Abstract
Key points
Recent evidence indicates a role for group III/IV muscle afferents in reflex control of the human ventilatory response to exercise.
Dyspnoea in chronic obstructive pulmonary disease (COPD) may be linked to this reflex response.
This study shows that activation of the muscle metaboreflex causes a ventilatory response in COPD patients but not in healthy controls.
This indicates abnormal involvement of muscle afferents in the control of ventilation in COPD which may be a contributing factor to exercise dyspnoea.
Abstract
Blockade of thin fibre muscle afferent feedback during dynamic exercise reduces exercise hyperpnoea in health and chronic obstructive pulmonary disease (COPD). Therefore, we hypothesised that activation of the muscle metaboreflex at rest would cause hyperpnoea. We evaluated the effect of muscle metaboreflex activation on ventilation, in resting COPD patients and healthy participants. Following a bout of rhythmic hand grip exercise, post exercise circulatory occlusion (PECO) was applied to the resting forearm to sustain activation of the muscle metaboreflex, in 18 COPD patients (FEV1/FVC ratio < 70%), 9 also classified as chronically hypercapnic, and 9 age‐ and gender‐matched controls. The cardiovascular response to exercise and the sustained blood pressure elevation during PECO was similar in patients and controls. During exercise ventilation increased by 6.64 ± 0.84 in controls and significantly (P < 0.05) more, 8.38 ± 0.81 l min−1, in patients. During PECO it fell to baseline levels in controls but remained significantly (P < 0.05) elevated by 2.78 ± 0.51 l min−1 in patients until release of circulatory occlusion, with no significant difference in responses between patient groups. Muscle metaboreflex activation causes increased ventilation in COPD patients but not in healthy participants. Chronic hypercapnia in COPD patients does not exaggerate this response. The muscle metaboreflex appears to be abnormally involved in the control of ventilation in COPD and may be a contributing factor to exercise dyspnoea.
Key points
Recent evidence indicates a role for group III/IV muscle afferents in reflex control of the human ventilatory response to exercise.
Dyspnoea in chronic obstructive pulmonary disease (COPD) may be linked to this reflex response.
This study shows that activation of the muscle metaboreflex causes a ventilatory response in COPD patients but not in healthy controls.
This indicates abnormal involvement of muscle afferents in the control of ventilation in COPD which may be a contributing factor to exercise dyspnoea.
Abbreviations
- COPD
chronic obstructive pulmonary disease
- DBP
diastolic blood pressure
- f
respiratory frequency
- FEV1
forced expiratory volume in 1 s
- FVC
forced vital capacity
- HCO3−
bicarbonate
- HF
heart failure
- HR
heart rate
- MAP
mean arterial blood pressure
- MVC
maximal voluntary contraction
partial pressure of arterial carbon dioxide
partial pressure of arterial oxygen
- PECO
post exercise circulatory occlusion
partial pressure of end‐tidal carbon dioxide
- SBP
systolic blood pressure
minute ventilation
Introduction
It is well established that cardiovascular responses to exercise are controlled in part by central command (Goodwin et al. 1972) and partly by metabolically and mechanically sensitive thin fibre afferents (groups III and IV) arising from the exercising skeletal muscle (Coote et al. 1971; McCloskey & Mitchell, 1972). Selective experimental stimulation of metaboreceptive afferents is commonly achieved via the technique of post exercise circulatory occlusion (PECO). In this technique, the occlusion of blood flow immediately post exercise traps metabolites including ATP, lactic acid/H+, bradykinin, arachidonic acid and its cyclo‐oxygenase products (Kaufman et al. 1983; Rotto & Kaufman, 1988; Hanna & Kaufman, 2004) within the previously exercising muscle and so stimulates metabosensitive fibres. It has been consistently shown that stimulation of these afferents via PECO induces an increased activation of the sympathetic nervous system and a resultant pressor reflex (Alam & Smirk, 1937; Seals et al. 1988).
Whilst the role of central command in driving exercise hyperpnoea is also well established (Krogh & Lindhard, 1913; Eldridge et al. 1981; Green et al. 2007), the role of skeletal muscle afferent feedback in controlling the exercise hyperpnoea is more controversial. Traditionally, it has been considered unimportant as evidenced by the classic finding in healthy humans that PECO does not prevent the recovery of ventilation to resting levels (Rowell et al. 1976; Innes et al. 1989; Haouzi et al. 2001). However, recent evidence, involving the blockade of afferent feedback during dynamic exercise has revealed a contribution of muscle afferents to ventilatory drive (Amann et al. 2010). Intrathecal administration of fentanyl reduced exercise hyperpnoea in healthy participants and chronic obstructive pulmonary disease (COPD) and heart failure (HF) patient groups (Gagnon et al. 2012; Olson et al. 2014). Additionally, we have shown that in healthy participants activation of muscle afferents using PECO, combined with mild hypercapnia, generates a ventilatory response and there is evidence of a synergistic interaction between central chemoreflex activation and muscle afferent feedback (Lykidis et al. 2010; Bruce & White, 2012, 2015).
Patients with COPD commonly develop skeletal muscle dysfunction; both in terms of a decreased force generating capacity and derangements in energy metabolism. There is an increasing body of evidence suggesting that, alongside the respiratory impairments, skeletal muscle dysfunction is an important contributing factor in the exercise intolerance associated with the disease (see Maltais et al. 2014). A slow to fast transition of muscle fibre types (Whittom et al. 1998; Jobin et al. 1998; Gosker et al. 2002), increased muscle glycolytic enzyme activity (Jakobsson et al. 1995; Gea et al. 2001), and reduced mitochondrial density and (accordingly) oxidative enzyme activity (Jakobsson et al. 1995; Gosker et al. 2002, 2007) have been demonstrated. These maladaptations may be important determinants in reducing fatigue resistance commonly found in COPD.
