Abstract
The main task of the immune system is to distinguish and respond accordingly to ‘danger’ or ‘non‐danger’ signals. This is of critical importance in the gastrointestinal tract in which immune cells are constantly in contact with food antigens, symbiotic microflora and potential pathogens. This complex mixture of food antigens and symbionts are essential for providing vital nutrients, so they must be tolerated by the intestinal immune system to prevent aberrant inflammation. Therefore, in the gut the balance between immune activation and tolerance should be tightly regulated to maintain intestinal homeostasis and to prevent hypersensitivity to harmless luminal antigens. Loss of this delicate equilibrium can lead to abnormal activation of the intestinal immune system resulting in devastating gastrointestinal disorders such as inflammatory bowel disease (IBD). Recent evidence supports the idea that the central nervous system interacts dynamically via the vagus nerve with the intestinal immune system to modulate inflammation through humoral and neural pathways, using a mechanism also referred to as the intestinal cholinergic anti‐inflammatory pathway. In this review, we will focus on the current understanding of the mechanisms and neuronal circuits involved in the intestinal cholinergic anti‐inflammatory pathway. Further investigation on the crosstalk between the nervous and intestinal immune system will hopefully provide new insights leading to the identification of innovative therapeutic approaches to treat intestinal inflammatory diseases.
Abbreviations
- 5‐HT4R
5‐hydroxytryptamine 4 receptor
- α7nAChR
α7‐subtype of the nicotinic acetylcholine receptor
- CAIP
cholinergic anti‐inflammatory pathway
- CD
Crohn's disease
- CSF‐1
colony stimulating factor 1
- DMV
dorsal motor nucleus of the vagus
- DNBS
2,4‐dinitrobenzenesulfonic acid
- DSS
dextran sulfate sodium
- IBD
inflammatory bowel disease
- IL‐6
interleukin‐6
- IL‐10
interleukin‐10
- JAK2
Janus kinase 2
- NF‐κB
nuclear factor κ‐light‐chain‐enhancer of activated B cells
- POI
postoperative ileus
- STAT3
signal transducer and activator of transcription 3
- TGF‐β
transforming growth factor β
- TNF‐α
tumour necrosis factor α
- UC
ulcerative colitis
- VNS
vagus nerve stimulation
Introduction
The gastrointestinal system is one of the largest vulnerable surfaces of our body continuously facing the external environment, including microbiota, nutrients, pollutants, and harmful pathogens. This result in mutual benefits represented by co‐habitation and at the same time it provides protection against pathogens. To maintain intestinal homeostasis, the mucosal immune system is able to recognize pathogenic insults and mount an inflammatory response; however, at the same time it is able to be ‘tolerant’ to innocuous food antigens and microbiota. This education and maturation of the intestinal immune system is the result of millions of years of co‐evolution with host‐specific microbiota and dietary intake. Disruption of these homeostatic mechanisms can result in undesired immune reactions leading to intestinal disorders, such as inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn's disease (CD) (Veldhoen & Brucklacher‐Waldert, 2012; Thorburn et al. 2014).
In the last two decades, it has become evident that the communication between the nervous and immune system can influence the inflammatory response via the release of neurotransmitters, cytokines and hormones (Steinman, 2004; Sternberg, 2006). Several mechanisms have been described within the nervous system that can execute this anti‐inflammatory effect. For example, the release of glucocorticoids via the hypothalamic–pituitary–adrenal axis and the adrenergic nervous system have been shown to modulate the immune system by reducing inflammation (Haddad et al. 2002; Silverman et al. 2005; Bellinger et al. 2008).
More recently, Tracey and coworkers demonstrated that stimulation of the parasympathetic nervous system via the vagus nerve led to lower cytokine production and increased survival in a rodent model of sepsis, introducing the novel concept of the ‘cholinergic anti‐inflammatory pathway’ (CAIP) (Borovikova et al. 2000; Tracey, 2002). This was the first experimental proof of the direct interaction between the central nervous system (CNS) and the immune system, with a direct regulation of inflammation by means of neuronal circuitry in peripheral tissues.
Currently, it is proposed that sensory neurons are activated by inflammatory mediators, released during local tissue inflammation, and subsequently send signals regarding the microenvironment to the CNS. In turn, efferent nerve fibres release neuromediators in the periphery to modulate local immune cells (Tracey, 2009). Taking into account the extensive innervation of the gastrointestinal tract, it is not surprising that the nervous system, via the vagus nerve, appears to play a major role in modulating immune activation in the gut wall (Fig. 1). In this review, we will focus on the anti‐inflammatory role of the parasympathetic nervous system in the gastrointestinal tract and discuss current knowledge and clinical implication of the intestinal CAIP.
Figure 1. Schematic overview of the crosstalk between the nervous and immune system in the gastrointestinal tract .
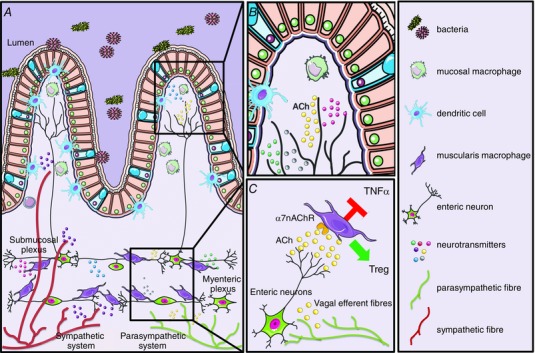
The gastrointestinal tract has extensive innervation provided by the enteric nervous system and by extrinsic fibres from the sympathetic and parasympathetic nervous system. A, schematic representation of the intestinal wall with its different layers, showing the distribution of the intrinsic and extrinsic innervation and their relationship with the intestinal immune cells. Parasympathetic efferent fibres innervate the intestinal wall by contacting exclusively the enteric neurons located in the myenteric plexus region. Instead, sympathetic efferent fibres are also in direct contact with the mucosa and with intestinal immune cells located in the submucosal and mucosal compartment. B, in the mucosal villi, several immune cells such as macrophages, dendritic cells and T cells are affected by the release of neurotransmitters, such as ACh, produced by neuronal fibres. C, in the myenteric plexus, close proximity between enteric neurons and resident macrophages has been described in several publications. Based on anatomical and functional evidence, it has been hypothesized that secretion of ACh by cholinergic enteric neurons influences the phenotype of resident macrophages resulting in inhibition of TNF‐α and induction of regulatory T cells.
