the purpose of this discussion, lymphatic pumping is defined as the net outflow of a collecting lymphatic segment due to active contraction of the LMC layer(s). Net outflow is equal to forward (centripetal) flow due to a propulsive contraction minus any reflux through the valves during the contraction cycle.
Pumping behaviour can be visualized in video movies of isolated lymphangions held at constant pressure. The images in Fig. 1 and linked movies (Movies S1 and S2 in the online Supporting information) show contractions of a popliteal afferent lymphatic from a mouse expressing green fluorescent protein (GFP) in lymphatic endothelial cells (LECs) under the control of the LEC‐specific transcription factor, Prox1. The outer edges of the fluorescent border demarcate the inner diameter of the vessel, which becomes clear when the fluorescence (Fig. 1 B) and brightfield (Fig. 1 A) images are compared. Both valve leaflets are visible as they open and close during the contraction cycle. Robust and nearly synchronous contractions of a single layer of LMCs are evident.
Figure 1. Brightfield (A) and fluorescence (B) images of a popliteal afferent lymphangion from a Prox1GFP mouse after dissection, cannulation and partial cleaning .
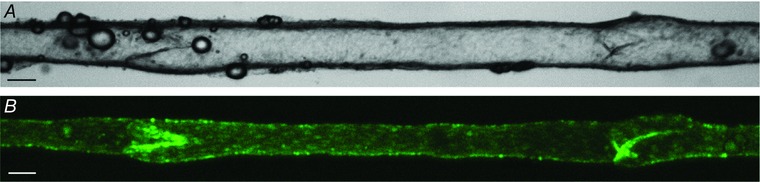
The vessel is pressurized to 3 cmH2O from cannulation pipettes on either end (out of field of view). Calibration bars = 50 μm. Movies S1 and S2 in the online Supporting information show contraction sequences in each imaging mode.
Lymphatic muscle is non‐striated and usually classified as vascular smooth muscle, but it shares biochemical and functional characteristics with both vascular and cardiac muscle (von der Weid & Zawieja, 2004). Like vascular smooth muscle, lymphatic muscle contraction is regulated primarily by the balance of myosin light chain kinase (MLCK)/myosin light chain phosphatase (MLCP) activity controlling myosin light chain phosphorylation (reviewed in Chakraborty et al. 2015 a). Lymphatic vessels resemble arterioles in that they develop basal tone and exhibit myogenic constriction to pressure elevation (Davis et al. 2009). Although the physiological role of the myogenic response in lymphatic vessels is not known, myogenic tone/constriction may serve primarily to preserve valve function (Scallan et al. 2012 a), as discussed below. Like blood vessels, lymphatic behaviour is regulated by nitric oxide (NO) and other endothelium‐derived factors such as prostaglandins and histamine (Gasheva et al. 2013; Nizamutdinova et al. 2014). Both lymphatic tone and spontaneous contractions are inhibited by NO produced as a result of shear stress on the endothelium (in response to either forward or reverse lymph flow) (Gashev et al. 2002). Like cardiac muscle, LMCs express troponin C and I as well as cardiac isoforms of tropomyosin (Muthuchamy et al. 2003); however, the functional roles of these contractile proteins remain unclear, as are the ways in which they might complement or interact with MLCK/MLCP to control the contraction cycle.
Lymphatic muscle shares several electrophysiological properties with both vascular smooth muscle (VSM) and cardiac muscle. LMC contractions depend predominantly on Ca2+ influx through L‐type, voltage‐gated Ca2+ channels (Telinius et al. 2014 c), while the resting membrane potential is influenced substantially by Cl− (van Helden, 1993; von der Weid et al. 2008 b) and voltage‐gated K+ channels (Allen & McHale, 1988; Telinius et al. 2014 b). Further, the activation of ATP‐sensitive potassium (KATP) channels (Mizuno et al. 1999; Mathias & von der Weid, 2013) and Ca2+‐activated K+ channels (Cotton et al. 1997) in lymphatic muscle can dramatically modulate spontaneous contractile activity. Like cardiac muscle, the spontaneous contractions of LMCs are initiated by action potentials (APs) that probably originate in LMCs, but also might be generated by a network of interstitial cells (McCloskey et al. 2002; Sanders & Ward, 2008; Briggs Boedtkjer et al. 2013). LMCs also express several types of ion channels that are similar to those that control pacemaking in the sino‐atrial node, e.g. fast Na+ channels (Hollywood et al. 1997 b; Telinius et al. 2015), T‐type Ca2+ channels (Hollywood et al. 1997 a; Lee et al. 2014), hyperpolarization‐activated cyclic nucleotide‐gated (HCN) channels (McCloskey et al. 1999) and ether‐à‐go‐go related gene (ERG) channels (Gui et al. 2014). The roles of these channels in lymphatic pacemaking are not yet fully understood.
Use of cardiac analyses to evaluate lymphatic contractile function
Functionally, many aspects of the lymphatic pump resemble those of the cardiac ventricular pump. At the beginning of a lymphatic pump cycle both valves are closed so that contraction of the LMC layer (i.e. systole) results in a rapid rise in intraluminal pressure; once pressure exceeds outflow pressure the outflow valve opens, ejecting lymph. When the LMC layer relaxes (i.e. diastole), intraluminal pressure falls, the outflow valve closes, and the inflow valve then opens to allow filling of the lymphangion. This sequence of events is illustrated by the experimental recording in Fig. 2 of two contraction cycles. Internal diameter is measured from high‐resolution video images of the vessel using edge detection while valve positions (inflow valve, blue arrow; outflow valve, red arrow) are measured using densitometry (Davis et al. 2012). Pressures at the inflow and outflow ends are controlled by a servo system and intraluminal pressure (P L) is measured through a 3 μm servo‐nulling pipette advanced through the wall. To evaluate pump function inflow and outflow pressures are manipulated independently or in unison. In this case, a slow rise in outflow pressure (P out) is imposed while holding inflow pressure (P in) constant. Both pressures are referenced to external pressure (atmospheric). The contractions of the entire lymphangion are essentially synchronized, with a delay of only a few milliseconds for spread of the contraction wave. The contraction amplitude (AMP) for this vessel, as determined from end‐diastolic diameter (EDD) minus end‐systolic diameter (ESD), is larger on the outflow than inflow side of the outflow valve; note that EDD slowly rises as P out is raised, but remains approximately constant on the inflow side because P in is held low and the central segment is protected from reverse flow in diastole by the outflow valve, which is closed. The ejection fraction (EF) is calculated as (EDD2 − ESD2)/EDD2, thereby converting the diameter change to a volume change over a constant length. EF can be as high as 80% for isolated lymphangions from rat and mouse (Scallan et al. 2012 b; Scallan & Davis, 2013). Although pump flow in mouse and rat vessels is not able to be measured directly with current methods (as it is in lymphatics from larger animals; McHale & Roddie, 1976; McHale & Meharg, 1992), pump output (FPF, fractional pump flow) can be estimated from the product of EF and contraction frequency (FREQ).
Figure 2. Pump cycle of an isolated, cannulated (2‐valve) lymphangion from rat mesentery when Pout is elevated ramp‐wise while Pin is held constant .
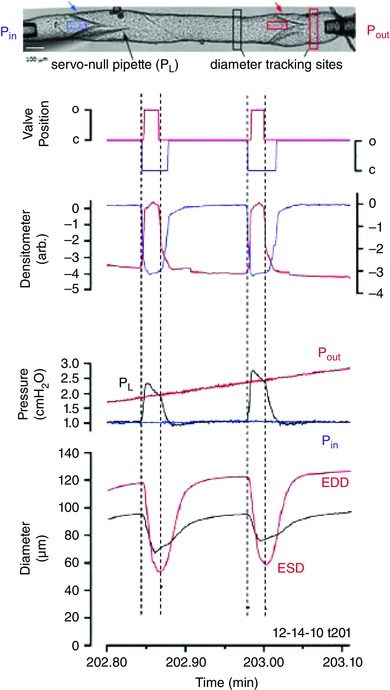
Normal direction of flow is left to right. Red and black diameter traces correspond to red and black tracking windows on each side of the output valve in the video image at the top. Blue and red densitometer traces correspond to blue and red densitometer windows positioned just upstream of the input and output valves, respectively. Valve position traces represent the binary state of each valve based on thresholding of the respective densitometer traces. Black pressure trace is the intraluminal pressure (between the valves) measured by a sharp servo‐nulling pipette advanced through (and sealed into) the wall. Modified from Davis et al. (2011).
Modulators of the lymph pump
Like the heart (West, 1991), lymphatic pumping is regulated by four major factors: preload, afterload, spontaneous contraction frequency and contractility. The influence of each factor is addressed briefly in the next section.
Preload
Preload, which is set by end‐diastolic pressure (or volume), is a significant determinant of lymphatic pump function. Increasing the filling pressure over a certain range enhances pump output, analogous to the Frank–Starling relationship for the heart (Smith, 1949; Mislin & Schipp, 1966; McHale & Meharg, 1992). In rat and mouse lymphangions FREQ increases with pressure over the range 0–5 cmH2O, reaching a plateau at higher pressures. AMP increases over 0–3 cmH2O and then declines at higher pressures (Gashev et al. 2004; Scallan et al. 2012 b). Examples are shown in Fig. 3 A. Like the cardiac ventricles, lymphatic EDD increases in a curvilinear fashion with pressure, reflecting the underlying passive pressure–diameter relationship, in contrast to ESD, which increases linearly with pressure (Fig. 3 B). FPF peaks at around 5 cmH2O, which is consistent with results from isolated chains of lymphatic segments from larger species (McHale & Roddie, 1976; Elias et al. 1990; Eisenhoffer et al. 1995; Li et al. 1998). The FREQ response to a change in preload is also rate sensitive, as shown in Fig. 3 C, where a rapid pressure step evokes a burst of contractions followed by a subsequent decline in FREQ; increasing preload at a slower rate will eliminate the bursting (Davis et al. 2008 a).
Figure 3. Effect of elevating preload independently of afterload on the contractile function of an ex vivo mesenteric lymphangion from rat .
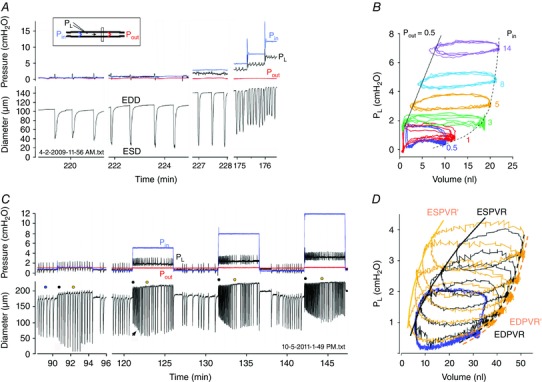
A, P in was elevated to various levels while P out was held constant. Pipette resistances were purposely kept to relatively high values to limit the inhibitory effect of forward flow produced by P in > P out gradient. Inset shows diagram of pressure and diameter measurement sites. B, pressure–volume loop constructed from a portion of the data in A, showing the curvilinear P–V relationship for EDD (dashed line) and linear P–V relationship for ESD. C, time course of spontaneous contraction AMP and FREQ changes after a series of step elevations in P in (P out held constant). After each step, AMP falls but then recovers (or gets even larger) over the course of ∼1 min (arrow). Also, a burst of high FREQ contractions occurs, with FREQ subsequently slowing slightly. D, P–V plot of some of the data in C; blue traces represent data from 3 contraction cycles prior to the P in steps (corresponding to time indicated by blue dot in C), black and gold traces represent single contraction cycles corresponding to the black and gold dots in C, immediately after the pressure step (black dots in C) or ∼1 min later (gold dots in C). The shift in the end‐systolic P–V relationship (ESPVR) with time after P in elevation, with unchanged end‐diastolic (ED)PVR reflects an increase in contractility. Modified from Scallan et al. (2012 b).