During exercise the increased glycolytic capacity and shift away from oxidative metabolism results in a greater and earlier onset muscle acidosis. (see Puente‐Maestu et al. 2013, for review). This would be expected to generate an augmented metaboreflex, and if muscle afferent feedback does provide a drive to ventilation, it could contribute to dyspnoea and exercise intolerance. However, to our knowledge, no controlled studies have yet examined the ventilation of COPD patients during metaboreflex activation using PECO. Therefore, the primary aim of this investigation is to examine the respiratory responses of patients with COPD to PECO, compared with age‐matched healthy control participants. We hypothesise that patients with COPD will demonstrate augmented ventilatory responses to metaboreflex activation.
Secondly, based on our previous observations of a synergistic interaction between experimentally induced hypercapnia and metaboreflex activation, we aim to examine whether any respiratory response to PECO in COPD patients relates to their level of CO2 retention. (Lykidis et al. 2010; Bruce & White, 2012, 2015). We hypothesise that chronically hypercapnic patients will generate a larger ventilatory response during PECO in comparison to normocapnic patients.
Methods
Ethical approval
All participants received written and verbal information regarding the experimental procedures prior to giving informed written consent. All subjects were habituated to all the experimental procedures, which conform to the Declaration of Helsinki and were approved by an NHS ethical committee.
Participants
Eighteen patients with stable moderate to severe COPD, defined according to Global Initiative for Obstructive Lung Disease criteria, were recruited. Patient pulmonary function data and participant characteristics are shown in Table 1. COPD was defined as FEV1/FVC ratio < 70% following bronchodilator medication. Moderate COPD was defined as a FEV1% predicted between 50 and 79% and severe COPD between 30 and 49%. The medications prescribed to the patients are listed in Table 2. Patients were not considered stable and excluded from participation if in the previous 6 weeks they had been hospitalised or suffered an exacerbation or infection. Patients with heart failure/disease, type II diabetes, renal disease or an active malignancy revealed by medical history were also excluded. In total, four COPD patients had controlled hypertension managed by the prescribed antihypertensive medications also listed in Table 2. All patients continued to adhere to their prescribed drug regimens throughout the study. Nine healthy age‐ and gender‐matched controls also volunteered for this study. Participants visited the laboratory for one trial day following habituation. Before the trial day participants refrained from consuming food and caffeine in the 4 h before the trial and from performing strenuous physical activity or consuming alcohol in the 12 h before the trial. This study followed a within‐subject design with all subjects participating in both trials. The order of each 8 min trial was randomised with a 30 min rest period in between.
Table 1.
Participant characteristics (± SD)
| Healthy | COPD | |
|---|---|---|
| N | 9 (5 male) | 18 (10 male) |
| Age | 67.7 ± 6.1 | 65.1 ± 5.1 |
| Height (cm) | 167.1 ± 4.9 | 162.2 ± 3.2 |
| Weight (kg) | 71.8 ± 3.7 | 75.6 ± 3.1 |
| MVC (N) | 298 ± 45 | 246 ± 37* |
| Resting SBP (mmHg) | 125 ± 3.2 | 119 ± 2.4 |
| Resting DBP (mmHg) | 78 ± 2.6 | 75 ± 1.9 |
| Resting MAP (mmHg) | 93 ± 3.1 | 89 ± 1.6 |
| Resting HR (bpm) | 71 ± 1.6 | 68 ± 1.3 |
| FEV1 (l) | — | 1.06 ± 0.3 |
| FEV1 (% predicted) | — | 42 ± 5.7 |
| FVC (l) | — | 2.35 ± 0.9 |
| FEV1/FVC (%) | — | 45 ± 3.4 |
| (mmHg) | — | 67.4 ± 9.2 |
| (mmHg) | — | 43.4 ± 6.5 |
| pH | — | 7.41 ± 0.03 |
| HCO3 − (mequiv l−1) | — | 27.8 ± 3.1 |
*Significant difference from Healthy participants (P < 0.05). FEV1, forced expiratory volume in one second; FVC, forced vital capacity; HCO3 −, arterial bicarbonate content; MVC, maximal voluntary contraction force.
Table 2.
List of medications prescribed to the COPD patients
| Medication | Total | Normocapnic | Hypercapnic |
|---|---|---|---|
| N | 18 | 9 | 9 |
| Salbutamol | 15 | 6 | 9 |
| Salmeterol | 5 | 3 | 2 |
| Seratide | 13 | 8 | 5 |
| Symbicort | 1 | 1 | 0 |
| Tiotropium | 9 | 4 | 5 |
| Theophyllin | 5 | 4 | 1 |
| Aspirin | 5 | 1 | 4 |
| ACE inhibitor | 2 | 1 | 1 |
| CCB | 3 | 1 | 2 |
| Statin | 4 | 2 | 2 |
CCB, calcium channel blocker.
Experimental procedures
Participants were seated in an upright position and held a custom made handgrip dynamometer with their right hand. The device was clamped to a surface top which also supported the participant's right arm. Maximal voluntary contractions (MVCs) were determined by instructing participants to perform 3–5 maximal handgrip efforts separated by 1 min and the highest was taken as the MVC. During the experimental protocol participants maintained the required percentage of their MVC by matching their force output to a target force displayed on a computer screen positioned in front of them at eye level. The rhythmic isometric handgrip exercise task was performed at a duty cycle of 1 s contraction at 50% MVC, to 1 s relaxation, and the rhythm was maintained via a metronome.
The experimental protocol is shown in Fig. 1. Prior to the start of each trial participants rested for 5 min in order to establish steady state ventilation and cardiovascular variables. Both trials then consisted of a 2 min baseline recording period before a 2 min rhythmic isometric handgrip exercise task. Participants then either (1) rested for a further 4 min (Control trial) or (2) a cuff was rapidly inflated to 200 mmHg around the upper right arm by a rapid cuff inflator commencing 2–3 contractions before cessation of the exercise period, ensuring that immediately after exercise circulatory occlusion was achieved and so participants went through a 2 min period of PECO. Then the cuff was deflated and participants rested for 2 min (PECO trial).
Figure 1.
Schematic diagram of the 8 min protocol for the control and PECO trials
The order of each 8 min trial was randomised with a 30 min rest period in between.