The cholinergic anti‐inflammatory pathway
In 2000, during the investigation of the anti‐inflammatory properties of the p38 MAP kinase inhibitor, semapimod (CNI‐1493), Tracey and colleagues revealed for the first time the vagal anti‐inflammatory effect using a model of carrageenan‐induced paw oedema (Borovikova et al. 2000). Both intracerebroventricular application of CNI‐1493 and electrical stimulation of the transected peripheral vagus nerve protected against the development of acute inflammation resulting in oedema. These anti‐inflammatory effects were abrogated by bilateral vagotomy, suggesting that the intact vagus nerve is necessary for CNI‐1493 activity. A few years later, Wang and coworkers identified the missing link between the vagus nerve and macrophage activation as the anti‐inflammatory action of vagal stimulation was absent in mice lacking the α7‐subtype of the nicotinic acetylcholine receptor (α7nAChR) (Wang et al. 2003). The cholinergic regulation of the inflammatory response seems to be mediated by the presence of the classical receptor from the neurotransmitter‐gated superfamily of ion channels, α7nAChR, on non‐neuronal cells such as macrophages (de Jonge & Ulloa, 2007). Besides the classical induction of ion channel fluxes, α7nAChR stimulation in macrophages results in the inhibition of lipopolysaccharide‐mediated activation of the nuclear factor κ‐light‐chain‐enhancer of activated B cells (NF‐κB) transcription factor (Wang et al. 2004). A critical component of the nicotinic anti‐inflammatory effect, at least in macrophages, is mediated via the activation of the Janus kinase 2 (JAK2)–signal transducer and activator of transcription 3 (STAT3) signalling pathway. Stimulation of α7nAChR with nicotine triggers activation of its catalytic intracellular domain leading to recruitment and phosphorylation of the tyrosine kinase JAK2, and subsequent activation of the transcription factor STAT3, resulting in direct inhibition of inflammatory cytokine production (de Jonge et al. 2005).
Since the spleen is one of the major sources of inflammatory mediators during systemic inflammation such as sepsis (Huston et al. 2006; Qin et al. 2006; Huston et al. 2008), it represents an ideal target for modulation of the immune response. Initial experiments indeed indicated the spleen as a target organ of the vagus nerve controlling tumour necrosis factor α (TNF‐α) production during sepsis (Huston et al. 2006). The anatomy of the splenic innervation, in particular the cholinergic or vagal component, is, however, still a matter of debate. Close interposition of immune cells with nerve terminals could be identified, but these fibres are of an adrenergic nature. Although these data indicate adrenergic modulation of splenic macrophages, in vitro data strongly suggest cholinergic inhibition of splenic macrophages via the expression of α7nAChR. In contrast to the initial hypothesis proposing direct contact between vagal nerve fibres and splenic macrophages (Tracey, 2002), it is now clear that in the spleen the vagus nerve rather indirectly modulates the innate immune response by activating adrenergic neurons in the prevertebral ganglia. In line with this hypothesis, only in mice with an intact and innervated spleen, vagus nerve stimulation (VNS) is able to exert its anti‐inflammatory effect (Huston et al. 2006). Recent studies suggest that ACh released by the vagus nerve in the celiac mesenteric ganglia activates postsynaptic α7nAChR on adrenergic neurons of the splenic nerve, leading to the release of noradrenaline in the spleen (Rosas‐Ballina et al. 2011). There, adrenergic nerve fibres stimulate ACh synthesis by memory splenic T cells interacting with α7nAChR located on adjacent macrophages (Rosas‐Ballina et al. 2011).
Thus, it is supposed that in the case of systemic inflammation, cytokines and/or endotoxins are present in detectable amounts in the circulation resulting in the activation of different areas of the CNS. As these areas project to the autonomic motor neurons in the brain stem connected to peripheral organs via the vagus nerve, this will result in activation of the cholinergic pathway, thereby modulating inflammatory response via the so‐called ‘inflammatory reflex’ (Tracey, 2002). In the case of more localized peripheral inflammation, circulating cytokines are absent or too low to activate the circumventricular organs. Yet, the CNS is informed on the presence of inflammation through ‘sensory’ neuronal fibres innervating the periphery (Andersson & Tracey, 2012).
The intestinal cholinergic anti‐inflammatory pathway
The intestinal immune system needs to preserve a delicate equilibrium between tolerance to harmless antigens and an effective immune response to pathogens. When this equilibrium is disrupted, an immune response against innocuous antigens can be induced leading to devastating chronic inflammatory conditions such as IBD. It has been shown that the microenvironment in the mucosa and submucosa plays a critical role in maintaining this equilibrium. Several factors, such as transforming growth factor β (TGF‐β), interleukin‐10 (IL‐10), retinoic acid and short chain fatty acids, have already been described in depth for their immunomodulatory effects in the intestine (Veldhoen & Brucklacher‐Waldert, 2012; Thorburn et al. 2014). Lately, experimental and clinical evidence suggest that an additional actor, the parasympathetic nervous system via the vagus nerve, is playing a crucial role in modulating the intestinal microenvironment, preserving tissue homeostasis and immune tolerance (Fig. 1 and Table 1).
Table 1.
