Abstract
Hypothalamic neurons expressing histamine and orexin/hypocretin (hcrt) are necessary for normal regulation of wakefulness. In Parkinson's disease, the loss of dopaminergic neurons is associated with elevated histamine levels and disrupted sleep/wake cycles, but the mechanism is not understood. To characterize the role of dopamine in the development of histamine neurons, we inhibited the translation of the two non-allelic forms of tyrosine hydroxylase (th1 and th2) in zebrafish larvae. We found that dopamine levels were reduced in both th1 and th2 knockdown, but the serotonin level and number of serotonin neurons remained unchanged. Further, we demonstrated that th2 knockdown increased histamine neuron number and histamine levels, whereas increased dopaminergic signaling using the dopamine precursor l-DOPA (l-3,4-dihydroxyphenylalanine) or dopamine receptor agonists reduced the number of histaminergic neurons. Increases in the number of histaminergic neurons were paralleled by matching increases in the numbers of hcrt neurons, supporting observations that histamine regulates hcrt neuron development. Finally, we show that histaminergic neurons surround th2-expressing neurons in the hypothalamus, and we suggest that dopamine regulates the terminal differentiation of histamine neurons via paracrine actions or direct synaptic neurotransmission. These results reveal a role for dopaminergic signaling in the regulation of neurotransmitter identity and a potential mechanism contributing to sleep disturbances in Parkinson's disease.
Keywords: dopamine, dopamine receptor, histamine, hypothalamus, zebrafish, morpholino, orexin
Introduction
Dopamine is a critically important neurotransmitter in the vertebrate brain. It is involved in motor functions, conditioned behaviors, and hormone regulation (1). There is also an increasing body of evidence to suggest that dopamine has neurotrophic functions in the central nervous system (2, 3). For example, dopamine from the brain promotes the generation of motoneurons in the zebrafish spinal cord (4). In human disease conditions affecting the dopaminergic system, marked changes in the histaminergic system have been observed (5). Histamine, a modulatory neurotransmitter, is necessary for sleep-wake cycle function, alertness, memory, and hormonal regulation (6), and its functions are disrupted by neurological diseases, including narcolepsy, Gilles de la Tourette syndrome, and schizophrenia (5). In Parkinson's disease, the nigrostriatal dopaminergic system is severely damaged, and a concomitant increase in brain histamine levels (7) and denser histaminergic fiber networks have been observed (8) in the striatum and substantia nigra. In schizophrenia, the modified dopamine hypothesis proposes decreased dopaminergic activity in some brain regions (9), and an increase in histamine turnover has been reported (10). The potential regulatory role of dopamine for histaminergic neuron development is difficult to study in mammals because tyrosine hydroxylase knockout mice do not survive (11, 12).
In zebrafish (Danio rerio), two non-allelic forms of th are expressed in the brain in a largely complementary manner (13–15). However, the biological roles of the two th forms are not clear. In this report, we studied the roles of th2 in the developing zebrafish brain. We used antisense morpholino oligonucleotides (MOs)2 to knock down th2 gene expression and dopamine receptor ligands to alter dopaminergic signaling and analyzed the effects on the catecholaminergic, histaminergic, serotonergic, and hypocretin systems. Our results show that th2 is essential in dopamine production and reveal a novel regulatory role for th2-dependent dopaminergic signaling during zebrafish development in the specification of hypothalamic histamine and hypocretin neurons.