Afterload
The lymphatic pump must adapt to elevated outflow pressures resulting from partial outflow obstruction, increased central venous pressure and/or gravitational shifts. Lymphangions in series can propel lymph against higher pressures than individual lymphangions (Jamalian et al. 2016), which is required in dependent extremities where outflow pressures can reach relatively high levels (Olszewski, 2002). An elevation in lymphatic outflow pressure is analogous to an elevation in aortic pressure for the ventricular pump, as it increases the load against which the pump must eject. For rat mesenteric vessels studied ex vivo, the limit against which a single lymphangion pumped was determined by slowly raising P out in ramp‐wise fashion while monitoring the opening of the output valve in systole (Davis et al. 2012). On average the P out level before reaching pump failure, denoted by cessation of valve opening with each contraction cycle, was ∼11 cmH2O higher than P in, with considerable variation between lymphangions (range 2–18). Given that the inter‐valve distance is only ∼1 mm and the difference in pressures between lymphangions is only 1–2 cmH2O in vivo (Zweifach & Prather, 1975), there is seemingly a large margin of safety built into the system.
Contraction frequency
In the heart, cardiac output = stroke volume × heart rate. The analogous expression for the lymphatic pump is FPF = EF × FREQ. The contraction FREQ of collecting lymphatics is exquisitely sensitive to pressure, and changes as small as 0.5 cmH2O can double FREQ (Scallan et al. 2012 b). In some cases FREQ increases 10‐fold over the pressure range 0–5 cmH2O. Striking examples of this response can be observed in Fig. 3 C at time = 132 and 142 min. As in the heart, high FREQ can limit filling (West, 1991) and thus have a negative effect on AMP. This effect is illustrated in Fig. 4 B at time = 391 min, where an extended pause between contractions allows EDD to increase to a higher value.
Figure 4. Effect of elevating afterload in an ex vivo mesenteric lymphangion from rat .
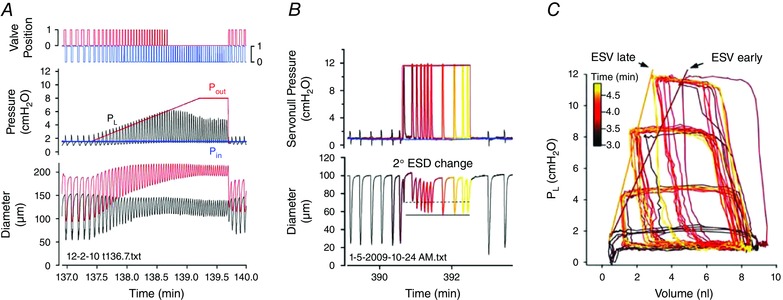
A, ramp‐wise elevation in P out (with P in held constant) leads to a progressive increase in the peak systolic pressure developed in the lumen (P L, black trace). Note also a modest, progressive constriction on the input side of the valve during the pressure ramp. Opening of the output valve (top red trace) is indicative of ejection during systole, until P out reaches ∼6.2 cmH2O, at which point the pump limits (fails). B, response to a step increase in P out. Contraction AMP declines initially but then partially recovers over the next ∼1 min. C, P–V plot of the data in B showing time‐dependent leftward shift in the curves after a P out step (data in B represent the top set of curves). See diagram in Fig. 2 for explanation of pressure, diameter and valve position measurements. ESV, end‐systolic volume. Modified from Davis et al. (2012).
Contractility
‘Contractility’ is often used in a broad sense in the lymphatic literature to describe the enhancement of AMP or FREQ in response to a pressure increase or agonist activation (McHale et al. 1980; Benoit et al. 1989; Gashev et al. 2002; Muthuchamy & Zawieja, 2008; von der Weid et al. 2008 a). In contrast, the term ‘contractility’, and the related term, ‘positive inotropy’, have very specific definitions in the cardiac literature. Positive inotropic agents produce an increase in cardiac muscle contractility: an increase in the strength and velocity of force development at constant preload. Likewise, an increase in aortic pressure (afterload) leads to an intrinsic increase in contractility, which is also called the ANREP effect (Sarnoff et al. 1960; West, 1991). A similar phenomenon can be observed in isolated lymphangions in response to elevated outflow pressure. Ramp‐wise elevation in P out is associated with a constriction on the upstream side of the valve and a gradual decline in AMP; however, when P out is returned to control, unusually large amplitude contractions are observed for a limited period of time (see Fig. 8 in Davis et al. (2012). Likewise, a step‐wise increase in P out produces an initial reduction in ESD that gradually recovers over the course of a few minutes (Fig. 4 B) and, if the pressure–diameter relationship is plotted (with diameter converted to volume), the line describing the end‐systolic volume (ESV) vs. pressure relationship shifts gradually, increasing its slope over time; the slope increase is indicative of an increase in contractility (Fig. 4 C). Interestingly, unlike in the heart, step increases in preload can also transiently increase contractility in ex vivo lymphangions (Fig. 3 D). The mechanistic bases of these responses are not known but may involve changes in calcium sensitivity, as shown for cardiac muscle (Solaro, 2011).
At least four additional factors have a significant impact on lymphatic pumping. Each of these is addressed in the following sections.
Neural modulation
While not required for lymphatic contractions per se, the effects of neural signalling molecules appear to be keenly involved in regulating lymphatic contractions. However, direct observations of lymphatic innervation and the mechanisms regulating this process are under‐studied, with much of our current understanding stemming largely from experiments performed in the 1980s and 90s in vessels from different regions and species. A comprehensive discussion of this topic merits its own review (Zawieja et al. 2011).
Sympathetic adrenergic nerve fibres appear to be the dominant neural innervation of the lymphatic vasculature (Todd & Bernard, 1973; Alessandrini et al. 1981; McHale, 1990; Hollywood & McHale, 1994). α‐Adrenergic stimulation of contractile lymphatic vessels consistently increases tone, AMP and FREQ (McHale, 1990), and these effects are countered by β‐adrenergic receptor activation (von der Weid, 1998). Muscarinic receptors on LMCs also promote an increase in FREQ; however, this action of a muscarinic receptor agonist is usually masked by a dominant, inhibitory effect of NO as a consequence of endothelial nitric oxide synthase (eNOS) activation in lymphatic endothelial cells (Ohhashi & Takahashi, 1991; Scallan & Davis, 2013). Interestingly, serotonin (5‐HT) can either inhibit or increase spontaneous lymphatic contractions depending on the species and the specific pattern of serotonin receptor expression (Miyahara et al. 1994; McHale et al. 2000; Chan & von der Weid, 2003). The inhibitory effects on contraction by both serotonin and vasoactive intestinal peptide (VIP) appear to be mediated through cAMP/cGMP and downstream activation of KATP channels (Ohhashi et al. 1983; Chan & von der Weid, 2003; von der Weid et al. 2012). Calcitonin gene related peptide also produces inhibitory effects on lymphatic contractions through signalling mechanisms in both LEC and LMC layers that appear to be mediated by the cAMP‐KATP axis (Hosaka et al. 2006). The neurotransmitter substance P (SP), commonly associated with afferent nerve endings, also has profound effects on lymphatic function, promoting extensive tone generation and increased FREQ (Amerini et al. 2004; Davis et al. 2008 b), although this enhancement comes at the expense of reduced AMP. Direct evidence for SP+ and VIP+ peptidergic innervation has been demonstrated in guinea pig (Guarna et al. 1991) and bovine (Ohhashi et al. 1983) mesenteric collecting lymphatics, but further work is required to determine if those findings are representative of other species and tissue beds. The human thoracic duct has both sympathetic and parasympathetic innervation (Mignini et al. 2012) that appears to decrease with ageing. This innervation is functional because the fibres can be activated via electrical field stimulation and affect contractions in isolated human thoracic ducts (Telinius et al. 2014 a), similar to previous results in sheep lymphatics (Hollywood & McHale, 1994).
Contraction synchrony
The LMC layer of a lymphangion must contract in a coordinated, nearly synchronized manner to generate a systolic pressure pulse that can open the outflow valve and eject lymph; synchronization may be even more critical as outflow pressure is elevated. After lymphatic contractions are triggered by an AP in one of the LMCs, the AP propagates rapidly from cell to cell (at ≥8 mm s−1), and in either direction, over the length of the lymphangion (McHale & Meharg, 1992; Zawieja et al. 1993; Venugopal et al. 2007). Synchronized contractions require electrical coupling between the LMCs, presumably through connexins that form intercellular gap junctions. Although electrical coupling between LM cells has been documented (von der Weid et al. 1996; Crowe et al. 1997), the connexin isoform(s) in the LMC layer have yet to be identified. In mesenteric lymphatic vessel segments studied either ex vivo or in vivo, the application of gap‐junction blockers (e.g. n‐heptanol, oleic acid) leads to uncoordinated contractions of different parts of a lymphatic chain (McHale & Meharg, 1992; Zawieja et al. 1993). However, the particular gap junction blockers used in early studies were notoriously non‐specific; in the future, targeted deletion of specific connexin isoforms using gene knockout models will probably clarify the specific gap junctions involved in coordinating contractions of the lymphatic muscle layer.
In blood vessels, signals for vasodilatation are conducted primarily along the endothelium because endothelial cells (ECs) are well‐coupled electrically, particularly by connexin (Cx) 40 (Cx40) (Simon & McWhorter, 2002; de Wit et al. 2003; Wagner et al. 2007). A focal hyperpolarization induces a hyperpolarization wave that spreads upstream rapidly along the EC and across the internal elastic lamina to the VSM cell layer, which is coupled through connexins in EC projections (myoendothelial gap junctions, MEGJs) to VSM cells (Emerson & Segal, 2000; Sandow et al. 2012). In contrast, LECs express Cx37, Cx47 and Cx43 but not Cx40 (Simon & McWhorter, 2002; Kanady et al. 2011). Presumably, hyperpolarizing current can spread along the LEC layer in the same manner as in blood vessel endothelium, but that has not been tested; neither is the functional benefit of conducted hyperpolarization in a collecting lymphatic vessel obvious. Interestingly, Cx43 and Cx47 mutations are associated with the development of human lymphoedema for reasons that could be related to contraction wave dyssynchrony, valve development and/or valve maturation (Ferrell et al. 2010; Finegold et al. 2012).
An important difference between arterioles and lymphatics is the very limited degree of electrical coupling between LECs and LMCs (von der Weid et al. 1996; Crowe et al. 1997). We have confirmed that LMCs of pressurized rat lymphatic vessels have a resting membrane potential around −40 mV and fire spontaneous APs, while LECs have a stable resting potential around −70 mV (von der Weid & Van Helden, 1997) that does not oscillate during contractions of the overlying muscle layer (J. P. Scallan, M. J. Davis & S. D. Zawieja, unpublished observations). A possible advantage of this arrangement is that weak electrical coupling between LECs and LMCs may promote more efficient spread of the AP along the LMC layer if less electrical signal is shunted to LECs, which, collectively, would act as an electrical sink.
Valve function
Collecting lymphatics contain bicuspid valves (Figs 1 and 2) whose leaflets extend from a ring‐shaped base and insert as two buttresses into the vessel wall (Schmid‐Schönbein, 1990; Bazigou et al. 2014). The valve opening is a tapered funnel (Fig. 4 in Davis et al. 2011 and Movie S3 in the online Supporting information). Downstream from the valve is an enlarged sinus that facilitates valve opening and partially balances the high resistance of the narrow orifice created by the valve leaflets (Bazigou & Makinen, 2013; Wilson et al. 2015). Valves are spaced at semi‐regular intervals and the factors that control their spacing are not known (Kanady et al. 2011), but may involve Notch1 (Murtomaki et al. 2014) and semiphorin3A/neuropilin‐1/plexinA1 (Bouvree et al. 2012; Jurisic et al. 2012). Valves begin developing at embryonic day (E)15–E16 in the mouse and mature postnatally under a genetic programme that includes the transcription factors GATA2, PROX1 and FOXC2 (Petrova et al. 2004; Bazigou & Makinen, 2013; Bazigou et al. 2014; Sabine & Petrova, 2014; Kazenwadel et al. 2015; Sweet et al. 2015). Fluid shear stress is a key regulator of valve development (Sabine et al. 2012, 2015; Sweet et al. 2015) and mature valves exhibit a differential distribution of connexins, eNOS and other proteins across the valve (Petrova et al. 2004; Bohlen et al. 2011; Sabine & Petrova, 2014; Sabine et al. 2015). Many of the same factors regulate the development of the lymphovenous valves (Srinivasan & Oliver, 2011; Geng et al. 2016), which form earlier (E12) and whose integrity is critical for preventing backflow of blood into the central lymphatic ducts and thus maintaining separation between the lymphatic and blood circulations (Hess et al. 2014; Sweet et al. 2015).