Overview of studies investigating the role of the CAIP in experimental models of gastrointestinal disorders
| Disease | Model/treatment | Effect | Authors |
|---|---|---|---|
| Colitis | α7nAChR−/− mice | Increased colitis severity | Ghia et al. 2009 |
| Vagotomy and α7nAChr antagonist | Increased colitis severity | Ghia et al. 2006, 2008, 2011; Bianchi, 2007; O'Mahony et al. 2009; Ji et al. 2014 | |
| Adoptive transfer of macrophages from vagotomized mice | Increased colitis severity | Ghia et al. 2011 | |
| VNS | Decreased colitis severity | Sun et al. 2013 | |
| α7nAChR agonist | Decreased colitis severity | Bianchi, 2007; Ghia et al. 2007, 2009, 2011; Snoek et al. 2010 | |
| AChE inhibitors | Decreased colitis severity | Miceli & Jacobson, 2003; Ji et al. 2014 | |
| Nicotine | Decreased colitis severity | Galitovskiy et al. 2011; Hayashi et al. 2014 | |
| Ileus | Vagotomy | Increased ileus severity | de Jonge et al. 2005 |
| α7nAChR agonist | Reduced intestinal inflammation and improved postoperative ileus | de Jonge et al. 2005 | |
| Activation of cholinergic enteric neurons | Reduced intestinal inflammation and improved postoperative ileus | Tsuchida et al. 2011; Gomez‐Pinilla et al. 2014 | |
| VNS | Reduced intestinal inflammation and improved postoperative ileus | Matteoli et al. 2014; The et al. 2007 | |
| VNS in α7nAChR−/− mice | VNS is ineffective in α7nAChr−/− mice during post‐operative ileus | Matteoli et al. 2014 | |
| Pancreatitis | α7nAChr agonist GTS‐21 | Decreased pancreatitis severity | van Westerloo et al. 2006 |
| Vagotomy and α7nAChr antagonist | Increased pancreatitis severity | van Westerloo et al. 2006 | |
| Nicotine treatment | Decreased pancreatitis severity | Schneider et al. 2014 |
In contrast to the spleen, a direct vagal communication between the gut wall and the CNS has been experimentally proven in the gastrointestinal tract. Using anterograde tracers injected into the dorsal motor nucleus of the vagus (DMV), efferent vagal nerve terminals were shown to directly synapse with postganglionic neurons located in the enteric nervous system, rather than interacting with neurons in the prevertebral ganglia (Berthoud et al. 1990, 1991). Using the same experimental approach, a typical rostro‐caudal gradient of vagal preganglionic innervation has been reported with the highest density of vagal fibres observed in the stomach followed by a subsequent decrease in the small bowel and colon (Berthoud et al. 1990).
Vagal innervation appears to be vital for maintaining functional and anatomical intestinal homeostasis and informing the CNS concerning the immunological and nutritional status of the gastrointestinal tract. Fascinatingly, the vagus nerve mediates signalling from the gut microbiota linking emotional and cognitive centres of the brain with peripheral intestinal functions, resulting during dysbiosis in central nervous disorders such as autism and anxiety‐depressive behaviours (Carabotti et al. 2015; Mayer et al. 2015). Similarly, during local intestinal inflammation, direct activation of vagal nuclei such as the nucleus of the solitary tract has been reported by our group in a murine model of postoperative ileus (POI) (Cailotto et al. 2011). Interestingly, motor neurons of the dorsal nucleus of the vagus nerve, directly connected to the inflamed area, were also activated, which is compatible with the existence of a hard‐wired ‘inflammatory reflex’ (Cailotto et al. 2011).
Although many intestinal immune cells express nicotinic receptors to react on the CAIP (de Jonge et al. 2005), macrophages and dendritic cells are described as the main effectors of this pathway. In line with this, it has been demonstrated that vagotomized mice developed more severe dextran sulfate sodium (DSS)‐induced colitis as compared with control mice. Moreover, the same group showed that vagotomy had no effect in macrophage‐deficient mice, indicating a critical role for the presence of macrophages (Ghia et al. 2006). In addition, they have shown that adoptive transfer of macrophages isolated from vagotomized wild‐type mice into colony stimulating factor 1 deficient (CSF1−/−) mice resulted in severe colitis, suggesting again a central role of macrophages in the vagal anti‐inflammatory effect (Ghia et al. 2011). In a more recent study, the pro‐inflammatory effect of vagotomy during colitis was correlated with a reduction of splenic CD4+CD25+Foxp3+ regulatory T cells (O'Mahony et al. 2009). Interestingly, in the same study adoptive transfer of splenic CD4+CD25− T lymphocytes isolated from vagotomized mice increased the severity of colitis in DSS‐treated recipients (O'Mahony et al. 2009).
In support of these findings, two recent studies by Ghia's research group elegantly showed that also central activation of the cholinergic pathway, via the administration of the acetylcholinesterase inhibitor galantamine or treatment with muscarinic acetylcholine receptor agonist, reduced mucosal inflammation both in DSS and in 2,4‐dinitrobenzenesulfonic acid (DNBS)‐induced colitis (Ji et al. 2014; Munyaka et al. 2014). This effect was the direct consequence of reduced pro‐inflammatory cytokine secretion and maturation of splenic dendritic cells in an α7nAChR‐dependent fashion. Of note, the anti‐inflammatory effect was abolished in mice with vagotomy, splenic neurectomy, or splenectomy, indicating that central cholinergic activation of a vagus nerve‐to‐spleen circuit controls intestinal inflammation and this regulation can be explored to develop novel therapeutic strategies (Ji et al. 2014; Munyaka et al. 2014).
In line with these data, systemic nicotine treatment can suppress acute DSS‐induced colitis by downregulation of pro‐inflammatory cytokines interleukin‐6 (IL‐6) and TNF‐α (Ghia et al. 2006; Hayashi et al. 2014). Moreover, the acetylcholine esterase inhibitors neostigmine and physostigmine were able to significantly attenuate macroscopic damage, influx of myeloperoxidase positive cells and smooth muscle thickness in a rodent DNBS model of colitis (Miceli & Jacobson, 2003).