Results
Hypothalamic Histaminergic and Serotonergic Neurons Are Distinct from th2 Dopaminergic Neurons
Tg(f.TH:egfp) transgenic fish express GFP under the control of the fugu th promoter (16). In the 5-dpf zebrafish brain, GFP expression was seen in olfactory bulbs, the preoptic region, the paraventricular organ, and caudal periventricular hypothalamic zones (Fig. 1A). To further characterize the identity of GFP-positive cells in the hypothalamus, we performed in situ hybridization (ISH) with th2 riboprobes, followed by immunohistochemistry (IHC) using TH1, TH2, histamine, or 5-HT antibodies on 5-dpf Tg(f.TH:egfp) fish brain. We found that GFP-positive cells in the caudal hypothalamus expressed th2 mRNA (Fig. 1, B–D) and showed TH2 immunoreactivity (Fig. 1E). Larger magnification of single-channel and merged images depicted GFP and th2/TH2 co-localization in TH2 group 10b (Fig. 1, D' and E'), suggesting that GFP-ir cells co-expressing th2/TH2 in group 10b represent th2-containing populations (dopamine cell population 10/10b; the nomenclature used in this study is based on Sallinen et al. (17) and Chen et al. (13); or DC7 group according to Rink and Wullimann (18)). GFP-positive but TH2-negative cells were found in the olfactory bulb, telencephalon, preoptic region, and caudal hypothalamus (Fig. 1, A–D, white arrows). This may be caused by the differences and insufficiency of regulatory motifs driving GFP expression between zebrafish and fugu. Histamine immunoreactivity (his-ir), a histaminergic neuron marker, was confined to neurons in the caudal hypothalamus around the catecholaminergic cell group in the nucleus of the posterior recess (19, 20). The his-ir cells were located peripherally surrounding the GFP-immunoreactive (ir) cells of the population 10/10b in the caudal hypothalamus region (Fig. 1F, larger magnification shown in Fig. 1F'), and no co-existence of GFP and histamine was found in any single optical scanning frame. Serotonergic (5-HT) cells, which also reside in this region (19, 21), did not show any GFP-ir (Fig. 1G; a single section image shown in Fig. 1G'). Furthermore, we performed triple immunostaining on 5-dpf zebrafish brains using anti-TH1, anti-TH2, and anti-5-HT antibodies (Fig. 1H). The staining result showed that none of the TH1-ir or TH2-ir cells were immunoreactive for 5-HT (Fig. 1H'), suggesting that TH2-ir cells are distinct from serotonergic neurons. In summary, GFP-ir-th2/TH2-expressing cells were surrounded by histaminergic neurons and intermingled with serotonergic neurons in the nucleus of the posterior recess in the caudal hypothalamus. These results are also in agreement with TH2-ir distribution in the adult zebrafish brain (22).