Respiratory and cardiovascular measures
Ventilation was continuously monitored with a pneumotachograph (Flowmetrics, BRDL, fr‐41 s, ultasonic pneumotachograph, CA, USA) attached to the inspiratory side of a breathing valve (T‐Valve) with a mouthpiece. From this the respiratory frequency (f) and inspiratory airflows (l s−1) were measured and so minute ventilation () was calculated. All volumes recorded were converted to BTPS. The pneumotachograph was calibrated with a 3 l syringe to give a linear output over the range of 0.2–3 l. The partial pressure of end‐tidal CO2 () was recorded throughout the protocol with a rapid gas analyser (Servomex, 1440, Sussex, UK) sampling the end‐tidal gases in the breathing valve. The analyser was calibrated using gases with a known concentration of CO2 (between 0 and 10%). Heart rate (HR) was derived from a 3 lead electrocardiogram (ECG; Cardiorator CR7, Cardiac Records Ltd, London, UK) in the lead II position. From this, heart rate (HR) was continuously measured. Blood pressure was measured using finger photoplethysmography (Portapress, Finapress Medical Systems, Amsterdam, The Netherlands) with the cuff placed on the middle finger, and the hand supported at heart level on an adjustable table.
Data analysis
Mean averages for , f, , HR and mean arterial pressure (MAP) during each minute of the trials were calculated. The cardiovascular and ventilatory responses to exercise and PECO were assessed with an analysis of variance with repeated measures, and then when appropriate multiple comparison post hoc analysis. Comparisons between the control participants and COPD patients in both trials were assessed using a Student's unpaired t test. Data are expressed as means ± SEM and statistical significance was taken as (P < 0.05). Statistical analysis was conducted using a standard statistical package (SPSS, Chicago, IL, USA).
Results
Participant characteristics
Participant characteristics are presented in Table 1. In respect of age, resting cardiovascular variables and body size there were no significant differences between healthy participants and the patients. However, COPD patients produced a 17% lower handgrip force than healthy participants (P < 0.05).
Differences between trials at baseline
The mean , f, HR, MAP and during baseline are presented in Table 3. There were no significant differences in any of these variables between the controls and COPD participants.
Table 3.
Mean , HR, MAP and values recorded during the 2 min baseline period in the Control and PECO trials for the Healthy and COPD patients
| Trial | (l min−1) | f (breaths min−1) | HR (beats min−1) | MAP (mmHg) | (mmHg) | |
|---|---|---|---|---|---|---|
| Healthy | Control | 8.72 ± 0.92 | 12.3 ± 2.3 | 68 ± 1.3 | 93 ± 3.1 | 39 ± 1.6 |
| PECO | 9.09 ± 1.02 | 13.4 ± 2.8 | 69 ± 1.4 | 95± 3.8 | 39 ± 0.9 | |
| COPD | Control | 9.94 ± 0.6 | 14.5 ± 3.9 | 71 ± 1.6 | 89 ± 1.6 | 45 ± 2.2 |
| PECO | 10.37 ± 0.54 | 15.2 ± 3.1 | 72 ± 1.6 | 90 ± 1.9 | 44 ± 2.3 |
Respiratory responses
Changes in mean relative to baseline recorded during each of the four trials are shown in Fig. 2 A and B. Exercise caused significant increases in during the first and second minute of exercise in all trials. During the second minute of exercise, the of healthy participants increased by 6.36 ± 0.79 l min−1 and 6.64 ± 0.84 l min−1 in the control and PECO trials, respectively. In the COPD patients increased by 8.1 ± 0.64 l min−1 and 8.38 ± 0.81 l min−1 in control and PECO trials, respectively, with higher response in the final 30 s of exercise in patients compared to the healthy participants (P < 0.05). returned to baseline levels in both the healthy control and healthy PECO trials following exercise. remained significantly above baseline during the first minute after exercise in the COPD control trial (2.23 ± 0.33 l min−1) but returned to baseline in the second minute. During the COPD PECO trial, remained significantly elevated above baseline throughout PECO (3.82 ± 0.68 l min−1 and 2.78 ± 0.51 l min−1) only returning to baseline after cuff deflation. during PECO in the COPD PECO trial was significantly higher than that in the healthy PECO trial. During the recovery period, was not significantly different from baseline in all four trials (P > 0.05).
Figure 2. Changes in respiratory and cardiovascular variables during control and PECO trials in COPD patients and healthy participants .
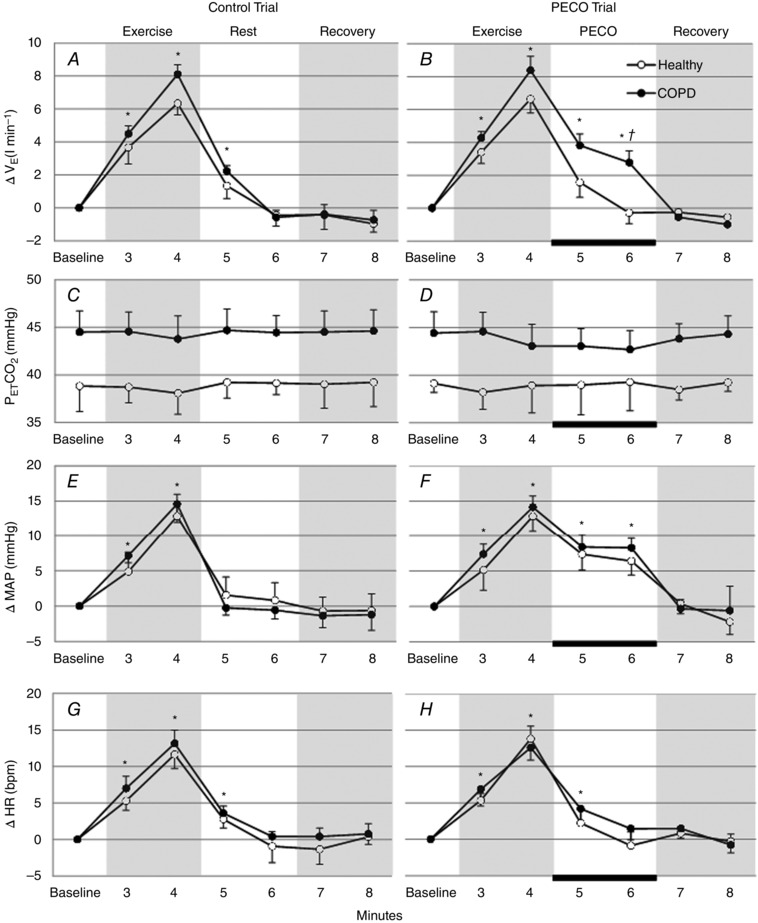
Change in , MAP and HR from baseline, and during each minute of the control trial and PECO trial in COPD patients and healthy participants. The black bar indicates the period of circulatory occlusion. *Significant difference from Baseline value (P < 0.05). †Significant difference between COPD patients and healthy participants.