Contrary to sepsis, the crucial role of the α7nAChR still remains ambiguous in colitis. In a model of depression‐induced colitis, α7nAChR−/− mice had a higher severity of acute DSS‐induced colitis, which was reduced after treatment with choline chloride (an α7nAChR specific agonist) (Ghia et al. 2009). On the other hand, Snoek et al. described that treatment with specific α7nAChR agonists (AR‐R17779 and GSK1345038A) reduced inflammation, inhibiting NF‐κB activity and cytokine expression without improving the clinical signs of colitis (Snoek et al. 2010). In Table 1 an overview of the role of the CAIP in the gastrointestinal tract is shown. So far, no conclusive data support the involvement of the α7nAChR in the vagal anti‐inflammatory effect in colitis. Thus, possible therapeutic approaches based on the activation of α7nAChR for the treatment of colitis should be evaluated with caution.
Another subset of intestinal resident macrophages, which are located between the muscle layers of the intestinal wall at the level of the myenteric plexus, is also regulated by the CAIP. Since these macrophages are located in a highly innervated area of the gut, they might therefore be strongly affected by secreted neuronal factors. By means of a murine model of POI, a disease characterized by localized muscularis externa inflammation, it has been shown that electrical stimulation of the vagus nerve lowered intestinal muscular inflammation, reduced cytokine production and decreased recruitment of inflammatory immune cells (de Jonge et al. 2005). This protective effect was later demonstrated to be independent of the spleen but mediated by local release of ACh inhibiting activation of resident macrophages expressing α7nAChR (Matteoli et al. 2014). Indeed, direct pharmacological engagement of α7nAChR on these resident macrophages reduces ATP‐induced Ca2+ increase in situ, pointing towards an anti‐inflammatory effect of cholinergic neurotransmitters (Matteoli et al. 2014).
In the intestinal wall, the vagus nerve contacts immune cells only indirectly, since vagal efferents solely synapse with enteric neurons. However, recent anatomical evidence revealed a close proximity between cholinergic neuronal fibres and intestinal resident macrophages both at the level of the myenteric plexus and in the lamina propria (Cailotto et al. 2011; Nemethova et al. 2013; Matteoli et al. 2014). This suggests that stimulation of the vagus nerve leads to the activation of enteric neurons that subsequently release factors, such as ACh, that might affect the local immune system.
Interestingly, two independent studies have recently demonstrated that activation of cholinergic enteric neurons via specific chemical compounds lowered intestinal inflammation and restored gastrointestinal motility in POI (Gomez‐Pinilla et al. 2014; Tsuchida et al. 2011). In both studies, 5‐hydroxytryptamine 4 receptor (5‐HT4R) agonists were used to increase ACh release from cholinergic enteric neurons, which subsequently was able to inhibit monocyte and macrophage activation in an α7nAChR‐dependent manner (Tsuchida et al. 2011; Gomez‐Pinilla et al. 2014). Altogether, these data strongly indicate that pharmacological engagement of cholinergic enteric neurons by means of 5‐HT4R agonists might represent a novel therapeutic approach to treat intestinal immune‐mediated diseases.
Clinical significance of the intestinal cholinergic anti‐inflammatory pathway
Inflammatory bowel disease
Patients with IBD suffer from chronic inflammation in the gastrointestinal tract that can be divided into two major forms based on their clinical presentation (Rutgeerts et al. 2009). Although the inflammation in UC is mainly superficial and found in the colon, patients with CD have a transmural inflammation throughout the whole gastrointestinal tract. Despite the fact that treatment of IBD patients has been improved in the last decade with the introduction of, for example, anti‐TNF antibodies (Rutgeerts et al. 2009; Van Assche et al. 2011), there is still much room for improvement as patients still often have complications or need to undergo intestinal resection.
Various studies have tried to correlate autonomic dysfunction, such as alteration of the vagal tone, with clinical outcome in IBD patients (Jerndal et al. 2010; Rubio et al. 2014). Bonaz and colleagues reported a negative correlation between low vagal tone and increased plasma levels of TNF‐α, suggesting that the CAIP may be altered in these patients (Pellissier et al. 2014). However, a clear correlation between IBD and vagal tone has still not been convincingly verified. Recently, Clarencon et al. (2014) described the first attempt of VNS in a patient with CD. The patient, subjected to long‐term low frequency VNS, showed significant improvement with reduced clinical disease activity index and endoscopic remission (Clarencon et al. 2014). This beneficial effect was correlated with an increased parasympathetic tone. Even though a therapeutic role for VNS in IBD is proposed in this report, results should be taken with caution considering the size of the study and the fact that a placebo effect could not be ruled out using this experimental approach.
Another strong suggestion that the CAIP could have a significant impact on the pathogenesis of IBD comes from the effect of smoking in UC and CD patients. Whereas UC patients have beneficial effects of smoking with a reduced disease severity (Gheorghe et al. 2004; Hoie et al. 2007; Bastida & Beltran, 2011), CD patients will increase their risk of relapses, repeat surgeries and the need for more aggressive immunosuppressive treatment after smoking (Cosnes et al. 1996; Yamamoto, 2005; Nos & Domenech, 2011). Although the exact mechanisms of these differences in outcome are not known yet, Galitovskiy et al. (2011) have recently tried to clarify this discrepancy in mice. Within this study they have examined the different effects of nicotine in two different mouse models of colitis mimicking a Th1 or Th2 type of inflammation. Within the Th2 model of inflammation, the expression of α7nAChR was induced on CD4+ T cells after nicotine treatment, leading to increased regulatory T cells and reduced inflammation. On the contrary, the mice with a Th1 inflammation demonstrated no increase in expression of this receptor or diminished inflammation (Galitovskiy et al. 2011). These findings may explain the differential effect of smoking in UC and CD patients mediated via the expression of α7nAChR.