FIGURE 1.
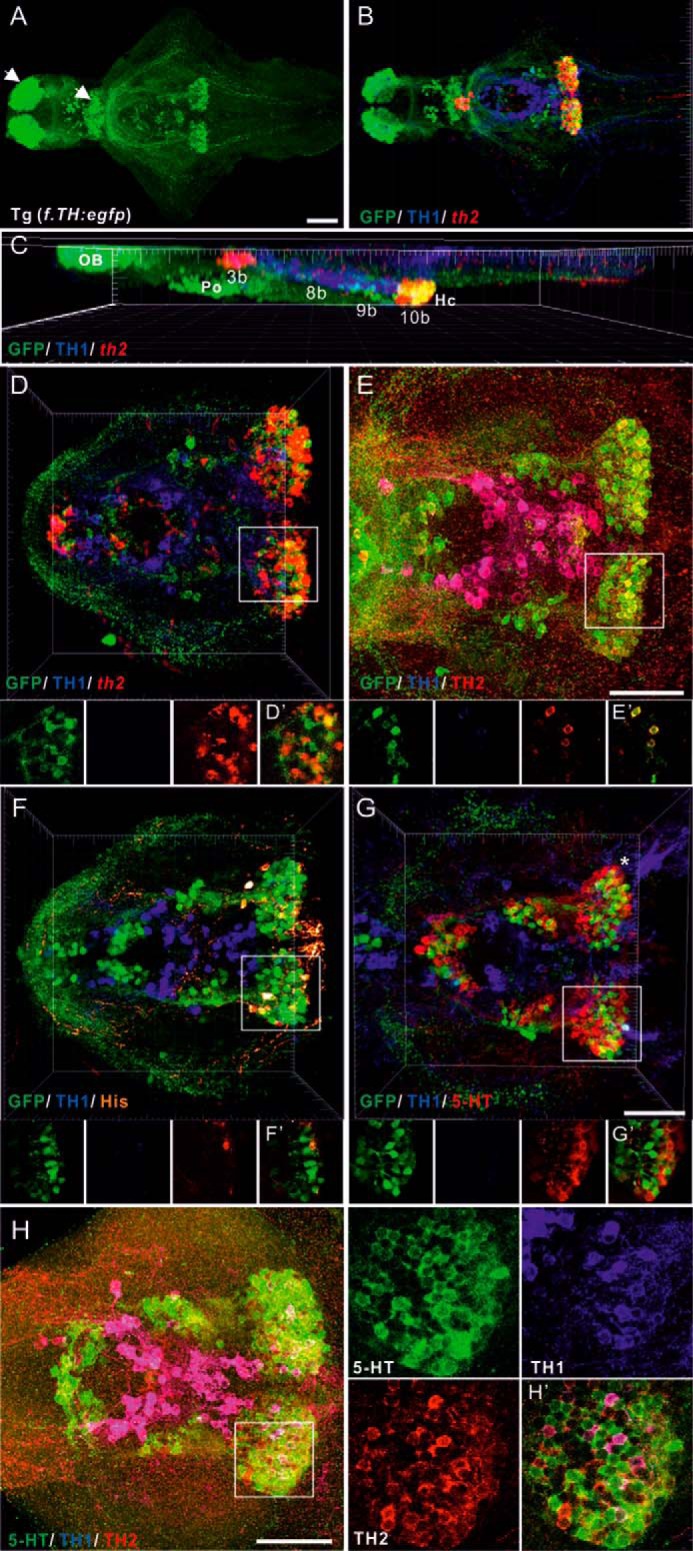
Multiple labeling of catecholaminergic, histaminergic, and serotoninergic neurons and GFP distribution in 5-dpf brains of the Tg(f.TH:egfp) transgenic line and Turku WT. A, ventral view of GFP distribution in a 5-dpf brain. The specimens initially hybridized (ISH) with th2 antisense riboprobes were processed for double immunostaining with chicken GFP and mouse TH1 antibodies (shown in B–D). B, ventral view of triple staining showing GFP-ir in green, TH1-ir in blue, and the th2 mRNA expression pattern in red. C, lateral view of B. The distribution patterns in the diencephalic and hypothalamic regions of GFP and TH1 with TH2, histamine (His), or 5-HT are shown in E–G, respectively. A triple immunostaining image with anti-TH1, anti-TH2, and anti-5HT on a 5-dpf brain is shown in H and H'. Larger magnification and single section images of group 10b (white rectangle) in D–H are shown in D'–H', respectively. The GFP-ir signal is shown in green, TH1-ir in blue, and th2-ish, TH2-ir, His-ir, and 5-HT-ir in red in the transgenic line (D–G). 5-HT-ir is shown in green in H. White arrows indicate GFP-positive but th2/TH2-negative populations. Cells labeled with both TH1 and th2 are shown in magenta. Cells labeled with both GFP and TH2 are shown in yellow. OB, olfactory bulb. 3b, preoptic group (Po); 8b, paraventricular organ; 9b, nucleus of lateral recess; 10b, caudal hypothalamus (Hc). TH2 group numbers are based on Ref. 13. Scale bars = 50 μm.
Both TH1 and TH2 Contribute to Catecholamine Synthesis
Based on the amino acid identity and phylogenetic analysis among invertebrate and vertebrate species, zebrafish th1 and th2 are classified as counterparts of the mammalian th (23, 24). However, it is still unclear whether TH2 has tyrosine hydroxylase activity because Ren et al. (25) reported that zebrafish th2 acts as tryptophan hydroxylase during development. To investigate the impact of th1 and th2 on catecholamine and 5-HT metabolism, the concentrations of dopamine, norepinephrine, epinephrine, and 5-HT were measured by HPLC using 5-dpf zebrafish heads following translation inhibition of th1 or th2. The efficacy of MOs used in this study was verified by Western blotting using an anti-TH2 antibody that recognizes both zebrafish TH1 and TH2 populations (22). The robust specific signals around 55 kDa representing the expected sizes of TH1 and TH2 proteins were observed in the 5-dpf head homogenate (supplemental Fig. S1). On the other hand, several strong bands between 40 and 55 kDa that were detected in the 36-hpf whole-embryo homogenate were clearly less prominent in the 36-hpf deyolked embryo (supplemental Fig. S1), suggesting that these nonspecific signals can be due to the interactions between the TH2 antibody and yolk proteins. In Fig. 2A, besides the specific bands around 55 kDa of TH1/TH2, the uneven signals between 40 and below 55 kDa among groups may thus be due to yolk differences in different morphants. Although the use of deyolked embryo homogenates is possible, the manual deyolking procedure of 5-dpf larvae is time-consuming and may easily cause protein degradation during sample preparation. To avoid preparation bias, we used whole larval protein lysates for the Western blotting analysis in this study. The Western blot showed that the TH1 protein was reduced 70% in the th1 morphants, whereas the amount of TH2 protein was essentially unchanged. On the other hand, only 45% of TH2 protein remained in 5-dpf th2 morphants. When th2 mRNA was co-injected with th2 MO1 + 2 (th2 rescue) in th2 morphants, a 40% increase in TH2 protein was detected in th2 morphants compared with the control-injected group (Fig. 2A). The th2 morphants developed with a normally sized head, eyes, trunk (Fig. 2B, F(5, 169) = 2.139, p = 0.0632), and notochord without any apparent defects or any distinguishable gross phenotype (Fig. 2C). The levels of p53 and delta113p53, recognized as off-targeting markers usually induced by MO knockdown (26), were not elevated in the th2 morphants (Fig. 2D, F(3, 8) = 1.216, p = 0.3648; Fig. 2E, F(2, 8) = 1.394, p = 0.3106), suggesting that th2 MOs specifically knocked down TH2 protein expression without causing obvious off-targeting effects. The HPLC results showed that the dopamine, norepinephrine, and epinephrine levels were significantly decreased in th1 morphants (Figs. 2, F–I). A significant reduction in dopamine level was also detected in th2 morphant groups (Fig. 2F, F(7, 16) = 8.942, p = 0.0002), but the norepinephrine and epinephrine levels were unaffected compared with those of control morphants (Fig. 2G, F(7, 16) = 9.372, p = 0.0001; Fig. 2H, F(7, 16) = 6.754, p = 0.0008). 5-HT levels were unaffected in both th1 and th2 morphants (Fig. 2I, F(7, 16) = 2.575, p = 0.0556). The group treated with p-chlorophenylalanine (an inhibitor of tryptophan hydroxylase) was used as a 5-HT intervention control. The MO efficiency was documented here similarly as described in detail earlier (22). Our data show that TH1 is responsible for catecholamine biosynthesis, including dopamine, norepinephrine, and epinephrine, whereas TH2 is responsible only for dopamine synthesis. This result is concordant with the expression pattern of dopamine beta-hydroxylase (dbh); only th1 is expressed in cells that also express dbh (required for the synthesis of norepinephrine and epinephrine) (19).