The ultrastructure of lymphatic valves was described extensively in the 1970s–1980s (Lauweryns, 1971; Vajda & Tomcsik, 1971; Gnepp & Green, 1980; Albertine et al. 1982), yet no functional studies were published until recently. We developed tests of isolated valves for rat (Davis et al. 2011) and mouse vessels (Sabine et al. 2015) in which ex vivo segments contain a single valve to enable pressure control through cannulation pipettes on either side. A servo‐nulling pipette, inserted through the wall on the input side and positioned upstream from the valve leaflets, allows measurement of small, local pressure changes upstream from the valve (Fig. 5). With this protocol the adverse pressure gradient (ΔP) required to close the valve can be determined with high precision. The first surprising observation is that the valves have an open bias, meaning they are predisposed to be open when the trans‐valve pressure gradient is zero. Although this property may seem inefficient from a conceptual viewpoint (permitting reflux at certain times in the contraction cycle), it results in lower resistance to forward flow under conditions of little or no adverse pressure gradient. A second surprising observation is that the ΔP for valve closure is substantially dependent on the vessel diameter. At low diameters (associated with systole or a high level of basal tone), adverse pressures as small as 0.1–0.3 cmH2O are sufficient to close the valve; however, as vessel diameter approaches its maximum (as it does when the vessel is distended in chronic lymphoedema; Olszewski, 2002), pressures of several cmH2O are required. One caveat of the valve closure measurement is that the resistances of the small micropipettes used to cannulate rodent vessels can lead to an overestimation of the ΔP for valve closure when substantial flow occurs. During the normal lymphangion pump cycle, the diameter at the end of systole/early diastole reduces to a value that will facilitate valve closure if there is even a slight (<0.3 cmH2O) adverse pressure gradient. This implies that the valve may not close properly in lymphangions that are dilated and/or unable to generate a systolic pressure greater than that in the adjacent lymphangion downstream. The simple observation of reflux occurring in a collecting lymphatic network in vivo (Brice et al. 2002; Kriederman et al. 2003; Normen et al. 2009), when pressures are unknown, does not provide incontrovertible evidence of an abnormal valve. Even modest valve defects can have enormous effects on the relationship between ΔP for closure and vessel diameter (Sabine et al. 2015), as discussed below.
Figure 5. Ex vivo test for valve closure .
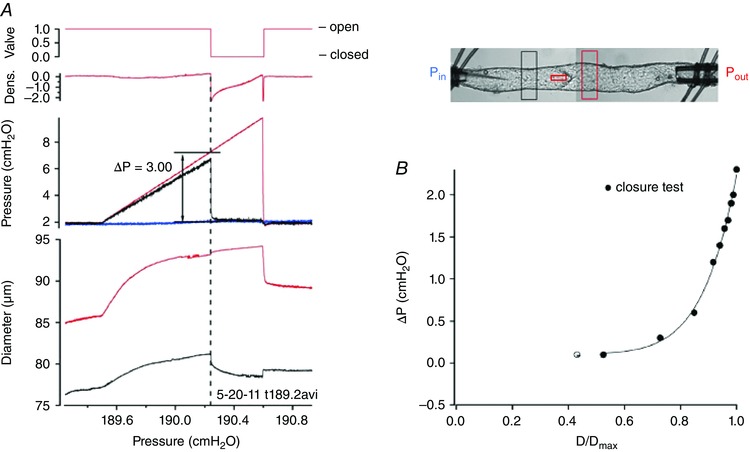
A, valve closure test for a 1‐valve mouse popliteal lymphatic. An image of the mouse vessel with diameter tracking windows and position of the servo‐nulling pipette from which the black pressure trace in A was obtained is shown to the right. To measure the adverse pressure gradient required for valve closure, both pressures are set equal (valve is open), then P out is elevated ramp‐wise (red pressure trace) until the valve snaps closed, as indicated by a rapid drop in the black trace; after a few more seconds, the P out ramp is terminated. Dotted line shows alignment of changes in densitometer window positioned upstream from leaflets, servo‐null pressure and diameter on the input side of the valve at the point of closure. The ΔP for closure is the difference between P out and P in at the moment of valve closure. B, summary data for a single rat mesenteric lymphatic valve. ΔP for closure is <0.3 cmH2O at low vessel diameters but rises to 2.2 cmH2O when the vessel is near‐maximally distended. D represents diameter. Panel B modified from Davis et al. (2011).
Barrier function
It is now appreciated that filtration disequilibrium exists across blood capillaries in skin, muscle and other tissues, where the combined hydrostatic and oncotic pressure gradient favours net filtration of fluid into the tissues. The steady reabsorption of this fluid by the venous circulation cannot occur in most organs, necessitating a lymphatic vasculature that absorbs and transports this fluid. Thus, the lymphatic vasculature serves a vital, rather than accessory, role in preventing tissue oedema (Levick & Michel, 2010). Lymphatic vessels have widely been regarded as ‘impermeable’ to fluid and solute, which would suggest that these vessels must have special junction adhesion proteins to constitute a very tight barrier. However, detailed analyses of collecting lymphatic endothelial junction proteins reveal no major differences from those of blood vessels (Baluk et al. 2007). Recent studies designed to directly quantify solute flux across the vessel wall of intact lymphatic vessels in vivo (Scallan & Huxley, 2010) and ex vivo (Scallan et al. 2015) demonstrate that collecting lymphatics are not only permeable to solute and fluid, but that their albumin permeability is comparable to that of postcapillary venules; further, lymphatic permeability is actively regulated because it can be modified by several signalling pathways, including nitric oxide (Scallan et al. 2013, 2015).
Lymphatic capillaries are an order of magnitude more permeable than collecting lymphatic vessels (Scallan & Huxley, 2010), most likely due to their discontinuous pattern of junctional adhesion proteins (Leak & Burke, 1968; Baluk et al. 2007), facilitating fluid and solute absorption from the interstitium. Although permeable collecting vessels may seem inefficient compared to idealized vessels that would retain all fluid and solute, the basal permeability leads to several interesting consequences. For example, it has been hypothesized that this extravasation allows antigen transported by the collecting lymphatics to reach local immune cells to mediate immune responses (Kuan et al. 2015). The basal permeability may therefore serve as the means of communication between lymph contents and regulation of vessel contraction through the recruitment and activation of immune cells. Activation of these immune cells may then lead to the production of nitric oxide through inducible nitric oxide synthase (iNOS) or other vasoactive molecules, which would inhibit lymphatic contractions and reduce lymph flow. Another consequence of having permeable collecting lymphatics is that lipids carried by these vessels will be distributed to the tissues, which may help explain why there is always adipose tissue located adjacent to collecting lymphatics and lymph nodes (Harvey, 2008). Indeed, this supports the finding that mice heterozygous for the lymphatic identity transcription factor, Prox1, develop late onset obesity as a result of pathological lymphatic leakage (Harvey et al. 2005).
Lymphatic pump dysfunction in pathological states
Lymphoedema can result from inherited (primary) or acquired (secondary) defects of the lymphatic system. Mutations in a number of genes including PROX1, GATA2, FOXC2 and VEGFR3/FLT4 lead to malformations in lymphatic vessels and/or valves, resulting in primary lymphoedema in humans (Brice et al. 2005; Ferrell et al. 2010; Mellor et al. 2011; Finegold et al. 2012; Sabine & Petrova, 2014). However, the majority of lymphoedema cases in developed countries occur secondary to other pathologies, for example breast cancer radiation/surgery (Szuba et al. 2003), where 30–50% of breast cancer survivors undergoing axillary node dissection ultimately suffer from lymphoedema (Rockson, 1998; Armer, 2005). Lymphatic pump dysfunction in this context could result from a number of factors, including contractile dysfunction, abnormal lymphangiogenesis, barrier dysfunction and valve defects. Each is discussed in the next section.
Contractile dysfunction
Contractile dysfunction is a common theme among several pathologies that involve the lymphatic system. Pioneering studies of human patients with chronic lymphoedema provide evidence of lymphatic contractile dysfunction. Olszewski and colleagues cannulated collecting lymphatic vessels in the dependent extremities of such patients and recorded pressures, either end‐on or side‐on, when the limbs were placed in various positions (Olszewski et al. 1968; Olszewski, 2002). Vessels in patients with lymphoedema were enlarged, exhibited weak contractions and elevated lymphatic diastolic pressures with the limbs in a dependent position. More recently, another group studying lymphatic transport in humans with breast cancer lymphoedema using lymphoscintigraphy has demonstrated that a component of the lymphatic dysfunction observed in these patients is lymphatic pump failure (Modi et al. 2007). That group is now employing genetic screening of human patients presenting with various forms of lymphoedema to identify new genetic mutations (Fotiou et al. 2015), some of which may regulate lymphatic contractile function.
Whether lymphatic pump dysfunction precedes the development of lymphoedema, or lymphoedema overloads the capacity of the lymphatics to precipitate contractile dysfunction, is difficult to determine. This question is particularly challenging in humans as patients are not seen in the clinic until lymphoedema is already established. Animal models of lymphoedema can, in principle, be used to distinguish between cause and effect, but the severity of lymphoedema produced in rodent models is usually quite limited (Shin et al. 2003). More recent rodent models (Mendez et al. 2012 a,b) consistently produce lymphoedema, and those models might be useful particularly if combined with newly developed imaging approaches to assess lymphatic contractile function in vivo (Liao et al. 2011; Proulx et al. 2013; Kwon et al. 2014). Likewise, ex vivo methods for quantifying murine lymphatic contractile function (Scallan & Davis, 2013) could be used in conjunction with non‐invasive, in vivo imaging techniques to assess collecting lymphatic contractile function at various time points during and after the development of lymphoedema (Dongaonkar et al. 2013).
Inflammation
Lymphatic contractile dysfunction is often contingent with tissue inflammation, and post‐surgical infection is a significant risk factor for developing secondary lymphoedema. Histological changes in lymphatic capillary size and density are widely assessed in both human disease states, yet experimental animal models often fail to address the pump function of the collecting vessels. The lymphatic expansion seen in disease states may actually suggest a deficit in lymphatic pumping, resulting in an activation of a compensatory lymphangiogenesis that nevertheless fails to resolve the pumping insufficiency (Tammela et al. 2007). Inflammatory modulation of lymphatic pump function was first described in the 1980s as a consequence of endotoxin infusion in sheep (Elias et al. 1987), where the actions of prostaglandins (Johnston et al. 1983; Ohhashi & Azuma, 1984) were hypothesized to contribute to the oedema observed in sepsis. The formation of reactive oxygen species (ROS), a hallmark of inflammation, also negatively influences lymphatic contractions (Zawieja et al. 1991) and ROS production as a result of fluorescent dye excitation for prolonged periods during in vivo imaging sessions could become a significant confounding variable when assessing lymphatic contractile function.