Selective α7nAChR agonists are currently being evaluated as potential therapeutic drugs for the treatment of cognitive impairments in schizophrenia and Alzheimer's disease. Only a very limited number of studies have been performed to evaluate their anti‐inflammatory potential in humans. Based on the observation that the selective α7nAChR agonist GTS‐21 attenuated cytokine production from whole blood and human monocytes more potently than nicotine (Kox et al. 2009; Rosas‐Ballina et al. 2009), its effect was tested in a human endotoxin model. However, no significant reduction in cytokine response to endotoxin injection in healthy subjects was observed between GTS‐21 and placebo treated subjects (Kox et al. 2011). Clinical studies evaluating the specific effect of α7nAChR activation are therefore awaited with great interest.
Postoperative ileus and other GI disorders
Despite numerous advances in surgical techniques and perioperative care, POI remains one of the most common side‐effects of abdominal surgery. It is associated with an abnormal pattern of gastrointestinal motility with additional symptoms such as nausea, vomiting, abdominal distension, intolerance to food and constipation. Many articles propose that POI is an essential phase of recovery after any abdominal procedure. However, POI has been shown to slow patient recovery and imparts a substantial financial burden on the health care system.
The pathophysiology of POI is not yet fully understood. However, it is accepted that activation of tissue resident macrophages in the myenteric plexus results in microscopic inflammation of the intestinal muscularis followed by influx of inflammatory cells such as neutrophils and monocytes (Eskandari et al. 1997; Kalff et al. 1998, 1999). The modulation of this inflammatory response by CAIP might represent an effective strategy to prevent POI (The et al. 2007). Previous preclinical studies support this hypothesis, as stimulation of the vagus nerve via electrical current (The et al. 2007), high‐fat enteral feeding (Lubbers et al. 2009) or peripheral α7nAChR activation (The et al. 2007) reduces cytokine production and infiltration of immune cells into the muscularis externa and ameliorates gastrointestinal motility.
In addition to a direct effect on immune cells, VNS also has a beneficial effect on preserving the intestinal epithelial barrier during inflammation via the release of local ACh (MacFie et al. 1999; Clark & Coopersmith, 2007). This effect is crucial in the resolution of various intestinal pathologies such as ischaemia–reperfusion or severe burn injury where a ‘leaky gut’ is the major effector in the pathogenesis of the disease. The vagal tone has been shown to have a marked anti‐inflammatory effect in several other gastrointestinal conditions as well, including pancreatitis (van Westerloo et al. 2006; Schneider et al. 2014) and leaking gut syndrome (Costantini et al. 2012). For instance, electrical stimulation of the vagus nerve was able to prevent loss of intestinal barrier function after burn injury mainly via sustaining epithelial tight junction protein expression, which is essential to prevent the translocation of microbial products into the systemic circulation (Costantini et al. 2012).
Conclusion and perspective
Increasing evidence has demonstrated that the intestinal microenvironment dictates the phenotype of mucosal and circulating immune cells at steady state and during inflammation. Nowadays, it is clear that the interaction between the nervous system and the intestinal immune cells is of great importance in maintaining tissue homeostasis and in regulating mucosal inflammatory responses. In the gastrointestinal tract, the vagus nerve seems to play a major role in setting the anti‐inflammatory cholinergic tone, via both a direct modulation of enteric neurons and an indirect interaction with splenic immune cells.
Despite the fact that the exact mechanisms and neuronal circuits of the intestinal CAIP are not yet completely understood, solid preclinical studies have demonstrated that an increased cholinergic tone (via electrical or chemical vagal nerve stimulation) reduces the inflammatory response, while alterations in this pathway result in increased disease severity and intestinal inflammation. Although data from animal studies are of great promise, so far there is no conclusive evidence that the vagus nerve plays a crucial role in controlling the human intestinal immune system. Proof of the existence of the vagal anti‐inflammatory pathway in humans will be a significant breakthrough for the clinical management of intestinal immune‐mediated inflammatory diseases. The fact that electrical stimulation of the vagus nerve is already a well‐established therapeutic option for intractable epilepsy and treatment‐resistant depression may anticipate possible clinical applications in intestinal disorders (Table 2).
Table 2.
Overview of clinical trials investigating the CAIP in gastrointestinal disorders
| Clinical Trial | Recruitment | Principle | |
|---|---|---|---|
| Identifier: | Official title | status | investigator |
| NCT01569503 | Vagus Nerve Stimulation a New Approach in the Treatment of Crohn's Disease | Recruiting | Bruno Bonaz |
| NCT01572155 | Pilot Study: Anti‐inflammatory Effect of Peroperative Stimulation of the Vagus Nerve | Completed | Guy Boeckxstaens |
| NCT02524626 | Anti‐inflammatory Effect of Peroperative Stimulation of the Vagus Nerve | Recruiting | Guy Boeckxstaens |
| NCT02311660 | Vagus Nerve Stimulation in Crohn's Disease | Recruiting | Geert D'Haens |
| NCT02620176 | The Effect of Transcutaneous Vagal Nerve Stimulation on Reducing Oesophageal Pain Hypersensitivity | Recruiting | Adam Farmer |
| NCT02388269 | Non‐invasive Vagus Nerve Stimulator Device for Treatment of Functional Dyspepsia or Irritable Bowel Syndrome | Completed | Owen Epstein |
| NCT02359188 | Transcutaneous Vagal Nerve Stimulation: Influence on miRNA, Inflammation, Cerebral Resting State and Gastric Motility | Not yet recruiting | Felix Rosenow |
| NCT02420158 | Trans‐cutaneous Vagal Nerve Electrical Stimulation and Irritable Bowel Syndrome | Recruiting | François Mion |
| NCT02425774 | Anti‐inflammatory Effect of Pre‐operative Stimulation of the Cholinergic Anti‐Inflammatory Pathway | Recruiting | Guy Boeckxstaens |
| NCT02367729 | Efficacy of Ear Neurostimulation for Adolescents With Functional Abdominal Pain | Recruiting | Katja Kovacic |
| NCT02648191 | Preoperative Treatment With Noninvasive Intra‐auricular Vagus Nerve Stimulation Pending Bariatric Surgery | Not yet recruiting | Radwan Kassir |
Of note, several ongoing clinical studies are evaluating the effect of vagal nerve stimulation in patients suffering from intestinal inflammatory diseases such as CD and POI (Table 2). Results from these studies may represent the final proof that activation of the CAIP is a powerful tool to be included in the therapeutic armamentarium against chronic intestinal immune‐mediated diseases.