The increased ventilation during exercise was related to change in both tidal volume and f; however, only the increases in f reached statistical significance, during the last minute of exercise, in all trials. In the COPD patients these increases were significantly (P < 0.05) greater in comparison to the healthy participants in the control (10.6 ± 3.1 and 6.7 ± 2.2 breaths min−1) and PECO trials (11.6 ± 2.8 and 7.2 ± 2.9 breaths min−1). Following exercise, during the last minute of rest in the control trials, f recovered to baseline levels in both groups (1.9. ± 3.3 and 0.4 ± 1.9 breaths min−1). In the PECO trials f remained significantly elevated above baseline levels in the COPD patients in contrast to the recovery to baseline levels seen in the healthy participants (4.9 ± 2.5 and 2.1 ± 2.2 breaths min−1).
Panels C and D in Fig. 2 show the mean values during throughout the four trials. There was no significant change in from baseline levels within each trial and no difference between the trials.
Cardiovascular responses
Changes in mean HR MAP relative to baseline during the four trials are shown in Fig. 2 E–H. Both MAP and HR significantly increased during exercise in the healthy control trial (13 ± 2.3 mmHg and 12 ± 1.5 beats min−1 (bpm); P < 0.05 versus baseline), in the healthy PECO trial (13 ± 1.6 mmHg and 14 ± 1; P < 0.05 versus baseline), in the COPD control trial (14 ± 1.1 mmHg and 13 ± 1 beats min−1; P < 0.05 versus baseline) and in the COPD PECO trial (13 ± 1 mmHg and 12 ± 1.2; P < 0.05 versus baseline). MAP and HR returned to baseline levels after exercise in the healthy control and COPD control trials. However, in both the PECO trials MAP fell from exercise levels but remained significantly elevated above baseline during the 2 min post exercise (P < 0.05) and only returned to baseline after cuff deflation. There were no significant differences in the blood pressure responses during PECO in both the healthy PECO and COPD PECO trials. During the recovery period, both MAP and HR values were not significantly different from baseline in all four trials (P > 0.05).
Hypercapnic vs. normocapnic COPD patients
COPD patients were separated into hypercapnic patients ( > 45 mmHg) and normocapnic patients. Table 4 shows that participants in the hypercapnic group had a significantly higher resting , arterial bicarbonate content and lower values than those in the normocapnic group. No other significant differences between groups were observed.
Table 4.
Characteristics of patients (± SD) separated into hypercapnic and normocapnic groups
| COPD normocapnic | COPD hypercapnic | |
|---|---|---|
| N | 9 (5 male) | 9 (5 male) |
| Age | 64.3 ± 6.1 | 67.6 ± 5.1 |
| Height (cm) | 160.1 ± 3.8 | 164.6 ± 3.2 |
| Weight (kg) | 76.8 ± 3.7 | 74.6 ± 3.1 |
| MVC (N) | 239 ± 56 | 250 ± 51 |
| Resting SBP (mmHg) | 116 ± 4.6 | 121 ± 3.9 |
| Resting DBP (mmHg) | 77 ± 2.6 | 74 ± 1.9 |
| Resting MAP (mmHg) | 91 ± 3.2 | 89 ± 3.6 |
| Resting HR (bpm) | 71 ± 1.6 | 67 ± 1.3 |
| FEV1 (l) | 1 ± 0.2 | 1.13 ± 0.3 |
| FEV1 (% predicted) | 43.5 ± 5.1 | 40.5 ± 6.4 |
| FVC (l) | 2.24 ± 0.7 | 2.45 ± 0.8 |
| FEV1/FVC (%) | 44 ± 5.1 | 47 ± 4.3 |
| (mmHg) | 71.6 ± 5.3 | 63.3 ± 6.4* |
| (mmHg) | 37.7 ± 3.3 | 49.2 ± 2.7* |
| pH | 7.41 ± 0.04 | 7.4 ± 0.03 |
| HCO3 (mequiv l−1) | 25.3 ± 1.4 | 30.2 ± 1.6* |
*Significant difference from normocapnic participants (P < 0.05). FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HCO3 −, arterial bicarbonate content; MVC, maximal voluntary contraction force.
Respiratory and cardiovascular responses during the PECO trial
Baseline , f, , HR and MAP in hypercapnic and normocapnic COPD patients during baseline of the PECO trials are shown in Table 5. As expected the of hypercapnic patients was significantly higher than normocapnic patients during baseline (+15 ± 1.3 mmHg, P < 0.05) and throughout the trial (Fig. 3 B). There were no significant differences between any other baseline variables.
Table 5.
Mean , HR, MAP and values recorded during the 2 min baseline period in the PECO trials of the normocapnic and hypercapnic COPD patients
| (l min−1) | f (breaths min−1) | HR (beats min−1) | MAP (mmHg) | (mmHg) | |
|---|---|---|---|---|---|
| Normocapnia | 9.9 ± 0.9 | 14.6 ± 4.1 | 70 ± 1.6 | 92 ± 3.4 | 37 ± 1.7 |
| Hypercapnia | 10.8 ± 0.8 | 15.7 ± 3.6 | 74 ± 1.2 | 89 ± 1.6 | 51 ± 2.3* |
*Significant difference from normocapnia (P < 0.05).
Figure 3. Changes in respiratory and cardiovascular variables during the PECO trials in normocapnic and hypercapnic COPD patients .