Current research on lymphatic contractile dysfunction in inflammation has focused heavily on the production of NO by regional iNOS positive immune cells (Liao et al. 2011). Recent studies have demonstrated the significant investiture of immune cells, primarily myeloid‐derived cells, in the adventitia of lymphatic collecting vessels, and their presence appears to be dynamically regulated in disease states and associated with adverse lymphatic function (Cromer et al. 2015; Chakraborty et al. 2015 b). Even after removal from the tissue, a typical collecting lymphatic vessel still retains significant populations of immune cells (neutrophils, macrophages, monocytes, dendritic cells, mast cells) that reside on and within the wall, and can modulate contraction (Chatterjee & Gashev, 2012, 2014; Chakraborty et al. 2015 b). As described above, NO is a potent inhibitor of lymphatic contractions and is produced in abundance by iNOS‐positive murine myeloid cells such as monocytes, macrophages and dendritic cells. Interleukin (IL)‐1β increases iNOS expression in cultured LECs (Cromer et al. 2014) and impairs lymphatic pump function through PGE2 production (Johnston et al. 1983; Hanley et al. 1989; Al‐Kofahi et al. 2015). However, iNOS expression and NO production can vary between different mouse strains and this must be taken into consideration when using in vivo models to assess the consequences of inflammation and lymphatic pumping. Furthermore, the lack of iNOS expression or significant NO production by human macrophages (Schneemann et al. 1993; Gross et al. 2014) in response to classical inflammatory agents raises critical questions concerning the translation of lymphatic dysfunction in rodent inflammatory models to human disease.
Lymphatic dysfunction is also present in lymphatic networks of the ear in the apolipoprotein E knockout (ApoE −/−) mouse model of hypercholesterolaemia (Lim et al. 2009). Dysfunction results in part from profound structural abnormalities in the lymphatic vasculature: initial lymphatic vessels are enlarged and collecting vessels assume an immature phenotype, with greatly decreased smooth muscle cell coverage, impaired valve formation or maintenance, and upregulation of the lymphatic capillary marker, Lyve1. It has yet to be demonstrated whether loss of mural cell coverage occurs in tissue beds outside of the ear, but if so, impairment of contractile function and lymph transport would certainly be expected.
Rheumatoid arthritis is a chronic inflammatory joint disease. In tumour necrosis factor (TNF)α‐overexpressing mice, the popliteal lymph node enlarges during the pre‐arthritic ‘expanding’ phase, and subsequently ‘collapses’. The collapsed phase is associated with the loss of the intrinsic lymphatic pulse in vivo (which may represent pumping strength, contraction frequency, or both) as assessed using a pressurized occluding cuff in conjunction with near infrared fluorescence (NIRF) imaging (Bouta et al. 2014). Consistent with contractile dysfunction, the pumping pressure of popliteal afferent lymphatics in those mice was found to be significantly elevated compared to the pumping pressure in wild‐type (WT) control mice in the expanding phase; however, pressure decreased below that of WT vessels in the collapsed phase. Interestingly, lymph node pressure followed the inverse pattern. Altogether, these changes result in a severely compromised lymph flow associated with collapsed lymph nodes in this mouse model.
Crohn's disease and inflammatory bowel disease (IBD)
The earliest descriptions of Crohn's disease or ‘regional ileitis’ by prominent clinical pathologists involved observations of lymphangectasia, oedema and lymphatic‐associated granulomas. The regional heterogeneity of the disease was suggested to be due to obstruction or failure of the lymphatics draining that region; however, the majority of current IBD research fails to account for an underlying component of lymphatic dysfunction (Van Kruiningen & Colombel, 2008). Artificial sclerosing or damage of the mesenteric collecting lymphatic vessels is able to recapitulate many of the phenotypes of Crohn's disease (Kalima, 1970) and chemically induced rodent models of IBD exhibit lymphatic contractile dysfunction. Guinea pig mesenteric vessels isolated from a 2,4,6‐trinitrobenzenesulfonic acid (TNBS)‐induced model of ileitis exhibit a dramatic loss of spontaneous contractions and enlarged collecting vessels (Wu et al. 2006), both of which are linked to NO‐dependent activation of KATP channels (Mathias & von der Weid, 2013). Mesenteric vessels from rats with TNBS‐induced colitis also show impaired lymphatic contraction regulation despite being isolated from the ileum and remote from the active site of TNBS administration (Cromer et al. 2015). While IBD studies in mice replicate the increased lymphatic density and remodelling observed in human disease, there are currently no data available on mouse lymphatic contractile function in this disease (Alexander et al. 2010; Ganta et al. 2010; Rahier et al. 2011). This is in part due to the lack of propulsive lymphatic contractions of murine mesenteric collecting lymphatics, which raises important questions regarding the role of lymphatic contractile dysfunction in the pathology of these diseases in the mouse and the usefulness of that species to replicate this particular aspect of human pathology.
Obesity and metabolic syndrome
Despite being a historical risk factor for the development of post‐surgical lymphoedema in cancer patient survivors (Ahmed et al. 2011), we know relatively little about the effects of obesity on lymphatic function (Chakraborty et al. 2010; Mehrara & Greene, 2014). Clinical data continue to accumulate, linking obesity to lymphatic dysfunction with the emergence of massive localized lymphoedema in the morbidly obese population, which appears to be functionally, but not structurally, related to the obesity (Vasileiou et al. 2011). As mentioned above, mice heterozygous for the lymphatic transcription factor Prox1 consistently develop adult onset obesity (Harvey et al. 2005; Sabine et al. 2015). While functional characterization of the lymphatic pump has not been performed in Prox1 +/− mice, those mice display significant structural abnormalities. However, an obese phenotype has not been observed in other models of disrupted lymphatic development (Harvey et al. 2005). In humans, massive localized lymphoedema appears to be largely predicated on body mass index, although obese patients probably also fall under the spectrum of the metabolic syndrome, an amalgamation of metabolic impairments that are largely driven by obesity (Grundy, 2004). Mesenteric lymphatic vessels isolated from a high‐fructose‐fed, non‐obese rat model of metabolic syndrome were shown to have a significant reduction in lymphatic pumping as a consequence of reduced contraction frequency (Zawieja et al. 2012). The high‐fructose fed Sprague–Dawley rat does not gain weight and adiposity changes are controversial, despite consistent presentation with elevated serum insulin, glucose, cholesterol and triglycerides (Oron‐Herman et al. 2008). However, a diet‐induced obesity mouse model recapitulates the impaired pressure–FREQ response of lymphatic collecting vessels observed in vivo using NIRF imaging (Blum et al. 2014), and interstitial flow and lymphatic capillary recruitment may be impaired in the tissue remodelling associated with the disease (Weitman et al. 2013). The primary defect in contractile function in both of these cases appears to be a reduction in FREQ. This effect is similar to the lymphatic dysfunction observed in aged rats, which also display a reduced FREQ (Akl et al. 2011) associated with increased ROS production in the vessel wall (Thangaswamy et al. 2012). These observations may point to a common underlying mechanism of dysfunction under conditions of obesity or metabolic stress, perhaps suggesting a role for a metabolic sensor such as the KATP channel.
Fibrosis and chronic inflammation
A clinical hallmark in lymphoedema patients is a predisposition for, and the presence of, elevated extracellular matrix deposition and fibrosis (Rockson, 2013). Unsurprisingly, fibrosis is a significant factor in the aetiology of both human obesity (Henegar et al. 2008; Divoux et al. 2010) and Crohn's disease (Lockhart‐Mummery & Morson, 1960), where fibrosis may contribute to the inhibition of fluid return and lymphatic function in those disease states. Fibrosis is often the result of chronic inflammation and typically is associated with the activation of the Th2 inflammatory paradigm and the production of IL‐6, IL‐13, IL‐4 and transforming growth factor (TGF)β. These cytokines have profound influences on altered macrophage accumulation and polarization (Mosser & Edwards, 2008; Ghanta et al. 2015), fibroblast activation and muscle cell dedifferentiation into a secretory phenotype. Recent studies that have attempted to delineate the role of fibrosis in lymphoedema have relied heavily on gross observations using the mouse tail model and oxazolone‐induced swelling in the rat axillary lymph node dissection model. The resolution of lymphoedema in the mouse tail model depends on the ability of the wound to fill in and for superficial lymphatic vessels to reform connections. This process is accelerated by the application of vascular endothelial growth factor C (VEGF‐C), which promotes lymphangiogenesis (Yoon et al. 2003; Rutkowski et al. 2006), or the blockade of IL‐4 and TGFβ, which otherwise inhibit lymphangiogenesis (Clavin et al. 2008; Avraham et al. 2010, 2013). Similar patterns emerge in experiments using the rat axillary node oxazolone/dissection model, suggesting that fibrosis plays an important role in inflammation‐induced swelling in that preparation (Lynch et al. 2015).
These studies highlight the negative impact of fibrosis on lymphatic function and raise important questions for further research. What are the effects of Th2 cytokines on the lymphatic pump function and/or contractile activity of the remaining lymphatic vessels in these models? Is the LMC phenotype and/or contractile function impaired due to the exposure to these cytokines or vessel wall remodelling? To what extent do fibrosis‐associated changes in the adventitia/matrix covering the collecting lymphatic vessels alter their function? As previously discussed, both initial and collecting lymphatic vessels are populated by multiple types of immune cells, and the influence of those cells and the cytokines they produce on LECs and LMCs are areas of active research.
Lymphatic network development/maturation/disruption
Defects in lymphangiogenesis occur in numerous mouse models deficient in genes responsible for lymphatic development, maturation, or valve formation/maintenance. Several of these models are characterized by a lack, or augmented recruitment, of smooth muscle cells to lymphatic capillaries or collecting lymphatic vessels. Aberrant LMC recruitment has been reported in mice deficient for the genes Foxc2 (Petrova et al. 2004), Reln (Lutter et al. 2012), Angpt2 (Makinen et al. 2005; Dellinger et al. 2008) and Akt1 (Zhou et al. 2010). Additionally, platelet‐specific deletion of Clec2 disrupts lymph flow, which indirectly leads to enhanced smooth muscle cell (SMC) recruitment to collecting lymphatic vessels and the thoracic duct (Sweet et al. 2015). Whether enhanced SMC recruitment or decreased SMC investiture would lead to augmented or inhibited contractile function, respectively, has yet to be directly explored.
Lymphatic vessel dilatation and network hyperplasia have also been observed in some of these and other models (Lapinski et al. 2012; Sevick‐Muraca & King, 2014) and might be predicted to have a negative impact on lymphatic pump function; however, that idea has yet to be tested. Additionally, as lymph is propelled against an adverse pressure gradient, failure of the lymphovenous valve(s) and/or collecting lymphatic valves may lead to elevation of intralymphatic pressures that the lymph pump system may be unable to overcome; this could result in regurgitation and lymph stasis in the initial lymphatic network. Prolonged exposure to elevated pressure could lead to proliferation, remodelling and contractile muscle phenotype loss. A combination of in vivo imaging techniques and intra‐lymphatic pressure recordings in intact animals will ultimately help answer these questions.
Barrier dysfunction
Collecting lymphatic permeability is regulated and maintained at a low level (∼2 × 10−7 cm s−1), but in certain disease states the barrier properties change and filtration has been shown to increase dramatically. In mouse mesenteric lymphatics studied ex vivo, comparisons of WT and eNOS −/− vessels suggest that basal nitric oxide (NO) production increases lymphatic permeability. Exogenous NO donors increase lymphatic permeability further, while inhibitors of NO synthase decrease basal lymphatic permeability. Histamine, which elicits endogenous NO production, exerts a similar effect on lymphatic permeability (Scallan et al. 2015). Diabetic mice deficient in the leptin receptor exhibit a >100‐fold increase in lymphatic permeability. Unexpectedly, this barrier dysfunction is rescued by exposing the vessels to l‐arginine to augment NO production, indicating that severely impaired, as well as elevated, NO production can both lead to disrupted lymphatic barrier function. A plausible explanation for this result is that cAMP, which stabilizes and lowers permeability, may be reduced in LECs of diabetic animals because it is normally degraded by the enzyme phosphodiesterase 3, which is overactive in those animals due to impaired NO production. Thus, treating the diabetic lymphatic vessels with a chemical inhibitor specific for phosphodiesterase 3 rescues the barrier dysfunction in leptin receptor knockout (db/db) vessels in mice (Scallan et al. 2015).