Additional information
Competing interests
The authors declare no competing financial interests.
Author contributions
All the authors wrote part of the manuscript and revised the entire manuscript. All authors approved the final version of the manuscript. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
G.G. and G.M. are supported by a postdoctoral research fellowship from the Flemish Fund for Scientific Research (FWO), Belgium. G.M. and M.S. are supported by a research grant of the KU Leuven, Belgium.
Acknowledgements
The authors would like to thank all members past and present of the Translational Research Centre for Gastrointestinal Disorders, KU Leuven for their valuable contributions and critical discussion about the data reviewed herein.
Biography
Gianluca Matteoli was recently appointed as Assistant Professor of Mucosal Immunology at the University of Leuven (Belgium) at the Translational Research Centre for Gastrointestinal Disorders. The main interests of his lab are the molecular mechanisms involved in the maintenance of intestinal immune tolerance and their possible therapeutic application in inflammatory bowel disease. Gera Goverse obtained her PhD at the VU University Medical Centre (the Netherlands) by studying the environmental control of the mucosal immune system. After her PhD, she joined the lab of Gianluca Matteoli and became intrigued by the new and emerging concepts of neuro‐immune interactions in the gut, applying her expertise in mucosal immunology to investigate the role of the enteric nervous system on the tolerogenic imprinting of intestinal immune cells. Recently, Michelle Stakenborg joined the group as a PhD student to further elucidate neuro‐immune modulation during intestinal inflammation.

This review was presented at the symposium “The vagal pathway to the viscera: from basic mechanisms to therapeutic applications”, which took place at the meeting of International Society for Autonomic Neuroscience in Stresa, Italy, 26–29 September 2015.
References
- Andersson U & Tracey KJ (2012). Neural reflexes in inflammation and immunity. J Exp Med 209, 1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastida G & Beltran B (2011). Ulcerative colitis in smokers, non‐smokers and ex‐smokers. World J Gastroenterol 17, 2740–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, Molinaro C, Lubahn C & Lorton D (2008). Sympathetic modulation of immunity: relevance to disease. Cell Immunol 252, 27–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Carlson NR & Powley TL (1991). Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol Regul Integr Comp Physiol 260, R200–R207. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Jedrzejewska A & Powley TL (1990). Simultaneous labeling of vagal innervation of the gut and afferent projections from the visceral forebrain with dil injected into the dorsal vagal complex in the rat. J Comp Neurol 301, 65–79. [DOI] [PubMed] [Google Scholar]
- Bianchi ME (2007). DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81, 1–5. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Nardi D, Zhang M, Yang H, Ombrellino M & Tracey KJ (2000). Role of vagus nerve signaling in CNI‐1493‐mediated suppression of acute inflammation. Auton Neurosci 85, 141–147. [DOI] [PubMed] [Google Scholar]
- Cailotto C, Costes LM, van der Vliet J, van Bree SH, van Heerikhuize JJ, Buijs RM & Boeckxstaens GE (2011). Neuroanatomical evidence demonstrating the existence of the vagal anti‐inflammatory reflex in the intestine. Neurogastroenterol Motil 24, 191–200. [DOI] [PubMed] [Google Scholar]
- Carabotti M, Scirocco A, Maselli MA & Severi C (2015). The gut‐brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28, 203–209. [PMC free article] [PubMed] [Google Scholar]
- Clarencon D, Pellissier S, Sinniger V, Kibleur A, Hoffman D, Vercueil L, David O & Bonaz B (2014). Long term effects of low frequency (10 hz) vagus nerve stimulation on EEG and heart rate variability in Crohn's disease: a case report. Brain Stimul 7, 914–916. [DOI] [PubMed] [Google Scholar]
- Clark JA & Coopersmith CM (2007). Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock 28, 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosnes J, Carbonnel F, Beaugerie L, Le QY & Gendre JP (1996). Effects of cigarette smoking on the long‐term course of Crohn's disease. Gastroenterology 110, 424–431. [DOI] [PubMed] [Google Scholar]
- Costantini TW, Krzyzaniak M, Cheadle GA, Putnam JG, Hageny AM, Lopez N, Eliceiri BP, Bansal V & Coimbra R (2012). Targeting α‐7 nicotinic acetylcholine receptor in the enteric nervous system: a cholinergic agonist prevents gut barrier failure after severe burn injury. Am J Pathol 181, 478–486. [DOI] [PubMed] [Google Scholar]
- de Jonge WJ & Ulloa L (2007). The α7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol 151, 915–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge WJ, Van Der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, Van den Wijngaard RM & Boeckxstaens GE (2005). Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2‐STAT3 signaling pathway. Nat Immunol 6, 844–851. [DOI] [PubMed] [Google Scholar]
- Eskandari MK, Kalff JC, Billiar TR, Lee KK & Bauer AJ (1997). Lipopolysaccharide activates the muscularis macrophage network and suppresses circular smooth muscle activity. Am J Physiol Gastrointest Liver Physiol 273, G727–G734. [DOI] [PubMed] [Google Scholar]