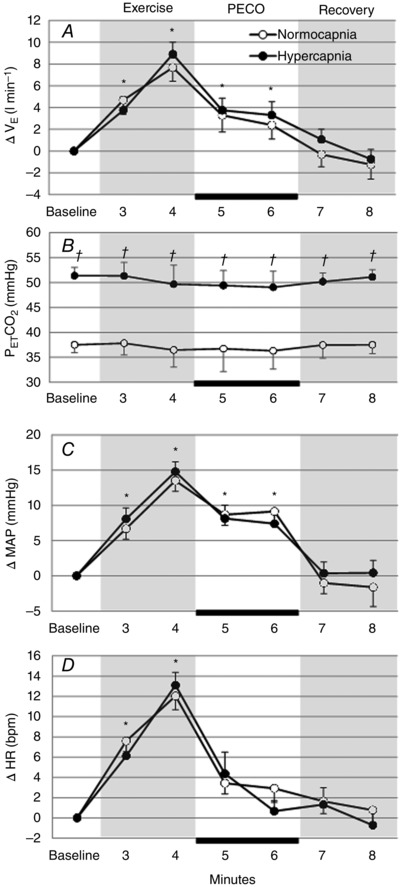
Change in , MAP and HR from baseline, and during each minute of the PECO trials in normocapnic and hypercapnic COPD patients. The black bar indicates the period of circulatory occlusion. *Significant difference from baseline value (P < 0.05). †Significant difference between normocapnic and hypercapnic groups.
Changes in mean relative to baseline in hypercapnic and normocapnic COPD patients during each minute of the PECO trial are shown in Fig. 3 A. No significant differences in were observed between the hypercapnic and normocapnic patients at any time point in the trial. Changes in mean HR and MAP relative to baseline in hypercapnic and normocapnic COPD patients during each minute of the PECO trial are shown in Fig. 3 C and D. No significant differences in HR and MAP were observed between the hypercapnic and normocapnic patients at any time point in the trial.
Discussion
This study investigated the ventilatory and cardiovascular responses of patients with moderate–severe COPD and healthy age‐matched controls, to rhythmic handgrip exercise and activation of the muscle metaboreflex. The main findings are that activation of the metaboreflex via PECO maintained ventilation significantly above baseline in COPD patients but not in the healthy controls. However, there was no difference in this response between hypercapnic and normocapnic COPD patients.
During PECO, where volition and muscle force are absent, only the activity of the muscle metaboreflex remains increased. The magnitude of the blood pressure response generated during PECO is known to be a reliable indicator of the level of muscle metaboreflex activation (Coote et al. 1971; McCloskey & Mitchell 1972; Kaufman et al. 1983). Therefore, our observation of similar blood pressure elevation during PECO in the patients and healthy participants is good evidence of a comparable level of muscle metaboreflex activation in both groups.
As expected, using PECO to maintain metaboreflex activation in isolation from central command and muscle mechanoreceptor activation failed to sustain the ventilation of healthy participants above baseline values. This finding has been well documented in many studies of healthy individuals using circulatory occlusion following prior exercise, e.g. handgrip and cycling exercise (Rowell et al. 1976; Innes et al. 1989; Haouzi et al. 2001). This evidence forms a major component of the classical view that in humans, muscle afferent activation does not contribute to respiratory drive in exercise.
However, in our patients we demonstrated, for the first time, a sustained elevation of ventilation during PECO, which suggests that activation of the muscle metaboreflex can generate and maintain a respiratory response following forearm exercise. This is further supported by the finding that ventilation only returned to baseline values post arm‐cuff deflation and thus removal of metaboreflex activation. Our observation of a ventilatory response, largely driven by increased f, to activation of the muscle metaboreflex is consistent with other recent evidence based upon removal of afferent input during exercise. Amann et al. (2010) showed that the hyperpnoea in response to dynamic cycling exercise can be attenuated by inhibiting the neurotransmission of group III and IV afferents from the exercising muscles via intrathecal administration of fentanyl. In addition, administration of this spinal anaesthesia to patients with COPD (Gagnon et al. 2012) and HF (Olson et al. 2014) reduced the exercise ventilatory response, f and sensations of dyspnoea, while enhancing cycling exercise endurance time. When considered together, this evidence, based on both removal and addition of muscle afferent feedback, reveals a potentially important role for the feedback in human respiratory control. In addition, whereas Gagnon et al. demonstrated that muscle afferent feedback plays an important role in the control of the exercise hyperpnoea and may limit exercise capacity in COPD, our methodology has the advantage of showing that the sensitivity of the muscle afferent mediated control of ventilation may be increased in COPD. Furthermore, our use of PECO selectively activates the muscle metaboreflex and so clearly defines its contribution in the absence of muscle mechanoreceptor input.
A disproportionate exercise ventilatory response in COPD is well documented and indeed our patients did produce a significantly greater ventilatory response during the rhythmic handgrip exercise task compared with healthy age‐matched controls. As these patients also displayed an augmented ventilatory response to PECO compared with healthy controls, our results suggest that metaboreflex activation may contribute to this characteristic excessive exercise ventilatory response in COPD. This contribution could be mediated by two mechanisms. First, a greater local metabolite accumulation in the muscle causing enhanced activation of the muscle metaboreflex. Second, there could be a greater central sensitivity to the same level of muscle afferent input.
The first explanation is plausible as COPD is associated with the down‐regulation of skeletal muscle oxidative enzyme activity, an increased lactic acidosis and a lower muscle pH in exercise (Casaburi et al. 1991; Kutsuzawa et al. 1992; Gosker et al. 2002). Indeed, Kutsuzawa et al. found an exaggerated decrease in the pH of forearm muscles during a standardised handgrip exercise in patients with COPD compared with healthy controls. Animal studies show that the arterial injection of lactic acid/H+ causes an increase in the discharge of group III and IV muscle afferent fibres (Rotto & Kaufman, 1988) and an increase in ventilation and blood pressure (Rotto et al. 1989). This is likely to be mediated, in part, by the stimulation of acid sensing ion channels located on the free nerve endings of muscle afferent fibres (Li et al. 2004; Hayes et al. 2008). Therefore the skeletal muscle dysfunction associated with COPD may result in a standard exercise bout producing increased stimulation of metabosensitive receptors, an augmented metaboreflex, and so an exaggerated ventilatory response.