Two other metabolic diseases in which lymphatic barrier function has been shown to be compromised are obesity, as discussed above, and hypercholesterolaemia. Injection of Evans Blue dye into the ears of ApoE −/− mice reveals gross leakage of dye out of the lymphatic vessels of the ear (Lim et al. 2009). This defect is not due to the loss of the ApoE gene itself, since it can be rescued by pharmacological block of cholesterol absorption from the gut (Lim et al. 2013). Whether lymphatic leakage occurs in other organs during hypercholesterolaemia, and the mechanisms behind the leakage, remain to be elucidated. In atherosclerotic ApoE −/− mice, the lymphatic vasculature is required for reverse cholesterol transport even from the aortic wall. Without a functional lymphatic network near the aorta, labelled cholesterol was retained within the aortic plaques, presumably due to a defect in macrophage egress (Martel et al. 2013).
Valve dysfunction
Normal lymphatic pumping requires adequate contractile strength and synchrony of lymphatic muscle as well as proper valve function. If a lymphangion is undergoing robust, large amplitude contractions, but either valve has abnormal reflux, or is completely incompetent, then forward lymph propulsion will be compromised. When lymphatic output is calculated from the product of EF and FREQ (i.e. FPF), as it is in rodent vessels (Gashev et al. 2002, 2004; Liao et al. 2011; Nagai et al. 2011), that calculation assumes normally functioning valves; output will be grossly overestimated if the valves are in any way dysfunctional.
Defects in valve development underlie multiple types of primary lymphoedema (Sabine et al. 2015). Mice engineered with complete loss of Gata2 function develop lymphoedema due to defective lymphovenous and lymphatic valves (Kazenwadel et al. 2015). Deletion of integrin α9, which with fibronectin lines the core of the lymphatic valve leaflet, results in truncated leaflets and retrograde flow from collectors to precollectors (Bazigou et al. 2009). Likewise, loss of function mutations of one allele of FOXC2 in humans result in lymphoedema distichiasis, which is characterized by lymphoedema in dependent extremities (Mellor et al. 2007). Foxc2 +/− mice lack about 50% of lymphatic (and venous) valves and exhibit many of the same systemic defects (Petrova et al. 2004). Inducible Foxc2 null (Foxc2 lecKO) mice have a more severe phenotype, dying within a few days if induced shortly after birth, or at ∼5 months of age if induced at 4 weeks, providing evidence that Foxc2 is critical for lymphatic valve maintenance (Sabine et al. 2015). We recently applied some of the functional tests described above to valves in Fox2 lecKO mice. The valves exhibited a 4‐ to 9‐fold increase in pressure back‐leak compared to WT mice when outflow pressure was raised (Sabine et al. 2015). Defects in the valve closure–diameter relationship were also noted (M. J. Davis, unpublished observations), which would predict that pump output should be severely compromised even in the absence of changes in lymphatic muscle contractile function. Other than this example, pump function tests have not been reported for any genetically altered mice with valve defects; however, the application of such tests to newly developed mouse models of human primary lymphoedema (Lapinski et al. 2012; Kazenwadel et al. 2015; Geng et al. 2016) will be important for assessing the mechanism and severity of valve and/or pump dysfunction in those models.
Conclusions
Lymphatic pump function is essential for normal lymph transport, particularly in dependent extremities. Pump function is determined by the intrinsic properties of lymphatic muscle, which is regulated by lymphatic preload, afterload, spontaneous contraction rate, contractility and neural influences. In addition, normal pump function depends on a coordinated LMC contraction wave, and proper LEC valve and barrier function. Each of these parameters may be compromised to a different extent in inherited and acquired forms of lymphoedema, as well as in diseases that have a secondary component of lymphatic dysfunction, including inflammatory diseases such as IBD, Crohn's disease, rheumatoid arthritis, obesity, diabetes and metabolic syndrome. The tools for investigating the roles of each factor are becoming available and can be applied to the study of lymphatic vessels in genetically modified mice, offering hope for the development of therapeutic strategies to target lymphatic dysfunction in humans, even in diseases that were previously unknown to contain a lymphatic component.
Additional information
Competing interests
None declared.
Funding
This work was supported by NIH grants K99 HL124142 to J.P.S., and R01‐HL‐120867, HL‐122608 and HL‐122578 to M.J.D.
Supporting information
Movie S1. Several spontaneous contraction cycles of a popliteal afferent collecting lymphatic vessel (pressurized to 3 cm H2O, ex vivo) from a Prox1GFP mouse under brightfield illumination.
Movie S2. Same vessel as in Movie S1 recorded under confocal fluorescence illumination, with excitation at 499 nm and emission at 530± 10 nm.
Movie S3. Confocal fluorescence recording of lymphatic valve leaflets from a Prox1GFP mouse during a contraction cycle.
Acknowledgements
Prox1GFP mice were kindly provided by Y. K. Hong at the University of Southern California.
Biographies
Joshua P. Scallan is an Assistant Professor of Molecular Pharmacology and Physiology at the University of South Florida. His research focuses on the regulation of lymphatic endothelial permeability under physiological and pathophysiological conditions using transgenic models.
Michael J. Davis is a Margaret Proctor Mulligan Professor of Medical Research in the Department of Medical Pharmacology and Physiology at the University of Missouri. He has a long‐standing interest in mechanotransduction by vascular smooth muscle. His recent work focuses on the mechanical and electrophysiological properties of lymphatic smooth muscle and endothelium in genetically engineered mice.

References
- Ahmed RL, Schmitz KH, Prizment AE & Folsom AR (2011). Risk factors for lymphedema in breast cancer survivors, the Iowa Women's Health Study. Breast Cancer Res Treat 130, 981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akl TJ, Nagai T, Cote GL & Gashev AA (2011). Mesenteric lymph flow in adult and aged rats. Am J Physiol Heart Circ Physiol 301, H1828–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Kofahi M, Becker F, Gavins FN, Woolard MD, Tsunoda I, Wang Y, Ostanin D, Zawieja DC, Muthuchamy M, von der Weid PY & Alexander JS (2015). IL‐1β reduces tonic contraction of mesenteric lymphatic muscle cells, with the involvement of cycloxygenase‐2 and prostaglandin E2 . Br J Pharmacol 172, 4038–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertine KH, Fox LM & O'Morchoe CC (1982). The morphology of canine lymphatic valves. Anat Rec 202, 453–461. [DOI] [PubMed] [Google Scholar]
- Alessandrini C, Gerli R, Sacchi G, Ibba L, Pucci AM & Fruschelli C (1981). Cholinergic and adrenergic innervation of mesenterial lymph vessels in guinea pig. Lymphology 14, 1–6. [PubMed] [Google Scholar]
- Alexander JS, Chaitanya GV, Grisham MB & Boktor M (2010). Emerging roles of lymphatics in inflammatory bowel disease. Ann NY Acad Sci 1207, Suppl. 1, E75–85. [DOI] [PubMed] [Google Scholar]
- Allen JM & McHale NG (1988). The effect of known K+‐channel blockers on the electrical activity of bovine lymphatic smooth muscle. Pflugers Arch 411, 167–172. [DOI] [PubMed] [Google Scholar]
- Amerini S, Ziche M, Greiner ST & Zawieja DC (2004). Effects of substance P on mesenteric lymphatic contractility in the rat. Lymphat Res Biol 2, 2–10. [DOI] [PubMed] [Google Scholar]
- Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA & Randolph GJ (2004). Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity 21, 561–574. [DOI] [PubMed] [Google Scholar]
- Armer JM (2005). The problem of post‐breast cancer lymphedema: impact and measurement issues. Cancer Invest 23, 76–83. [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H & Alitalo K (2015). A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212, 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG & Mehrara BJ (2010). Blockade of transforming growth factor‐β1 accelerates lymphatic regeneration during wound repair. Am J Pathol 177, 3202–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham T, Zampell JC, Yan A, Elhadad S, Weitman ES, Rockson SG, Bromberg J & Mehrara BJ (2013). Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J 27, 1114–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E & McDonald DM (2007). Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 204, 2349–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E & Makinen T (2013). Flow control in our vessels: vascular valves make sure there is no way back. Cell Mol Life Sci 70, 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Wilson JT & Moore JE Jr (2014). Primary and secondary lymphatic valve development: molecular, functional and mechanical insights. Microvasc Res 96, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, Adams R, Muro AF, Sheppard D & Makinen T (2009). Integrin‐α9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell 17, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit JN, Zawieja DC, Goodman AH & Granger HJ (1989). Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol 257, H2059–H2069. [DOI] [PubMed] [Google Scholar]
- Blum KS, Karaman S, Proulx ST, Ochsenbein AM, Luciani P, Leroux JC, Wolfrum C & Detmar M (2014). Chronic high‐fat diet impairs collecting lymphatic vessel function in mice. PLoS One 9, e94713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen HG, Gasheva OY & Zawieja DC (2011). Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am J Physiol Heart Circ Physiol 301, H1897–H1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouta EM, Wood RW, Brown EB, Rahimi H, Ritchlin CT & Schwarz EM (2014). In vivo quantification of lymph viscosity and pressure in lymphatic vessels and draining lymph nodes of arthritic joints in mice. J Physiol 592, 1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvree K, Brunet I, Del Toro R, Gordon E, Prahst C, Cristofaro B, Mathivet T, Xu Y, Soueid J, Fortuna V, Miura N, Aigrot MS, Maden CH, Ruhrberg C, Thomas JL & Eichmann A (2012). Semaphorin3A, Neuropilin‐1, and PlexinA1 are required for lymphatic valve formation. Circ Res 111, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice G, Child AH, Evans A, Bell R, Mansour S, Burnand K, Sarfarazi M, Jeffery S & Mortimer P (2005). Milroy disease and the VEGFR‐3 mutation phenotype. J Med Genet 42, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice G, Mansour S, Bell R, Collin JRO, Child AH, Brady AF, Sarfarazi M, Burnand KG, Jeffery S, Mortimer PS & Murday VA (2002). Analysis of the phenotypic abnormalities in lymphoedema‐distichiasis syndrome in 74 patients with FOXC2 mutations or linkage to 16q24. J Med Genet 39, 478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs Boedtkjer D, Rumessen J, Baandrup U, Skov Mikkelsen M, Telinius N, Pilegaard H, Aalkjaer C & Hjortdal V (2013). Identification of interstitial Cajal‐like cells in the human thoracic duct. Cells Tissues Organs 197, 145–158. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Davis MJ & Muthuchamy M (2015. a). Emerging trends in the pathophysiology of lymphatic contractile function. Semin Cell Dev Biol 38, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Zawieja S, Wang W, Zawieja DC & Muthuchamy M (2010). Lymphatic system: a vital link between metabolic syndrome and inflammation. Ann NY Acad Sci 1207, Suppl. 1, E94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Zawieja SD, Wang W, Lee Y, Wang YJ, von der Weid PY, Zawieja DC & Muthuchamy M (2015. b). Lipopolysaccharide modulates neutrophil recruitment and macrophage polarization on lymphatic vessels and impairs lymphatic function in rat mesentery. Am J Physiol Heart Circ Physiol 309, H2042–H2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AK & von der Weid PY (2003). 5‐HT decreases contractile and electrical activities in lymphatic vessels of the guinea‐pig mesentery: role of 5‐HT7‐receptors. Br J Pharmacol 139, 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee V & Gashev AA (2012). Aging‐associated shifts in functional status of mast cells located by adult and aged mesenteric lymphatic vessels. Am J Physiol Heart Circ Physiol 303, H693–H702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee V & Gashev AA (2014). Mast cell‐directed recruitment of MHC class II positive cells and eosinophils towards mesenteric lymphatic vessels in adulthood and elderly. Lymphat Res Biol 12, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavin NW, Avraham T, Fernandez J, Daluvoy SV, Soares MA, Chaudhry A & Mehrara BJ (2008). TGF‐β1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol 295, H2113–H2127. [DOI] [PubMed] [Google Scholar]