- Galitovskiy V, Qian J, Chernyavsky AI, Marchenko S, Gindi V, Edwards RA & Grando SA (2011). Cytokine‐induced alterations of α7 nicotinic receptor in colonic CD4 T cells mediate dichotomous response to nicotine in murine models of Th1/Th17‐ versus Th2‐mediated colitis. J Immunol 187, 2677–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheorghe C, Pascu O, Gheorghe L, Iacob R, Dumitru E, Tantau M, Vadan R, Goldis A, Balan G, Iacob S, Dobru D & Saftoiu A (2004). Epidemiology of inflammatory bowel disease in adults who refer to gastroenterology care in Romania: a multicentre study. Eur J Gastroenterol Hepatol 16, 1153–1159. [DOI] [PubMed] [Google Scholar]
- Ghia JE, Blennerhassett P & Collins SM (2008). Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J Clin Invest 118, 2209–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghia JE, Blennerhassett P, Deng Y, Verdu EF, Khan WI & Collins SM (2009). Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology 136, 2280–2288. [DOI] [PubMed] [Google Scholar]
- Ghia JE, Blennerhassett P, El‐Sharkawy RT & Collins SM (2007). The protective effect of the vagus nerve in a murine model of chronic relapsing colitis. Am J Physiol Gastrointest Liver Physiol 293, G711–G718. [DOI] [PubMed] [Google Scholar]
- Ghia JE, Blennerhassett P, Kumar‐Ondiveeran H, Verdu EF & Collins SM (2006). The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology 131, 1122–1130. [DOI] [PubMed] [Google Scholar]
- Ghia JE, Park AJ, Blennerhassett P, Khan WI & Collins SM (2011). Adoptive transfer of macrophage from mice with depression‐like behavior enhances susceptibility to colitis. Inflamm Bowel Dis 17, 1474–1489. [DOI] [PubMed] [Google Scholar]
- Gomez‐Pinilla PJ, Di Giovangiulio M, Nemethova A, Stakenborg N, Farro G, Bosmans G, Matteoli G & Boeckxstaens GE (2014). Prucalopride activates the intestinal cholinergic anti‐inflammatory pathway and prevents postoperative ileus. Gastroenterology 146, 89. [Google Scholar]
- Haddad JJ, Saade NE & Safieh‐Garabedian B (2002). Cytokines and neuro‐immune‐endocrine interactions: a role for the hypothalamic‐pituitary‐adrenal revolving axis. J Neuroimmunol 133, 1–19. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Hamada T, Zaidi SF, Oshiro M, Lee J, Yamamoto T, Ishii Y, Sasahara M & Kadowaki M (2014). Nicotine suppresses acute colitis and colonic tumorigenesis associated with chronic colitis in mice. Am J Physiol Gastrointest Liver Physiol 307, G968–G978. [DOI] [PubMed] [Google Scholar]
- Hoie O, Wolters F, Riis L, Aamodt G, Solberg C, Bernklev T, Odes S, Mouzas IA, Beltrami M, Langholz E, Stockbrugger R, Vatn M & Moum B (2007). Ulcerative colitis: patient characteristics may predict 10‐yr disease recurrence in a European‐wide population‐based cohort. Am J Gastroenterol 102, 1692–1701. [DOI] [PubMed] [Google Scholar]
- Huston JM, Ochani M, Rosas‐Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch‐Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ & Ulloa L (2006). Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med 203, 1623–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston JM, Wang H, Ochani M, Ochani K, Rosas‐Ballina M, Gallowitsch‐Puerta M, Ashok M, Yang L, Tracey KJ & Yang H (2008). Splenectomy protects against sepsis lethality and reduces serum HMGB1 levels. J Immunol 181, 3535–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerndal P, Ringstrom G, Agerforz P, Karpefors M, Akkermans LM, Bayati A & Simren M (2010). Gastrointestinal‐specific anxiety: an important factor for severity of GI symptoms and quality of life in IBS. Neurogastroenterol Motil 22, 646‐e179. [DOI] [PubMed] [Google Scholar]
- Ji H, Rabbi MF, Labis B, Pavlov VA, Tracey KJ & Ghia JE (2014). Central cholinergic activation of a vagus nerve‐to‐spleen circuit alleviates experimental colitis. Mucosal Immunol 7, 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalff JC, Buchholz BM, Eskandari MK, Hierholzer C, Schraut WH, Simmons RL & Bauer AJ (1999). Biphasic response to gut manipulation and temporal correlation of cellular infiltrates and muscle dysfunction in rat. Surgery 126, 498–509. [PubMed] [Google Scholar]
- Kalff JC, Schraut WH, Simmons RL & Bauer AJ (1998). Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg 228, 652–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kox M, Pompe JC, Gordinou de Gouberville MC, van der Hoeven JG, Hoedemaekers CW & Pickkers P (2011). Effects of the α7 nicotinic acetylcholine receptor agonist GTS‐21 on the innate immune response in humans. Shock 36, 5–11. [DOI] [PubMed] [Google Scholar]
- Kox M, van Velzen JF, Pompe JC, Hoedemaekers CW, van der Hoeven JG & Pickkers P (2009). GTS‐21 inhibits pro‐inflammatory cytokine release independent of the Toll‐like receptor stimulated via a transcriptional mechanism involving JAK2 activation. Biochem Pharmacol 78, 863–872. [DOI] [PubMed] [Google Scholar]
- Lubbers T, Luyer MD, de Haan JJ, Hadfoune M, Buurman WA & Greve JW (2009). Lipid‐rich enteral nutrition reduces postoperative ileus in rats via activation of cholecystokinin‐receptors. Ann Surg 249, 481–487. [DOI] [PubMed] [Google Scholar]
- MacFie J, O'Boyle C, Mitchell CJ, Buckley PM, Johnstone D & Sudworth P (1999). Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut 45, 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli G, Gomez‐Pinilla PJ, Nemethova A, Di GM, Cailotto C, van Bree SH, Michel K, Tracey KJ, Schemann M, Boesmans W, Vanden Berghe P & Boeckxstaens GE (2014). A distinct vagal anti‐inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63, 938–948. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Tillisch K & Gupta A (2015). Gut/brain axis and the microbiota. J Clin Invest 125, 926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli PC & Jacobson K (2003). Cholinergic pathways modulate experimental dinitrobenzene sulfonic acid colitis in rats. Auton Neurosci 105, 16–24. [DOI] [PubMed] [Google Scholar]