FIGURE 2.
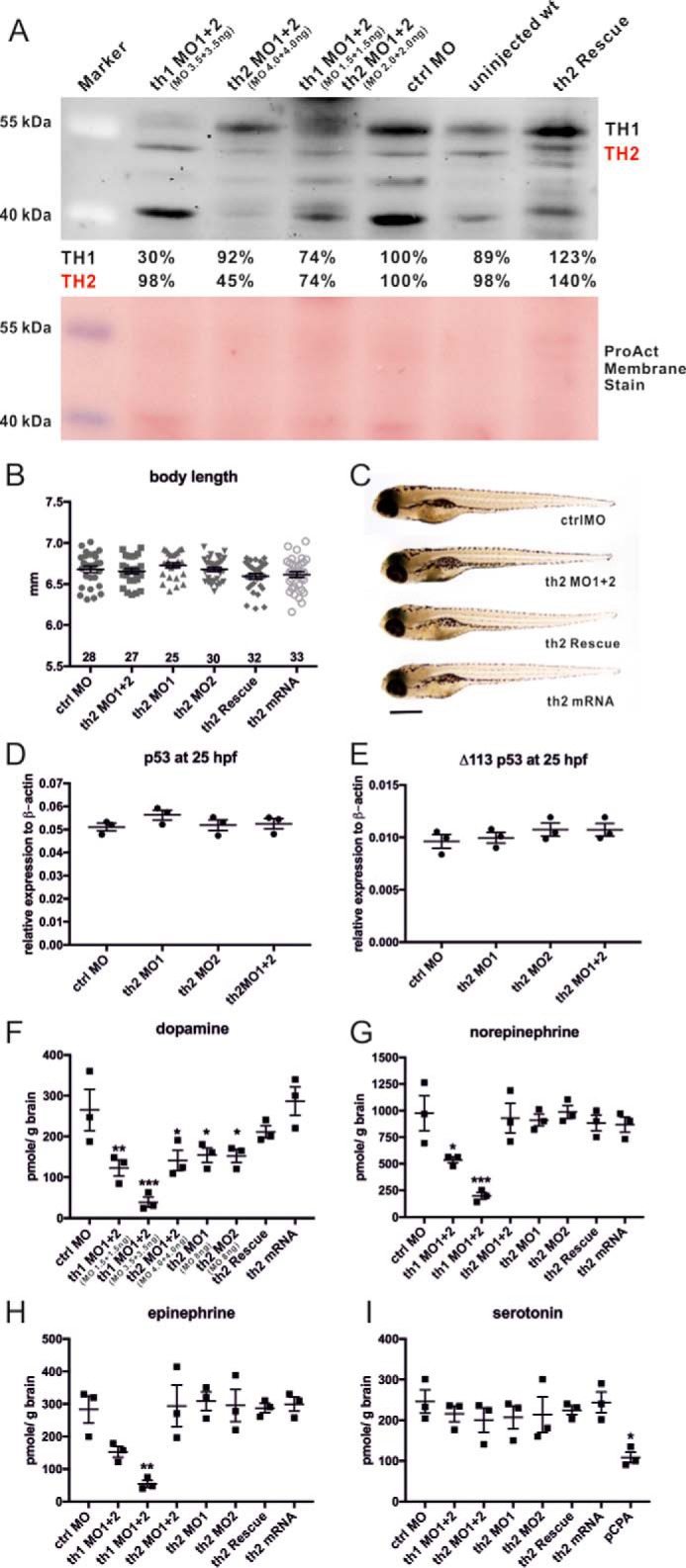
Western blotting, body length, and the concentration of catecholamines and serotonin in morphants. A, Western blotting analysis using 5-dpf larvae with anti-TH2 antibody. ProAct membrane stain was used as the loading control. B, quantification of body length. The sample number of each group and mean ± S.E. are indicated in the graph. C, bright-field image of the morphants. D and E, qPCR analysis of p53 and delta113p53 transcript levels (n = 3/group, Student's t test). Catecholamine and serotonin concentrations were measured using HPLC. Fifteen 5-dpf heads were homogenized for each group, and three individual groups (n = 3) per treatment were analyzed. F, dopamine. G, norepinephrine. H, epinephrine. I, serotonin. th2 rescue is th2 MO1 + 2 co-injected with th2 mRNA. *, p < 0.05; **, p < 0.01; ***, p < 0.001; one-way ANOVA with Tukey's test. Scale bar = 500 μm.
Dopaminergic and Serotonergic Markers in th2 Morphants
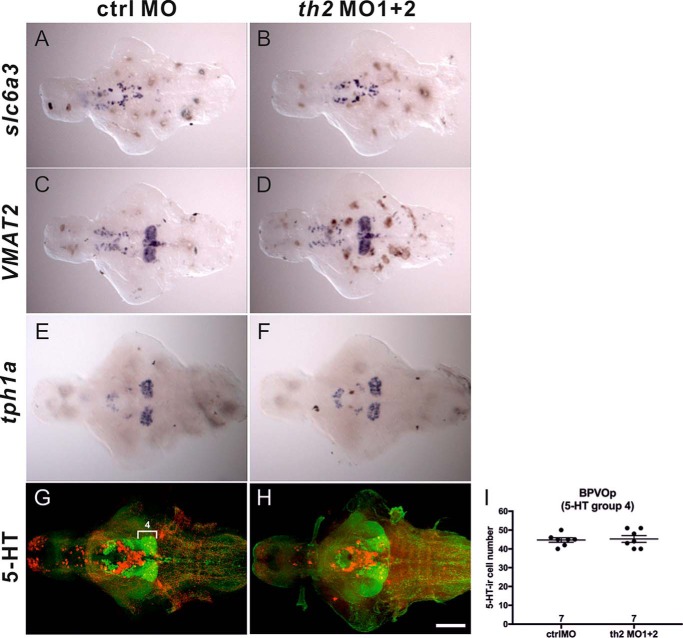
To study whether loss of TH2 function affects the development of catecholaminergic neurons and neighboring serotonergic neurons expressing tph1a, WISH was performed on 5-dpf fish brains. The dopamine transporter (slc6a3), responsible for dopamine reuptake from the synaptic cleft, is present in many dopaminergic cells and is commonly used as a marker of dopaminergic neurons (27). As shown in Fig. 3, A and B, the expression pattern of slc6a3 mRNA was intact in th2 morphants. Vesicular monoamine transporter 2 (VMAT2) is responsible for reuptake of monoamines, including dopamine, 5-HT, norepinephrine, and histamine, in nerve terminals (28). The VMAT2 mRNA expression pattern was not altered in th2 morphants (Fig. 3, C and D). Tryptophan hydroxylase is involved in the biosynthesis of serotonin. The expression pattern of tph1a (Fig. 3, E and F) and the number of serotonergic neurons in the ventrocaudal hypothalamus (5-HT group 4) (29), shown by 5-HT immunohistochemistry (Fig. 3, G–I; p = 0.7953, Student's t test) were largely unaffected in th2 morphants, in agreement with the unchanged 5-HT concentration in 5-dpf morphant brains (Fig. 2G), indicating that knockdown of th2 did not affect the serotonergic system.