- Cotton KD, Hollywood MA, McHale NG & Thorbury KD (1997). Outward currents in smooth muscle cells isolated from sheep mesenteric lymphatics. J Physiol 503, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer W, Wang W, Zawieja SD, von der Weid PY, Newell‐Rogers MK & Zawieja DC (2015). Colonic insult impairs lymph flow, increases cellular content of the lymph, alters local lymphatic microenvironment, and leads to sustained inflammation in the rat ileum. Inflamm Bowel Dis 21, 1553–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer WE, Zawieja SD, Tharakan B, Childs EW, Newell MK & Zawieja DC (2014). The effects of inflammatory cytokines on lymphatic endothelial barrier function. Angiogenesis 17, 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe MJ, von der Weid PY, Brock JA & Van Helden DF (1997). Co‐ordination of contractile activity in guinea‐pig mesenteric lymphatics. J Physiol 500, 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Davis AM, Ku CW & Gashev AA (2009). Myogenic constriction and dilation of isolated lymphatic vessels. Am J Physiol Heart Circ Physiol 296, H293–H302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Davis AM, Lane MM, Ku CW & Gashev AA (2008. a). Rate‐sensitive contractile responses of lymphatic vessels to circumferential stretch. J Physiol 587, 165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Lane MM, Davis AM, Durtschi D, Zawieja DC, Muthuchamy M & Gashev AA (2008. b). Modulation of lymphatic muscle contractility by the neuropeptide substance P. Am J Physiol Heart Circ Physiol 295, H587–H597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Rahbar E, Gashev AA, Zawieja DC & Moore JE (2011). Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am J Physiol Heart Circ Physiol 301, H48–H60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Scallan JP, Wolpers JH, Muthuchamy M, Gashev AA & Zawieja DC (2012). Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. Am J Physiol Heart Circ Physiol 303, H795–H808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit C, Roos F, Bolz SS & Pohl U (2003). Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol Genomics 13, 169–177. [DOI] [PubMed] [Google Scholar]
- Dellinger M, Hunter R, Bernas M, Gale N, Yancopoulos G, Erickson R & Witte M (2008). Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin‐2 deficient mice. Dev Biol 19, 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre‐Millo M, Poitou C, Zucker JD, Bedossa P & Clement K (2010). Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 59, 2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JB (2010). Lymphatic lipid transport: sewer or subway? Trends Endocrinol Metab 21, 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongaonkar RM, Nguyen TL, Quick CM, Hardy J, Laine GA, Wilson E & Stewart RH (2013). Adaptation of mesenteric lymphatic vessels to prolonged changes in transmural pressure. Am J Physiol Heart Circ Physiol 305, H203–H210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhoffer J, Kagal A, Klein T & Johnston MG (1995). Importance of valves and lymphangion contractions in determining pressure gradients in isolated lymphatics exposed to elevations in outflow pressure. Microvasc Res 49, 97–110. [DOI] [PubMed] [Google Scholar]
- Elias RM, Johnston MG, Hayashi A & Nelson W (1987). Decreased lymphatic pumping after intravenous endotoxin administration in sheep. Am J Physiol 253, H1349–H1357. [DOI] [PubMed] [Google Scholar]
- Elias RM, Wandolo G, Ranadive NS, Eisenhoffer J & Johnston MG (1990). Lymphatic pumping in response to changes in transmural pressure is modulated by erythrolysate/hemoglobin. Circ Res 67, 1097–1106. [DOI] [PubMed] [Google Scholar]
- Emerson GG & Segal SS (2000). Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries – Role in vasomotor control. Circ Res 87, 474–479. [DOI] [PubMed] [Google Scholar]
- Engeset A, Olszewski W, Jaeger PM, Sokolowski J & Theodorsen L (1977). Twenty‐four hour variation in flow and composition of leg lymph in normal men. Acta Physiol Scand 99, 140–148. [DOI] [PubMed] [Google Scholar]
- Ferrell RE, Baty CJ, Kimak MA, Karlsson JM, Lawrence EC, Franke‐Snyder M, Meriney SD, Feingold E & Finegold DN (2010). GJC2 missense mutations cause human lymphedema. Am J Hum Genet 86, 943–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold DN, Baty CJ, Knickelbein KZ, Perschke S, Noon SE, Campbell D, Karlsson JM, Huang D, Kimak MA, Lawrence EC, Feingold E, Meriney SD, Brufsky AM & Ferrell RE (2012). Connexin 47 mutations increase risk for secondary lymphedema following breast cancer treatment. Clin Cancer Res 18, 2382–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotiou E, Martin‐Almedina S, Simpson MA, Lin S, Gordon K, Brice G, Atton G, Jeffery I, Rees DC, Mignot C, Vogt J, Homfray T, Snyder MP, Rockson SG, Jeffery S, Mortimer PS, Mansour S & Ostergaard P (2015). Novel mutations in PIEZO1 cause an autosomal recessive generalized lymphatic dysplasia with non‐immune hydrops fetalis. Nat Commun 6, 8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganta VC, Cromer W, Mills GL, Traylor J, Jennings M, Daley S, Clark B, Mathis JM, Bernas M, Boktor M, Jordan P, Witte M & Alexander JS (2010). Angiopoietin‐2 in experimental colitis. Inflamm Bowel Dis 16, 1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashev AA, Davis MJ, Delp MD & Zawieja DC (2004). Regional variations of contractile activity in isolated rat lymphatics. Microcirculation 11, 477–492. [DOI] [PubMed] [Google Scholar]
- Gashev AA, Davis MJ & Zawieja DC (2002). Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 450, 1023–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasheva OY, Gashev AA & Zawieja DC (2013). Cyclic guanosine monophosphate and the dependent protein kinase regulate lymphatic contractility in rat thoracic duct. J Physiol 591, 4549–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Cha B, Mahamud MR, Lim KC, Silasi‐Mansat R, Uddin MK, Miura N, Xia L, Simon AM, Engel JD, Chen H, Lupu F & Srinivasan RS (2016). Multiple mouse models of primary lymphedema exhibit distinct defects in lymphovenous valve development. Dev Biol 409, 218–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanta S, Cuzzone DA, Torrisi JS, Albano NJ, Joseph WJ, Savetsky IL, Gardenier JC, Chang D, Zampell JC & Mehrara BJ (2015). Regulation of inflammation and fibrosis by macrophages in lymphedema. Am J Physiol Heart Circ Physiol 308, H1065–H1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnepp DR & Green FHY (1980). Scanning electron microscopic study of canine lymphatic vessels and their valves. Lymphology 13, 91–99. [PubMed] [Google Scholar]
- Gross TJ, Kremens K, Powers LS, Brink B, Knutson T, Domann FE, Philibert RA, Milhem MM & Monick MM (2014). Epigenetic silencing of the human NOS2 gene: rethinking the role of nitric oxide in human macrophage inflammatory responses. J Immunol 192, 2326–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM (2004). Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 89, 2595–2600. [DOI] [PubMed] [Google Scholar]
- Guarna M, Pucci AM, Alessandrini C, Volpi N, Fruschelli M, D'Antona D & Fruschelli C (1991). Peptidergic innervation of mesenteric lymphatics in guinea pigs: an immunocytochemical and pharmacological study. Lymphology 24, 161–167. [PubMed] [Google Scholar]
- Gui P, Li M, Hill MA & Davis MJ (2014). KCNQ and ERG channels control the rate of diastolic depolarization and electrical pacemaking frequency in lymphatic muscle. FASEB J 28, Suppl., 666.663. [Google Scholar]
- Hanley CA, Elias RM, Movat HZ & Johnston MG (1989). Suppression of fluid pumping in isolated bovine mesenteric lymphatics by interleukin‐1: interaction with prostaglandin E2 . Microvasc Res 37, 218–229. [DOI] [PubMed] [Google Scholar]
- Harvey NL (2008). The link between lymphatic function and adipose biology. Ann NY Acad Sci 1131, 82–88. [DOI] [PubMed] [Google Scholar]
- Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW & Oliver G (2005). Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult‐onset obesity. Nat Genet 37, 1072–1081. [DOI] [PubMed] [Google Scholar]
- Henegar C, Tordjman J, Achard V, Lacasa D, Cremer I, Guerre‐Millo M, Poitou C, Basdevant A, Stich V, Viguerie N, Langin D, Bedossa P, Zucker JD & Clement K (2008). Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol 9, R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess PR, Rawnsley DR, Jakus Z, Yang Y, Sweet DT, Fu J, Herzog B, Lu M, Nieswandt B, Oliver G, Makinen T, Xia L & Kahn ML (2014). Platelets mediate lymphovenous hemostasis to maintain blood‐lymphatic separation throughout life. J Clin Invest 124, 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollywood MA, Cotton KD, Thornbury KD & McHale NG (1997. a). Isolated sheep mesenteric lymphatic smooth muscle cells possess both T‐ and L‐type calcium currents. J Physiol 501.P, 109–110P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollywood MA, Cotton KD, Thornbury KD & McHale NG (1997. b). Tetrodotoxin‐sensitive sodium current in sheep lymphatic smooth muscle. J Physiol 503, 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollywood MA & McHale NG (1994). Mediation of excitatory neurotransmission by the release of ATP and noradrenaline in sheep mesenteric lymphatic vessels. J Physiol 481, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K, Rayner SE, von der Weid PY, Zhao J, Imtiaz MS & van Helden DF (2006). Calcitonin gene‐related peptide activates different signaling pathways in mesenteric lymphatics of guinea pigs. Am J Physiol Heart Circ Physiol 290, H813–H822. [DOI] [PubMed] [Google Scholar]
- Jamalian S, Davis MJ, Zawieja DC & Moore JE Jr (2016). Network scale modeling of lymph transport and its effective pumping parameters. PLoS One 11, e0148384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MG, Kanalec A & Gordon JL (1983). Effects of arachidonic acid and its cyclo‐oxygenase and lipoxygenase products on lymphatic vessel contractility in vitro. Prostaglandins 25, 85–98. [DOI] [PubMed] [Google Scholar]
- Jurisic G, Maby‐El Hajjami H, Karaman S, Ochsenbein AM, Alitalo A, Siddiqui SS, Ochoa Pereira C, Petrova TV & Detmar M (2012). An unexpected role of semaphorin3a‐neuropilin‐1 signaling in lymphatic vessel maturation and valve formation. Circ Res 111, 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalima TV (1970). Experimental lymphatic obstruction in the ileum. Ann Chir Gynaecol Fenn 59, 187–201. [PubMed] [Google Scholar]
- Kanady JD, Dellinger MT, Munger SJ, Witte MH & Simon AM (2011). Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev Biol 354, 253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazenwadel J, Betterman KL, Chong CE, Stokes PH, Lee YK, Secker GA, Agalarov Y, Demir CS, Lawrence DM, Sutton DL, Tabruyn SP, Miura N, Salminen M, Petrova TV, Matthews JM, Hahn CN, Scott HS & Harvey NL (2015). GATA2 is required for lymphatic vessel valve development and maintenance. J Clin Invest 125, 2979–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriederman BM, Myloyde TL, Witte MH, Dagenais SL, Witte CL, Rennels M, Bernas MJ, Lynch MT, Erickson RP, Caulder MS, Miura N, Jackson D, Brooks BP & Glover TW (2003). FOXC2 haploinsufficient mice are a model for human autosomal dominant lymphedema‐distichiasis syndrome. Hum Mol Genet 12, 1179–1185. [DOI] [PubMed] [Google Scholar]