However, the blood pressure response of the COPD patients to PECO was not significantly different to that of healthy controls, a finding consistent with other reports using static handgrip exercise (Roseguini et al. 2008; Sherman et al. 2011). This is consistent with a similar level of muscle afferent activation between patients and healthy controls. If so, then our second explanation for an augmented ventilatory response to PECO becomes more appealing. Indeed, in healthy participants, we have shown that it is possible to manipulate acutely the sensitivity of the respiratory control system to muscle afferent input (Lykidis et al. 2010; Bruce & White, 2012). These studies used exposure to mild hypercapnia to alter the level of central respiratory activity in combination with a controlled level of muscle afferent activation. Under conditions of mild hypercapnia, muscle metaboreflex activation using PECO generated a normal pressor response. However, the elevation of ventilation, caused by prior exercise was sustained, in a manner resembling the response of the COPD patients in the present study. This prior work suggests a differential response by central cardiovascular and respiratory control networks to muscle metaboreflex activation, and the present study supports this view.
In additional experiments this and hyperventilation, during PECO, was shown to be unrelated to exposure of the active muscle to hypercapnia and it was not attenuated by acute exposure to hyperoxia, thereby reducing peripheral chemoreflex stimulation (Bruce & White, 2015). These observations have been argued to be evidence of a synergistic interaction between the central chemoreflex and muscle afferent input (Coote, 2012). This was the rationale for comparing hypercapnic COPD patients, normocapnic COPD patients and healthy control participants. Knowing that some COPD patients retain CO2, we hypothesised that such patients might display an exaggerated response to PECO. However, we found no differences in the ventilatory responses to PECO between groups of hypercapnic and normocapnic COPD patients. It appears unlikely that the level of muscle metaboreflex differed between the two groups as their blood pressure responses to PECO were similar. So the explanation for these findings may relate to differences between chronic exposure and acute exposure to hypercapnia. It is known that chronic elevation of causes desensitisation of central chemoreceptors and this is classically found in CO2 retaining COPD patients (Richerson & Boron, 2005), but further studies are required to examine this mechanism more fully.
Implications
Physical inactivity is considered an important factor in the development of skeletal muscle dysfunction in COPD (Serres et al. 1998), playing a part in a ‘dyspnoea spiral’ (Prefaut et al. 1995). Patients avoid physical exertion to prevent sensations of dyspnoea, resulting in further deconditioning of skeletal muscles. A downward spiral may result, further exaggerating the exercise ventilatory response and exacerbating the sensations of dyspnoea in exercise with further deterioration in exercise tolerance over time. This is analogous to the ‘muscle hypothesis’ used to explain effort intolerance in HF (Coats et al. 1994), a patient group who share similar features of skeletal muscle dysfunction to COPD (Franssen et al. 2002), and produce augmented respiratory and cardiovascular responses to PECO (Piepoli et al. 1996, 1999; Notarius et al. 2001; Ponikowski et al. 2001; Scott et al. 2002; Crisafulli et al. 2007; Olson et al. 2010). Regardless of whether afferent feedback is increased in COPD patients, our results show, for the first time, an exaggerated respiratory response to metaboreflex activation in these patients, providing new insight into the neural link between skeletal muscle afferent activation and exercise dyspnoea in COPD.
Endurance training in COPD patients has been shown to improve the metabolic efficiency of skeletal muscle by increasing oxidative enzyme activities and reducing lactic acid production and skeletal muscle acidosis in exercise (Casaburi et al. 1991; Maltias et al. 1996; Whittom et al. 1998; Sala et al. 1999). Training of healthy subjects certainly results in attenuated muscle metaboreflex mediated changes in muscle sympathetic nerve activity (Sinoway et al. 1989) and blood pressure (Fisher & White, 1999). An improvement in skeletal muscle oxidative capacity and reduced muscle acidosis in exercise may therefore attenuate the stimulation of metabolically sensitive receptors in skeletal muscle and thus reduce the central respiratory drive and ventilatory response to the exercise. As such, the sensations of dyspnoea upon exertion may be attenuated and exercise tolerance may increase. Indeed a 6 week training programme of the forearm muscles in HF has shown to reduce the ventilatory and cardiovascular responses to PECO following rhythmic handgrip exercise (Piepoli et al. 1996), and this was thought to be achieved through improvements in skeletal muscle metabolism and the reduced stimulation of the muscle metaboreflex (Piepoli et al. 2008). However, similar studies evaluating muscle afferent adaptation to training are yet to be conducted in COPD patients.
Limitations
Although the primary objective of this study was to examine ventilatory responses to activation of the muscle metaboreflex, we evaluated the level of this reflex activation by measurement of the pressor response to PECO. Recording of muscle sympathetic nerve activity would provide a more direct estimation of the efferent outflow resulting from muscle metaboreflex activation, though this still would not be a direct measure of the muscle afferent activity, which remains impractical in human studies. The standardisation of exercise conditions also becomes an important issue in respect of comparing responses in groups with different muscle strengths. In the present study the MVCs of the patient group were on average 17% lower. Though they performed the same relative exercise intensity as the healthy controls (50% of MVC), we cannot be certain that this rhythmic isometric force × time integral produced the same metabolite accumulations during the crucial PECO phase of the experiments. If lower metabolite accumulation resulted in the patient group, due to lower absolute force production, then the similar pressor response to healthy participants might suggest an exaggerated cardiovascular response to metaboreceptor activation. However, the literature pertaining to sustained isometric contractions is clear that the exercise pressor response is unrelated to muscle strength under well controlled conditions (Fisher & White, 2004).
In our previous studies we had access to an in‐house dynamic end‐tidal forcing system to clamp at a given level. Constraints of ethical approval meant that the present study was performed in an off‐site, hospital environment where we were unable to transport this system. As a result, in the COPD patient trials where hyperventilation during PECO was observed there was a slight (non‐significant) fall in . However, if anything, this would be expected to cause a small underestimate of the influence of muscle metaboreflex on ventilation in the COPD patients. Though set against this, the known desensitisation of chemoreception in COPD patients makes its influence less likely. We also acknowledge that the study of ventilatory responses to muscle metaboreflex activation in the forearm muscles should be extended to study of the functionally more significant larger locomotor muscle masses of the legs.