FIGURE 3.
The expression pattern of slc6a3, VMAT2, and tph1a and the distribution of 5-HT-ir cells in 5-dpf ctrlMO and th2MO1 + 2 morphant brains. A and B, slc6a3 ISH. C and D, VMAT2 ISH. E and F, tph1a ISH. G and H, double staining of TH1 and 5-HT. I, quantification of 5-HT-ir cells in the PVOp (5-HT group 4). TH1-labeled cells are shown in red and 5-HT-positive cells in green. Scale bar = 100 μm.
TH2 Plays a Role in the Regulation of Histaminergic Neuron Development
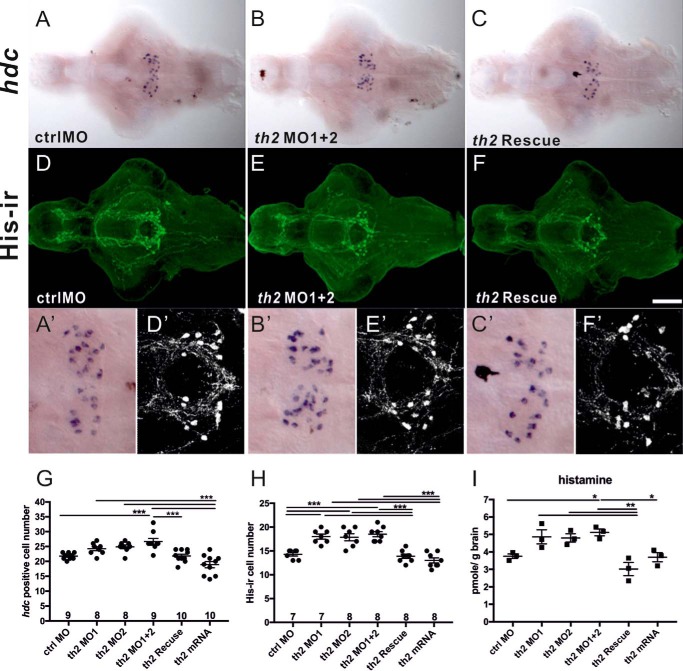
To study whether th2 deficiency affects the development of histaminergic neurons, hdc ISH and double-labeling immunohistochemistry were performed on 5-dpf brains. The number of histaminergic neurons (Fig. 4, A, B, and G (F(5, 48) = 12.58, p < 0.0001); Fig. 4, D, E, and H (F(5, 39) = 21.58, p < 0.0001) was significantly higher in th2 morphants than in control morphants. th2 mRNA co-injection efficiently normalized the number of histaminergic neurons in th2 morphants (Fig. 4, C and F–H), showing that the phenotype was specific for th2 MO1 + 2. Moreover, the histamine level measured with HPLC was significantly increased in th2 morphants compared with that in control morphants or in the th2 morphant mRNA rescue group (Fig. 4I, F(5, 12) = 8.649, p = 0.0011). These data indicate that the development of histaminergic neurons is driven in part by the catecholaminergic TH2 neurons in the zebrafish brain.
FIGURE 4.
A significant change in histaminergic neuron numbers in th2-deficient fish brains. The number of histaminergic neurons and histamine level is elevated in th2 morphants. hdc ISH and His immunostaining were carried out on 5-dpf fish brains. A–C, hdc ISH in 5-dpf brains of ctrl MO, th2 MO1 + 2, and th2 rescue. D–F, immunostaining with histamine in ctrl MO, th2 MO1 + 2, and th2 rescue brains. G, quantification of hdc cell number. H, quantification of his-ir cell number. I, histamine level measured by HPLC. Large magnifications of hdc-ish (A'–C') and His-ir cell images (D'–F') are depicted. Histamine-containing cells are labeled in green. The number of brains analyzed and the mean values of the cell numbers are shown in the columns. Scale bar = 100 μm. *, p < 0.05; **, p < 0.01; ***, p < 0.001; one-way ANOVA with Tukey's test.