- Kuan EL, Ivanov S, Bridenbaugh EA, Victora G, Wang W, Childs EW, Platt AM, Jakubzick CV, Mason RJ, Gashev AA, Nussenzweig M, Swartz MA, Dustin ML, Zawieja DC & Randolph GJ (2015). Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node – homing adipose tissue dendritic cells. J Immunol 194, 5200–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S, Agollah GD, Wu G & Sevick‐Muraca EM (2014). Spatio‐temporal changes of lymphatic contractility and drainage patterns following lymphadenectomy in mice. PLoS One 9, e106034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapinski PE, Kwon S, Lubeck BA, Wilkinson JE, Srinivasan RS, Sevick‐Muraca E & King PD (2012). RASA1 maintains the lymphatic vasculature in a quiescent functional state in mice. J Clin Invest 122, 733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauweryns J (1971). Stereomicroscopic funnel‐like architecture of pulmonary lymphatic valves. Lymphology 4, 125–132. [PubMed] [Google Scholar]
- Leak LV & Burke JF (1968). Electron microscopic study of lymphatic capillaries in the removal of connective tissue fluids and particulate substances. Lymphology 1, 39–52. [PubMed] [Google Scholar]
- Lee S, Roizes S & von der Weid PY (2014). Distinct roles of L‐ and T‐type voltage‐dependent Ca2+ channels in regulation of lymphatic vessel contractile activity. J Physiol 592, 5409–5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick JR & Michel CC (2010). Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res 87, 198–210. [DOI] [PubMed] [Google Scholar]
- Li B, Silver I, Szalai JP & Johnston MG (1998). Pressure‐volume relationships in sheep mesenteric lymphatic vessels in situ: response to hypovolemia. Microvasc Res 56, 127–138. [DOI] [PubMed] [Google Scholar]
- Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, Munn LL, Ruddle NH, Jain RK, Fukumura D & Padera TP (2011). Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci USA 108, 18784–18789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HY, Rutkowski JM, Helft J, Reddy ST, Swartz MA, Randolph GJ & Angeli V (2009). Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am J Pathol 175, 1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HY, Thiam CH, Yeo KP, Bisoendial R, Hii CS, McGrath KC, Tan KW, Heather A, Alexander JS & Angeli V (2013). Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR‐BI‐mediated transport of HDL. Cell Metab 17, 671–684. [DOI] [PubMed] [Google Scholar]
- Lockhart‐Mummery HE & Morson BC (1960). Crohn's disease (regional enteritis) of the large intestine and its distinction from ulcerative colitis. Gut 1, 87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH & Kipnis J (2015). Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter S, Xie S, Tatin F & Makinen T (2012). Smooth muscle‐endothelial cell communication activates Reelin signaling and regulates lymphatic vessel formation. J Cell Biol 197, 837–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch LL, Mendez U, Waller AB, Gillette AA, Guillory RJ 2nd & Goldman J (2015). Fibrosis worsens chronic lymphedema in rodent tissues. Am J Physiol Heart Circ Physiol 308, H1229–H1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R & Wilkinson GA (2005). PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev 19, 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, Bittman R, Tall AR, Chen SH, Thomas MJ, Kreisel D, Swartz MA, Sorci‐Thomas MG & Randolph GJ (2013). Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest 123, 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias R & von der Weid PY (2013). Involvement of the NO‐cGMP‐K(ATP) channel pathway in the mesenteric lymphatic pump dysfunction observed in the guinea pig model of TNBS‐induced ileitis. Am J Physiol Gastrointest Liver Physiol 304, G623–G634. [DOI] [PubMed] [Google Scholar]
- McCloskey KD, Hollywood MA, Thornbury KD, Ward SM & McHale NG (2002). Kit‐like immunopositive cells in sheep mesenteric lymphatic vessels. Cell Tissue Res 310, 77–84. [DOI] [PubMed] [Google Scholar]
- McCloskey KD, Toland HM, Hollywood MA, Thornbury KD & McHale NG (1999). Hyperpolarization‐activated inward current in isolated sheep mesenteric lymphatic smooth muscle. J Physiol 521, 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale NG (1990). Lymphatic innervation. Blood Vessels 27, 127–136. [DOI] [PubMed] [Google Scholar]
- McHale NG & Meharg MK (1992). Co‐ordination of pumping in isolated bovine lymphatic vessels. J Physiol 450, 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale NG & Roddie IC (1976). The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J Physiol 261, 255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale NG, Roddie IC & Thornbury KD (1980). Nervous modulation of spontaneous contractions in bovine mesenteric lymphatics. J Physiol 309, 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale NG, Thornbury KD & Hollywood MA (2000). 5‐HT inhibits spontaneous contractility of isolated sheep mesenteric lymphatics via activation of 5‐HT4 receptors. Microvasc Res 60, 261–268. [DOI] [PubMed] [Google Scholar]
- Mehrara BJ & Greene AK (2014). Lymphedema and obesity: is there a link? Plast Reconstr Surg 134, 154e–160e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor RH, Brice G, Stanton AWB, French J, Smith A, Jeffery S, Levick JR, Burnand KG & Mortimer PS (2007). Mutations in FOXC2 are strongly associated with primary valve failure in veins of the lower limb. Circulation 115, 1912–1920. [DOI] [PubMed] [Google Scholar]
- Mellor RH, Tate N, Stanton AWB, Hubert C, Mäkinen T, Smith A, Burnard KG, Jeffery S, Levick JR & Mortimer PS (2011). Mutations in FOXC2 in humans (lymphoedema distichiasis syndrome) cause lymphatic dysfunction on dependency. J Vasc Res 48, 397–407. [DOI] [PubMed] [Google Scholar]
- Mendez U, Brown EM, Ongstad EL, Slis JR & Goldman J (2012. a). Functional recovery of fluid drainage precedes lymphangiogenesis in acute murine foreleg. Am J Physiol Heart Circ Physiol 302, H2250–H2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez U, Stroup EM, Lynch LL, Waller AB & Goldman J (2012. b). A chronic and latent lymphatic insufficiency follows recovery from acute lymphedema in the rat foreleg. Am J Physiol Heart Circ Physiol 303, H1107–H1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignini F, Sabbatini M, Coppola L & Cavallotti C (2012). Analysis of nerve supply pattern in human lymphatic vessels of young and old men. Lymphat Res Biol 10, 189–197. [DOI] [PubMed] [Google Scholar]
- Mislin H & Schipp R (1966). Structural and functional relations of the lymph vessels In Rüttiman A. (ed.), Progress in Lymphology. Proceedings of the International Symposium on Lymphology, Zurich, Switzerland, Jul 19–23, New York: Hafner, pp. 360–365. [Google Scholar]
- Miyahara H, Kawai Y & Ohhashi T (1994). 5‐Hydroxytryptamine‐2 and ‐4 receptors located on bovine isolated mesenteric lymphatics. J Pharmacol Exp Ther 271, 379–385. [PubMed] [Google Scholar]
- Mizuno R, Ono N & Ohhashi T (1999). Involvement of ATP‐sensitive K+ channels in spontaneous activity of isolated lymph microvessels in rats. Am J Physiol 277, H1453–H1456. [DOI] [PubMed] [Google Scholar]
- Modi S, Stanton AWB, Svensson WE, Peters AM, Mortimer PS & Levick JR (2007). Human lymphatic pumping measured in healthy and lymphoedematous arms by lymphatic congestion lymphoscintigraphy. J Physiol 583, 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM & Edwards JP (2008). Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8, 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtomaki A, Uh MK, Katajewski C, Zhao J, Nagasaki T, Shawber CJ & Kitajewski J (2014). Notch signaling functions in lymphatic valve formation. Development 141, 2446–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuchamy M, Gashev A, Boswell N, Dawson N & Zawieja D (2003). Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J 17, 920–922. [DOI] [PubMed] [Google Scholar]
- Muthuchamy M & Zawieja D (2008). Molecular regulation of lymphatic contractility. Ann NY Acad Sci 1131, 89–99. [DOI] [PubMed] [Google Scholar]
- Nagai T, Bridenbaugh EA & Gashev AA (2011). Aging‐associated alterations in contractility of rat mesenteric lymphatic vessels. Microcirculation 18, 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizamutdinova IT, Maejima D, Nagai T, Bridenbaugh E, Thangaswamy S, Chatterjee V, Meininger CJ & Gashev AA (2014). Involvement of histamine in endothelium‐dependent relaxation of mesenteric lymphatic vessels. Microcirculation 21, 640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normen C, Ivanov KI, Cheng J, Zangger N, Delorenzi M, Jaquet M, Muira N, Puolakkainen P, Horsley V, Hu J, Augustin HG, Yla‐Herttuala S, Alitalo K & Petrova TV (2009). FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J Cell Biol 195, 439–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohhashi T & Azuma T (1984). Variegated effects of prostaglandins on spontaneous activity in bovine mesenteric lymphatics. Microvasc Res 27, 71–80. [DOI] [PubMed] [Google Scholar]
- Ohhashi T, Olschowka JA & Jacobowitz DM (1983). Vasoactive intestinal peptide inhibitory innervation in bovine mesenteric lymphatics. A histochemical and pharmacological study. Circ Res 53, 535–538. [DOI] [PubMed] [Google Scholar]
- Ohhashi T & Takahashi N (1991). Acetylcholine‐induced release of endothelium‐derived relaxing factor from lymphatic endothelial cells. Am J Physiol 260, H1172–H1178. [DOI] [PubMed] [Google Scholar]
- Olszewski WL (2002). Contractility patterns of normal and pathologically changed human lymphatics. Ann NY Acad Sci 979, 52–63. [DOI] [PubMed] [Google Scholar]
- Olszewski WL & Engeset A (1980). Intrinsic contractility of prenodal lymph vessels and lymph flow in human leg. Am J Physiol 239, H775–H783. [DOI] [PubMed] [Google Scholar]
- Olszewski WL, Kruszewski S, Sokolowski J, Zgliczynski L & Nielubowicz J (1968). Observations of movements of lymph vessels in patients with lymphoedema of the limbs. Polski Tygodnik Lekarski 23, 1345–1347. (In Polish.) [PubMed] [Google Scholar]
- Oron‐Herman M, Kamari Y, Grossman E, Yeger G, Peleg E, Shabtay Z, Shamiss A & Sharabi Y (2008). Metabolic syndrome: comparison of the two commonly used animal models. Am J Hypertens 21, 1018–1022. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer PS, Yla‐Herttuala S, Miura N & Alitalo K (2004). Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med 10, 974–981. [DOI] [PubMed] [Google Scholar]
- Proulx ST, Luciani P, Christiansen A, Karaman S, Blum KS, Rinderknecht M, Leroux JC & Detmar M (2013). Use of a PEG‐conjugated bright near‐infrared dye for functional imaging of rerouting of tumor lymphatic drainage after sentinel lymph node metastasis. Biomaterials 34, 5128–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahier JF, De Beauce S, Dubuquoy L, Erdual E, Colombel JF, Jouret‐Mourin A, Geboes K & Desreumaux P (2011). Increased lymphatic vessel density and lymphangiogenesis in inflammatory bowel disease. Aliment Pharmacol Ther 34, 533–543. [DOI] [PubMed] [Google Scholar]
- Renkin EM (1986). Some consequences of capillary permeability to macromolecules: Starling's hypothesis revisited. Am J Physiol Heart Circ Physiol 250, H706–H710. [DOI] [PubMed] [Google Scholar]
- Rockson SG (1998). Precipitating factors in lymphedema: myths and realities. Cancer 15, 2814–2816. [DOI] [PubMed] [Google Scholar]
- Rockson SG (2013). The lymphatics and the inflammatory response: lessons learned from human lymphedema. Lymphat Res Biol 11, 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski JM, Moya M, Johannes J, Goldman J & Swartz MA (2006). Secondary lymphedema in the mouse tail: Lymphatic hyperplasia, VEGF‐C upregulation, and the protective role of MMP‐9. Microvasc Res 72, 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabine A, Agalarov Y, Maby‐El Hajjami H, Jaquet M, Hagerling R, Pollmann C, Bebber D, Pfenniger A, Miura N, Dormond O, Calmes JM, Adams RH, Makinen T, Kiefer F, Kwak BR & Petrova TV (2012). Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic‐valve formation. Dev Cell 22, 430–445. [DOI] [PubMed] [Google Scholar]