Conclusion
In conclusion, this study demonstrated that the activation of the metaboreflex via PECO increases ventilation significantly above baseline in COPD patients but not in healthy controls. However, these ventilatory responses to PECO were not related to . The findings of this study further define the role of skeletal muscle afferent feedback in the augmented ventilation and sensations of dyspnoea that are associated with COPD during exercise. The interaction of muscle afferent feedback with other drivers of ventilation requires further study.
Additional information
Competing interests
The authors declare they have no competing interests.
Author contributions
R.M.B., A.T. and M.J.W. conceived and designed the research. R.M.B. performed the experiments and analysed the data. R.M.B., A.T. and M.J.W. interpreted the results of the experiments and drafted the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The authors did not receive funding.
References
- Alam M & Smirk FH (1937). Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89, 372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2010). Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109, 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RM & White MJ (2012). Muscle afferent activation causes ventilatory and cardiovascular responses during concurrent hypercapnia in humans. Exp Physiol 97, 208–218. [DOI] [PubMed] [Google Scholar]
- Bruce RM & White MJ (2015). The ventilatory response to muscle afferent activation during concurrent hypercapnia in humans: central and peripheral mechanisms. Exp Physiol 100, 896–904. [DOI] [PubMed] [Google Scholar]
- Casaburi R, Patessio A, Ioli F, Zanaboni S, Donner CF & Wasserman K (1991). Reduction in exercise lactic acidosis and ventilation as a result of exercise training in obstructive lung disease. Am Rev Respir Dis 143, 9–18. [DOI] [PubMed] [Google Scholar]
- Coats AJS, Clark AL, Piepoli M, Volterrani M & Poole‐Wilson PA (1994). Symptoms and quality of life in heart failure: the muscle hypothesis. Br Heart J 72, S36–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH, Hilton SM & Perez‐Gonzalez JF (1971). Reflex nature of pressor response to muscular exercise. J Physiol 215, 789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH (2012). Muscles bring breathing alive. Exp Physiol 97, 207. [DOI] [PubMed] [Google Scholar]
- Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P & Concu A (2007). Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol 292, H2988–H2996. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE & Waldrop TG (1981). Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science 211, 844–846. [DOI] [PubMed] [Google Scholar]
- Fisher WJ & White MJ (1999). Training‐induced adaptations in the central command and peripheral reflex components of the pressor response to isometric exercise of the human triceps surae. J Physiol 520, 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP & White MJ (2004). Muscle afferent contributions to the cardiovascular response to isometric exercise. Exp Physiol 89, 639–646. [DOI] [PubMed] [Google Scholar]
- Franssen FM, Wouters EF & Schols AM (2002). The contribution of starvation, deconditioning and ageing to the observed alterations in peripheral skeletal muscle in chronic organ diseases. Clin Nutr 21, 1–14. [DOI] [PubMed] [Google Scholar]
- Gagnon P, Bussières JS, Ribeiro F, Gagnon SL, Saey D, N Gagné, Provencher S & Maltais F (2012). Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186, 606–615. [DOI] [PubMed] [Google Scholar]
- Gea JG, Pasto M, Carmona MA, Orozco‐Levi M, Palomeque J & Broquetas J (2001). Metabolic characteristics of the deltoid muscle in patients with chronic obstructive pulmonary disease. Eur Respir J 17, 939–45. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI & Mitchell JH (1972). Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226, 173–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosker HR, Hesselink MKC, Duimel H, Ward KA & Schols AMWJ (2007). Reduced mitochondrial density in the vastus lateralis muscle of patients with COPD. Eur Respir J 30, 73–79. [DOI] [PubMed] [Google Scholar]
- Gosker HR, van Mameren H, van Dijk PJ, Engelen MP, van der Vusse GJ, Wouters EF & Schols AM (2002). Skeletal muscle fibre type shifting and metabolic profile in patients with chronic obstructive pulmonary disease. Eur Respir J 19, 617–625. [DOI] [PubMed] [Google Scholar]
- Green AL, Wang S, Purvis S, Owen SL, Bain PG, Stein JF, Guz A, Aziz TZ & Paterson DJ (2007). Identifying cardiorespiratory neurocircuitry involved in central command during exercise in humans. J Physiol 578, 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna RL & Kaufman MP (2004). Activation of thin‐fiber muscle afferents by a P2X agonist in cats. J Appl Physiol 96, 1166–1169. [DOI] [PubMed] [Google Scholar]
- Haouzi P, Chenuel B & Chalon B (2001). Control of breathing and muscle perfusion in humans. Exp Physiol 86, 759–768. [DOI] [PubMed] [Google Scholar]
- Hayes SG, McCord JL, Rainier J, Liu Z & Kaufman MP (2008). Role played by acid‐sensitive ion channels in evoking the exercise pressor reflex. Am J Physiol Heart Circul Physiol 295, H1720–H1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes JA, Solarte I, Huszczu A, Yeh E, Whipp BJ & Wasserman K (1989). Respiration during recovery from exercise: effects of trapping and release of femoral blood flow. J Appl Physiol 67, 2608–2613. [DOI] [PubMed] [Google Scholar]
- Jakobsson P, Jorfeldt L & Henriksson J (1995). Metabolic enzyme activity in the quadriceps femoris muscle in patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 151, 374–377. [DOI] [PubMed] [Google Scholar]
- Jobin J, Maltais F, Doyon JF, LeBlanc P, Simard AA & Simard C (1998). Chronic obstructive pulmonary disease: capillarity and fibre type characteristics of skeletal muscle. J Cardiopulm Rehabil 18, 432–437. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH & Mitchell JH (1983). Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55, 105–112. [DOI] [PubMed] [Google Scholar]