Effects of th2 Knockdown on Orexin/Hypocretin (hcrt) Neuron Development
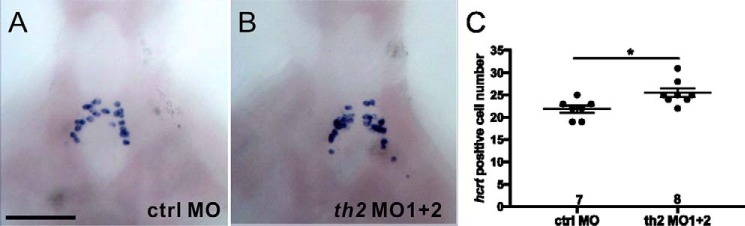
It has been reported that the histaminergic neurons regulate the development of hcrt neurons through histamine receptor H1 in zebrafish (20). Here, lack of th2 expression was associated with an increased number of histaminergic neurons. To learn whether hcrt neuron development was affected, possibly as a consequence of the histaminergic neuron alteration as described earlier (20), in the th2-deficient morphants, hcrt ISH was performed on 5-dpf fish brains. As shown in Figs. 5, A and B, a significant increase in hcrt-positive cells was observed in th2 MO1 + 2-injected fish (Fig. 5C, p = 0.0313, Student's t test) as the histaminergic hdc-containing cell number was increased (Fig. 4).
FIGURE 5.
A significant increase in hcrt cell numbers in th2-deficient fish brains. Results of hcrt ISH on 5-dpf dissected brains of a ctrl MO injected larva (A) and a th2 MO 1+2-injected larva (B) and counts of hcrt-positive cells in the brains of ctrl MO and th2 MO 1+2-injected larvae (C). The number of brains analyzed is shown above the horizontal axis in (C). *, p < 0.05 by Student's t test. Scale bar = 100 μm (for A and B).
Dopamine Receptor Agonists Inhibit the Development of Histaminergic Neurons
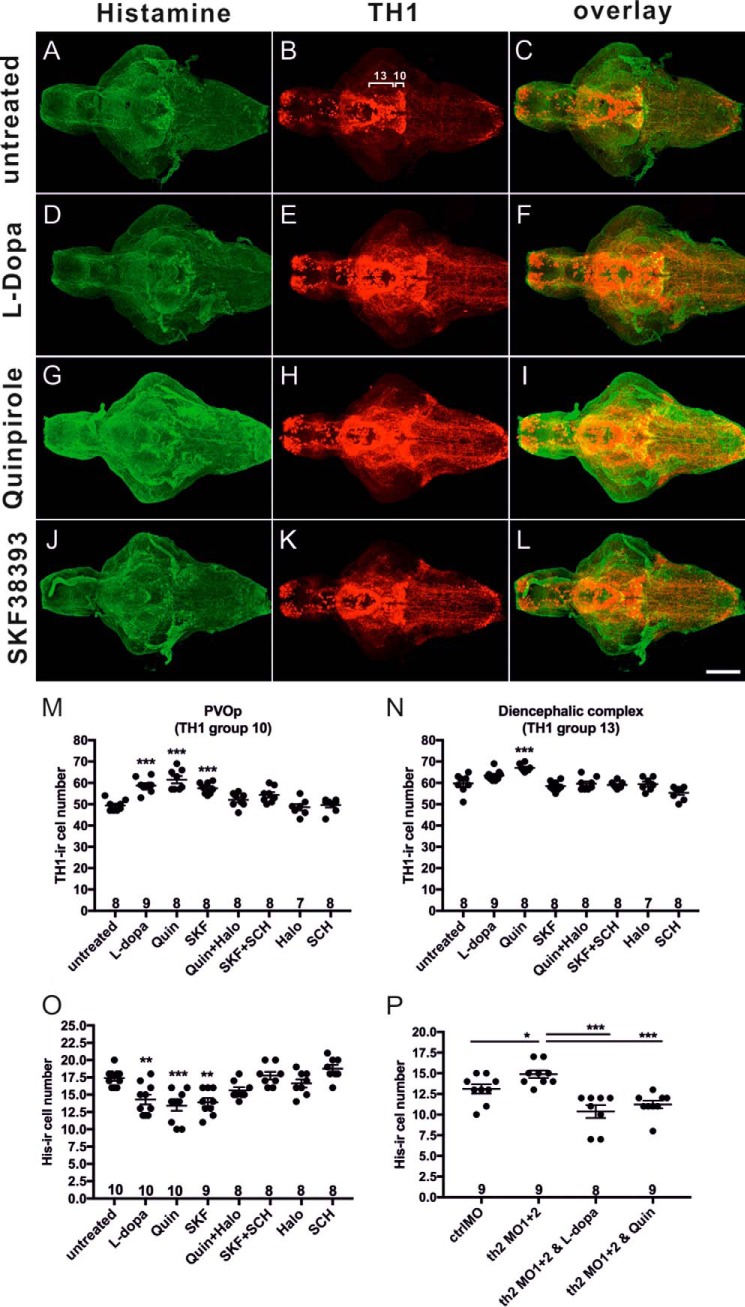
The dynamic changes of histaminergic neurons in th2 morphants suggest that dopamine might be important for the development of target neuron populations. Dopaminergic signaling is mediated by G protein-coupled dopamine receptors, which are grouped into two main subtypes. The D1-like receptors comprise D1 and D5 receptors coupled to stimulatory G proteins to activate adenylyl cyclase. The D2-like receptors, including D2, D3, and D4 receptors, are coupled to inhibitory G proteins and inhibit cAMP synthesis (1). To study whether dopamine or its precursor l-DOPA and specific subtypes of dopamine receptors are involved in the regulation of the histaminergic neuron development, 24-hpf fish embryos were treated with 10 mm l-DOPA (dopamine precursor), 10 μm SKF38393 (D1-like receptor agonist), 10 μm SCH23390 (D1-like receptor antagonist), 7.5 μm quinpirole (D2-like receptor agonist), and 7.5 μm haloperidol (D2-like receptor antagonist) until 5 dpf. Relevant drug concentrations were determined in preliminary experiments (data not shown) based