- Sabine A, Bovay E, Demir CS, Kimura W, Jaquet M, Agalarov Y, Zangger N, Scallan JP, Graber W, Gulpinar E, Kwak BR, Makinen T, Martinez‐Corral I, Ortega S, Delorenzi M, Kiefer F, Davis MJ, Djonov V, Miura N & Petrova TV (2015). FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J Clin Invest 125, 3861–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabine A & Petrova TV (2014). Interplay of mechanotransduction, FOXC2, connexins, and calcineurin signaling in lymphatic valve formation. Adv Anat Embryol Cell Biol 214, 67–80. [DOI] [PubMed] [Google Scholar]
- Sanders KM & Ward SM (2008). Interstitial cells of Cajal: a new perspective on smooth muscle function. J Physiol 576, 721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Senadheera S, Bertrand PP, Murphy TV & Tare M (2012). Myoendothelial contacts, gap junctions, and microdomains: anatomical links to function? Microcirculation 19, 403–415. [DOI] [PubMed] [Google Scholar]
- Sarnoff SJ, Mitchell JH & Remensnyder JP (1960). Homeometric autoregulation in the heart. Circ Res 8, 1077–1091. [DOI] [PubMed] [Google Scholar]
- Scallan JP & Davis MJ (2013). Genetic removal of basal nitric oxide enhances contractile activity in isolated murine collecting lymphatic vessels. J Physiol 591, 2139–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan JP, Davis MJ & Huxley VH (2013). Permeability and contractile responses of collecting lymphatic vessels elicited by atrial and brain natriuretic peptides. J Physiol 591, 5071–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan JP, Hill MA & Davis MJ (2015). Lymphatic vascular integrity is disrupted in type 2 diabetes due to impaired nitric oxide signalling. Cardiovasc Res 107, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan JP & Huxley VH (2010). In vivo determination of collecting lymphatic vessel permeability to albumin: a role for lymphatics in exchange. J Physiol 588, 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan JP, Wolpers JH & Davis MJ (2012. a). Constriction of isolated collecting lymphatic vessels in response to acute increases in downsteam pressure. J Physiol 591, 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan JP, Wolpers JH, Muthuchamy M, Zawieja DC, Gashev AA & Davis MJ (2012. b). Independent and interactive effects of preload and afterload on the pump function of the isolated lymphangion. Am J Physiol Heart Circ Physiol 303, H809–H824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid‐Schönbein GW (1990). Microlymphatics and lymph flow. Physiol Rev 70, 987–1028. [DOI] [PubMed] [Google Scholar]
- Schneemann M, Schoedon G, Hofer S, Blau N, Guerrero L & Schaffner A (1993). Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis 167, 1358–1363. [DOI] [PubMed] [Google Scholar]
- Schroedl F, Kaser‐Eichberger A, Schlereth SL, Bock F, Regenfuss B, Reitsamer HA, Lutty GA, Maruyama K, Chen L, Lutjen‐Drecoll E, Dana R, Kerjaschki D, Alitalo K, De Stefano ME, Junghans BM, Heindl LM & Cursiefen C (2014). Consensus statement on the immunohistochemical detection of ocular lymphatic vessels. Invest Ophthalmol Vis Sci 55, 6440–6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevick‐Muraca EM & King PD (2014). Lymphatic vessel abnormalities arising from disorders of Ras signal transduction. Trends Cardiovasc Med 24, 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin WS, Szuba A & Rockson SG (2003). Animal models for the study of lymphatic insufficiency. Lymphat Res Biol 1, 159–169. [DOI] [PubMed] [Google Scholar]
- Simon AM & McWhorter AR (2002). Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev Biol 251, 206–220. [DOI] [PubMed] [Google Scholar]
- Smith RO (1949). Lymphatic contractility; a possible intrinsic mechanism of lymphatic vessels for the transport of lymph. J Exp Med 90, 497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro RJ (2011). Regulation of Cardiac Contractility. Morgan & Claypool Life Sciences, San Rafael, CA, USA. [PubMed] [Google Scholar]
- Srinivasan RS & Oliver G (2011). Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev 25, 2187–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet DT, Jimenez JM, Chang J, Hess PR, Mericko‐Ishizuka P, Fu J, Xia L, Davies PF & Kahn ML (2015). Lymph flow regulates collecting lymphatic vessel maturation in vivo. J Clin Invest 125, 2995–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuba A, Shin WS, Strauss HW & Rockson SG (2003). The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med 44, 43–57. [PubMed] [Google Scholar]
- Tammela T, Saaristo A, Holopainen T, Lyytikka J, Kotronen A, Pitkonen M, Abo‐Ramadan U, Yla‐Herttuala S, Petrova TV & Alitalo K (2007). Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med 13, 1458–1466. [DOI] [PubMed] [Google Scholar]
- Telinius N, Baandrup U, Rumessen J, Pilegaard H, Hjortdal V, Aalkjaer C & Boedtkjer DB (2014. a). The human thoracic duct is functionally innervated by adrenergic nerves. Am J Physiol Heart Circ Physiol 306, H206–H213. [DOI] [PubMed] [Google Scholar]
- Telinius N, Kim S, Pilegaard H, Pahle E, Nielsen J, Hjortdal V, Aalkjaer C & Boedtkjer DB (2014. b). The contribution of K+ channels to human thoracic duct contractility. Am J Physiol Heart Circ Physiol 307, H33–H43. [DOI] [PubMed] [Google Scholar]
- Telinius N, Majgaard J, Kim S, Katballe N, Pahle E, Nielsen J, Hjortdal V, Aalkjaer C & Boedtkjer DB (2015). Voltage‐gated sodium channels contribute to action potentials and spontaneous contractility in isolated human lymphatic vessels. J Physiol 593, 3109–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telinius N, Mohanakumar S, Majgaard J, Kim S, Pilegaard H, Pahle E, Nielsen J, de Leval M, Aalkjaer C, Hjortdal V & Boedtkjer DB (2014. c). Human lymphatic vessel contractile activity is inhibited in vitro but not in vivo by the calcium channel blocker nifedipine. J Physiol 592, 4697–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaswamy S, Bridenbaugh EA & Gashev AA (2012). Evidence of increased oxidative stress in aged mesenteric lymphatic vessels. Lymphat Res Biol 10, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd GL & Bernard GR (1973). The sympathetic innervation of the cervical lymphatic duct of the dog. Anat Rec 177, 303–315. [DOI] [PubMed] [Google Scholar]
- Vajda J & Tomcsik M (1971). The structure of valves in the lymphatic vessels. Anat Rec 78, 521–531. [DOI] [PubMed] [Google Scholar]
- van Helden DF (1993). Pacemaker potentials in lymphatic smooth muscle of the guinea‐pig mesentery. J Physiol 471, 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kruiningen HJ & Colombel JF (2008). The forgotten role of lymphangitis in Crohn's disease. Gut 57, 1–4. [DOI] [PubMed] [Google Scholar]
- Vasileiou AM, Bull R, Kitou D, Alexiadou K, Garvie NJ & Coppack SW (2011). Oedema in obesity; role of structural lymphatic abnormalities. Int J Obes (Lond) 35, 1247–1250. [DOI] [PubMed] [Google Scholar]
- Venugopal AM, Stewart RH, Laine GA, Dongaonkar RM & Quick CM (2007). Lymphangion coordination minimally affects mean flow in lymphatic vessels. Am J Physiol Heart Circ Physiol 293, H1183–H1189. [DOI] [PubMed] [Google Scholar]
- von der Weid PY (1998). ATP‐sensitive K+ channels in smooth muscle cells of guinea‐pig mesenteric lymphatics: role in nitric oxide and β‐adrenoceptor agonist‐induced hyperpolarizations. Br J Pharmacol 125, 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid PY, Crowe MJ & Van Helden DF (1996). Endothelium‐dependent modulation of pacemaking in lymphatic vessels of the guinea‐pig mesentery. J Physiol 493, 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid PY, Rahman M, Imtiaz MS & van Helden DF (2008. a). Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: pharmacology and implication for spontaneous contractility. Am J Physiol Heart Circ Physiol 295, H1989–H2000. [DOI] [PubMed] [Google Scholar]
- von der Weid PY, Rahman M, Imtiaz MS & Van Helden DF (2008. b). Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: pharmacology and implication for spontaneous contractility. Am J Physiol Heart Circ Physiol 295, H1989–H2000. [DOI] [PubMed] [Google Scholar]
- von der Weid PY, Rehal S, Dyrda P, Lee S, Mathias R, Rahman M, Roizes S & Imtiaz MS (2012). Mechanisms of VIP‐induced inhibition of the lymphatic vessel pump. J Physiol 590, 2677–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid PY & Van Helden DF (1997). Functional electrical properties of the endothelium in lymphatic vessels of the guinea‐pig mesentery. J Physiol 504, 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid PY & Zawieja DC (2004). Lymphatic smooth muscle: the motor unit of lymph drainage. Int J Biochem Cell Biol 36, 1147–1153. [DOI] [PubMed] [Google Scholar]
- Wagner C, de Wit C, Kurtz L, Grunberger C, Kurtz A & Schweda F (2007). Connexin 40 is essential for the pressure control of renin synthesis and secretion. Circ Res 100, 556–563. [DOI] [PubMed] [Google Scholar]
- Weitman ES, Aschen SZ, Farias‐Eisner G, Albano N, Cuzzone DA, Ghanta S, Zampell JC, Thorek D & Mehrara BJ (2013). Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One 8, e70703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JB (1991). Best and Taylor's Physiological Basis of Medical Practice. Williams & Wilkins, Baltimore, MD, USA. [Google Scholar]
- Wiig H & Swartz MA (2012). Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev 92, 1005–1060. [DOI] [PubMed] [Google Scholar]
- Wilson JT, van Loon R, Wang W, Zawieja DC & Moore JE Jr (2015). Determining the combined effect of the lymphatic valve leaflets and sinus on resistance to forward flow. J Biomech 48, 3593–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TF, Carati CJ, MacNaughton WK & von der Weid PY (2006). Contractile activity of lymphatic vessels is altered in the TNBS model of guinea pig ileitis. Am J Physiol Gastrointest Liver Physiol 291, G566–G574. [DOI] [PubMed] [Google Scholar]
- Yoon YS, Murayama T, Gravereaux E, Tkebuchava T, Silver M, Curry C, Wecker A, Kirchmair R, Hu CS, Kearney M, Ashare A, Jackson DG, Kubo H, Isner JM & Losordo DW (2003). VEGF‐C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J Clin Invest 111, 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawieja DC, Davis KL, Schuster R, Hinds WM & Granger HJ (1993). Distribution, propagation, and coordination of contractile activity in lymphatics. Am J Physiol Heart Circ Physiol 264, H1283–H1291. [DOI] [PubMed] [Google Scholar]
- Zawieja DC, Greiner ST, Davis KL, Hinds WM & Granger HJ (1991). Reactive oxygen metabolites inhibit spontaneous lymphatic contractions. Am J Physiol 260, H1935–H1943. [DOI] [PubMed] [Google Scholar]
- Zawieja DC, von der Weid P‐Y & Gashev AA (2011). Microlymphatic biology In Comprehensive Physiology, ed. Tuma R, Duran W. & Ley K. Wiley. [Google Scholar]
- Zawieja SD, Wang W, Wu X, Nepiyushchikh ZV, Zawieja DC & Muthuchamy M (2012). Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol 302, H643–H653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Chang Z, Zhang L, Hong YK, Shen B, Wang B, Lu G, Tvorogov D, Hemmings BA, Yang Z & He Y (2010). Akt/protein kinase B is required for lymphatic network formation, remodeling, and valve development. Am J Pathol 1777, 2124–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach BW & Prather JW (1975). Micromanipulation of pressure in terminal lymphatics in the rat mesentery. Am J Physiol 228, 1326–1335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Several spontaneous contraction cycles of a popliteal afferent collecting lymphatic vessel (pressurized to 3 cm H2O, ex vivo) from a Prox1GFP mouse under brightfield illumination.
Movie S2. Same vessel as in Movie S1 recorded under confocal fluorescence illumination, with excitation at 499 nm and emission at 530± 10 nm.
Movie S3. Confocal fluorescence recording of lymphatic valve leaflets from a Prox1GFP mouse during a contraction cycle.