- Krogh A & Lindhard J (1913). The regulation of respiration and circulation during the initial stages of muscular work. J Physiol 47, 112–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuzawa T, Shioya S, Kurita D, Haida M, Ohta Y & Yamabayashi H (1992). 31P‐NMR study of skeletal muscle metabolism in patients with chronic respiratory impairment. Am Rev Respir Dis 146, 1019–1024. [DOI] [PubMed] [Google Scholar]
- Li J, Maile MD, Sinoway AN & Sinoway LI (2004). Muscle pressor reflex: potential role of vanilloid type 1 receptor and acid‐sensing ion channel. J Appl Physiol 97, 1709–1714. [DOI] [PubMed] [Google Scholar]
- Lykidis CK, Kumar P, Vianna LC, White MJ & Balanos GM (2010). A respiratory response to the activation of the muscle metaboreflex during concurrent hypercapnia in man. Exp Physiol 95, 194–201. [DOI] [PubMed] [Google Scholar]
- McCloskey DI & Mitchell JH (1972). Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigare R, Dekhuijzen PN, Franssen F, Gayan‐Ramirez G, Gea J, Gosker HR, Gosselink R, Hayot M, Hussain SN, Janssens W, Polkey MI, Roca J, Saey D, Schols AM, Spruit MA, Steiner M, Taivassalo T, Troosters T, Vogiatzis I & Wagner PD (2014). ATS/ERS Ad Hoc Committee on Limb Muscle Dysfunction in COPD: An official American Thoracic Society/European Respiratory Society statement: Update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 189, e15–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltais F, LeBlanc P, Simard C, Jobin J, C Bérubé, Bruneau J, Carrier L & Belleau R (1996). Skeletal muscle adaptation to endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 154, 442–447. [DOI] [PubMed] [Google Scholar]
- Notarius CF, Atchison DJ & Floras JS (2001). Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol 280, H969–H976. [DOI] [PubMed] [Google Scholar]
- Olson TP, Joyner MJ, Eisenach JH, Curry TB & Johnson BD (2014). Influence of locomotor muscle afferent inhibition on the ventilatory response to exercise in heart failure. Exp Physiol 99, 414–426. [DOI] [PubMed] [Google Scholar]
- Olson TP, Joyner MJ & Johnson BD (2010). Influence of locomotor muscle metaboreceptor stimulation on the ventilatory response to exercise in heart failure. Circ Heart Fail 3, 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P & Coats AJ (1996). Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation 93, 940–952. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Ponikowski P, Clark AL, Banasiak W, Capucci A & Coats AJ (1999). A neural link to explain the “muscle hypothesis” of exercise intolerance in chronic heart failure. American Heart Journal 137, 1050–1056. [DOI] [PubMed] [Google Scholar]
- Piepoli MF, Dimopoulos K, Concu A & Crisafulli A (2008). Cardiovascular and ventilatory control during exercise in chronic heart failure: Role of muscle reflexes. Int J Cardiol 130, 3–10. [DOI] [PubMed] [Google Scholar]
- Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJ & Piepoli MF (2001). Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation 104, 2324–2230. [DOI] [PubMed] [Google Scholar]
- Prefaut C, Varray A & Vallet G (1995). Pathophysiological basis of exercise training in patients with chronic obstructive pulmonary disease. Eur Respir Rev 5, 27–32. [Google Scholar]
- Puente‐Maestu L, Lázaro A & Humanes B (2013). Metabolic derangements in COPD muscle dysfunction. J Appl Physiol 114.9, 1282–1290. [DOI] [PubMed] [Google Scholar]
- Richerson GB & Boron WF (2005). Control of ventilation In Medical Physiology, 2nd edn, eds Boron WF. & Boulpaep EL, chapter 31, pp. 712–734. Elsevier Saunders, Philadelphia, USA. [Google Scholar]
- Roseguini BT, Alves C, Nchiappa GR, Stein R, Knorst MM & Ribeiro JP (2008). Attenuation of muscle metaboreflex in chronic obstructive pulmonary disease. Med Sci Sports Exerc 40, 1–6. [DOI] [PubMed] [Google Scholar]
- Rotto DM & Kaufman MP (1988). Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol 64, 2306–2313. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Stebbins CL & Kaufman MP (1989). Reflex cardiovascular and ventilatory responses to increasing H+ activity in cat hindlimb muscle. J Appl Physiol 67, 256–263. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Hermansen L & Blackmon JR (1976). Human cardiovascular and respiratory responses to graded muscle ischemia. J Appl Physiol 41, 693–701. [DOI] [PubMed] [Google Scholar]
- Sala E, Roca J, Marrades RM, Alonso J, Gonzalez De Suso JM, Moreno A, Barbera JA, Nadal J, de Jover L, Rodriguez‐Roisin R & Wagner PD (1999). Effects of endurance training on skeletal muscle bioenergetics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159, 1726–1734. [DOI] [PubMed] [Google Scholar]
- Scott AC, Davies LC, Coats AJ & Piepoli M (2002). Relationship of skeletal muscle metaboreceptors in the upper and lower limbs with the respiratory control in patients with heart failure. Clin Sci 102, 23–30. [PubMed] [Google Scholar]
- Seals DR, Chase PB & Taylor JA (1988). Autonomic mediation of the pressor responses to isometric exercise in humans. J Appl Physiol 64, 2190–2196. [DOI] [PubMed] [Google Scholar]
- Serres I, Gautier V, Varray A & Préfaut C (1998). Impaired skeletal muscle endurance related to physical inactivity and altered lung function in COPD patients. Chest 113, 900–905. [DOI] [PubMed] [Google Scholar]
- Sherman MFB, Road JD, McKenzie DC & Sheel W (2011). Preserved muscle metaboreflex in chronic obstructive pulmonary disease. Appl Physiol Nutr Metab 36, 821–830. [DOI] [PubMed] [Google Scholar]
- Sinoway L, Rea RF, Smith M & Mark AL ( 1989). Physical training induces desensitisation of muscle metaboreflex. Circulation 80 (Suppl. II), 290. [Google Scholar]
- Whittom F, Jobin J, Simard PM, Leblanc P, Simard CC, Bernard S, Belleau R & Maltais F (1998). Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exercise 30, 1467–1474. [DOI] [PubMed] [Google Scholar]