- Munyaka P, Rabbi MF, Pavlov VA, Tracey KJ, Khafipour E & Ghia JE (2014). Central muscarinic cholinergic activation alters interaction between splenic dendritic cell and CD4+. PLoS One 9, e109272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemethova A, Michel K, Gomez‐Pinilla PJ, Boeckxstaens GE & Schemann M (2013). Nicotine attenuates activation of tissue resident macrophages in the mouse stomach through the beta2 nicotinic acetylcholine receptor. PLoS One 8, e79264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nos P & Domenech E (2011). Management of Crohn's disease in smokers: is an alternative approach necessary? World J Gastroenterol 17, 3567–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony C, van der Kleij H, Bienenstock J, Shanahan F & O'Mahony L (2009). Loss of vagal anti‐inflammatory effect: in vivo visualization and adoptive transfer. Am J Physiol Regul Integr Comp Physiol 297, R1118–R1126. [DOI] [PubMed] [Google Scholar]
- Pellissier S, Dantzer C, Mondillon L, Trocme C, Gauchez AS, Ducros V, Mathieu N, Toussaint B, Fournier A, Canini F & Bonaz B (2014). Relationship between vagal tone, cortisol, TNF‐α, epinephrine and negative affects in crohn's disease and irritable bowel syndrome. PLoS One 9, e105328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas‐Ballina M, Czura CJ, Huston JM, Miller E, Lin X, Sherry B, Kumar A, LaRosa G, Newman W, Tracey KJ & Yang H (2006). Role of HMGB1 in apoptosis‐mediated sepsis lethality. J Exp Med 203, 1637–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas‐Ballina M, Goldstein RS, Gallowitsch‐Puerta M, Yang L, Valdes‐Ferrer SI, Patel NB, Chavan S, Al‐Abed Y, Yang H & Tracey KJ (2009). The selective α7 agonist GTS‐21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med 15, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas‐Ballina M, Olofsson PS, Ochani M, Valdes‐Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW & Tracey KJ (2011). Acetylcholine‐synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio A, Pellissier S, Picot A, Dantzer C & Bonaz B (2014). The link between negative affect, vagal tone, and visceral sensitivity in quiescent Crohn's disease. Neurogastroenterol Motil 26, 1200–1203. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, Vermeire S & Van AG (2009). Biological therapies for inflammatory bowel diseases. Gastroenterology 136, 1182–1197. [DOI] [PubMed] [Google Scholar]
- Schneider L, Jabrailova B, Soliman H, Hofer S, Strobel O, Hackert T, Buchler MW & Werner J (2014). Pharmacological cholinergic stimulation as a therapeutic tool in experimental necrotizing pancreatitis. Pancreas 43, 41–46. [DOI] [PubMed] [Google Scholar]
- Silverman MN, Pearce BD, Biron CA & Miller AH (2005). Immune modulation of the hypothalamic‐pituitary‐adrenal (HPA) axis during viral infection. Viral Immunol 18, 41–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoek SA, Verstege MI, Van Der Zanden EP, Deeks N, Bulmer DC, Skynner M, Lee K, Te Velde AA, Boeckxstaens GE & de Jonge WJ (2010). Selective α7 nicotinic acetylcholine receptor agonists worsen disease in experimental colitis. Br J Pharmacol 160, 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L (2004). Elaborate interactions between the immune and nervous systems. Nat Immunol 5, 575–581. [DOI] [PubMed] [Google Scholar]
- Sternberg EM (2006). Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol 6, 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Zhou KW, Wang S, Li P, Chen SJ, Lin GP, Zhao Y & Wang TH (2013). Involvement of MAPK/NF‐κB signaling in the activation of the cholinergic anti‐inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS One 8, e69424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The FO, Boeckxstaens GE, Snoek SA, Cash JL, Bennink R, Larosa GJ, Van den Wijngaard RM, Greaves DR & de Jonge WJ (2007). Activation of the cholinergic anti‐inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology 133, 1219–1228. [DOI] [PubMed] [Google Scholar]
- Thorburn AN, Macia L & Mackay CR (2014). Diet, metabolites, and “western‐lifestyle” inflammatory diseases. Immunity 40, 833–842. [DOI] [PubMed] [Google Scholar]
- Tracey KJ (2002). The inflammatory reflex. Nature 420, 853–859. [DOI] [PubMed] [Google Scholar]
- Tracey KJ (2009). Reflex control of immunity. Nat Rev Immunol 9, 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida Y, Hatao F, Fujisawa M, Murata T, Kaminishi M, Seto Y, Hori M & Ozaki H (2011). Neuronal stimulation with 5‐hydroxytryptamine 4 receptor induces anti‐inflammatory actions via α7nACh receptors on muscularis macrophages associated with postoperative ileus. Gut 60, 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Assche G, Vermeire S & Rutgeerts P (2011). Management of acute severe ulcerative colitis. Gut 60, 130–133. [DOI] [PubMed] [Google Scholar]
- van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, Tracey KJ & Van der Poll T (2006). The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology 130, 1822–1830. [DOI] [PubMed] [Google Scholar]
- Veldhoen M & Brucklacher‐Waldert V (2012). Dietary influences on intestinal immunity. Nat Rev Immunol 12, 696–708. [DOI] [PubMed] [Google Scholar]
- Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al‐Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ & Ulloa L (2004). Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 10, 1216–1221. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al‐Abed Y, Czura CJ & Tracey KJ (2003). Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421, 384–388. [DOI] [PubMed] [Google Scholar]
- Yamamoto T (2005). Factors affecting recurrence after surgery for Crohn's disease. World J Gastroenterol 11, 3971–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]