on previous publications (4, 30). The drug effect on the histaminergic system was studied by counting the histamine-ir cells. We found that wild-type larvae treated with l-DOPA, quinpirole, and SKF38393 showed a significant reduction in number of histaminergic hdc-expressing cells compared with the untreated control fish (Fig. 6, A, D, G, J, and O; F(7, 63) = 11.06, p < 0.0001), whereas a significant increase in TH1-ir cells was observed in l-DOPA- and quinpirole-treated groups (Fig. 6, B, E, H, K, and M; (F(7, 56) = 15.60, p < 0.0001); Fig. 6N (F(7, 56) = 12.51, p < 0.0001) in the posterior part of the paraventricular organ (PVOp) and diencephalic complex, including TH1 populations 10 and 13, where histaminergic neurons are neighboring (population number based on earlier descriptions). Interestingly, dopamine receptor antagonist treatment normalized the alterations of cell numbers caused by dopamine receptor agonist administrations, although antagonists alone did not affect the dopaminergic and histaminergic neurons (Fig. 6, M–O). Furthermore, l-DOPA and quinpirole treatment restored (normalized) the increased histaminergic neuron numbers in th2 morphants (Fig. 6P, F(3, 31) = 12.5, p < 0.0001), indicating that histaminergic neurons were affected by dopamine signaling activity during zebrafish development.
FIGURE 6.
Dopamine receptor agonists affect the histaminergic and dopaminergic neuron numbers in 5-dpf fish brains. The cell numbers were quantified following histamine and TH1 co-immunostaining. A–C, no treatment. D–F, 10 mm l-DOPA treatment. G–I, 7.5 μm quinpirole treatment. J–L, 10 μm SKF38393. Quantification of TH1-ir cell numbers after dopamine receptor agonist and antagonist administration is shown in PVOp (TH1 group 10, M) and in the diencephalic complex (TH1 group 13, N). Quantification of His-ir cell numbers is shown in O. Quantification of his-ir cell numbers in th2 MO1 + 2 morphant brains after drug treatments is shown in P. TH1 group numbers 10 and 13 are based on Ref. 61), corresponding to DC7 and DC6 in Ref. 36. His-ir positive cells are shown in green. TH1-labeled cells are shown in red. The number of brains analyzed and the mean value of the cell number are shown in the columns. *, p < 0.05; **, p < 0.01; ***, p < 0.001; one-way ANOVA with Tukey's test. Scale bar = 100 μm.
Wnt Signaling Affects Dopaminergic and Histaminergic Neuron Development
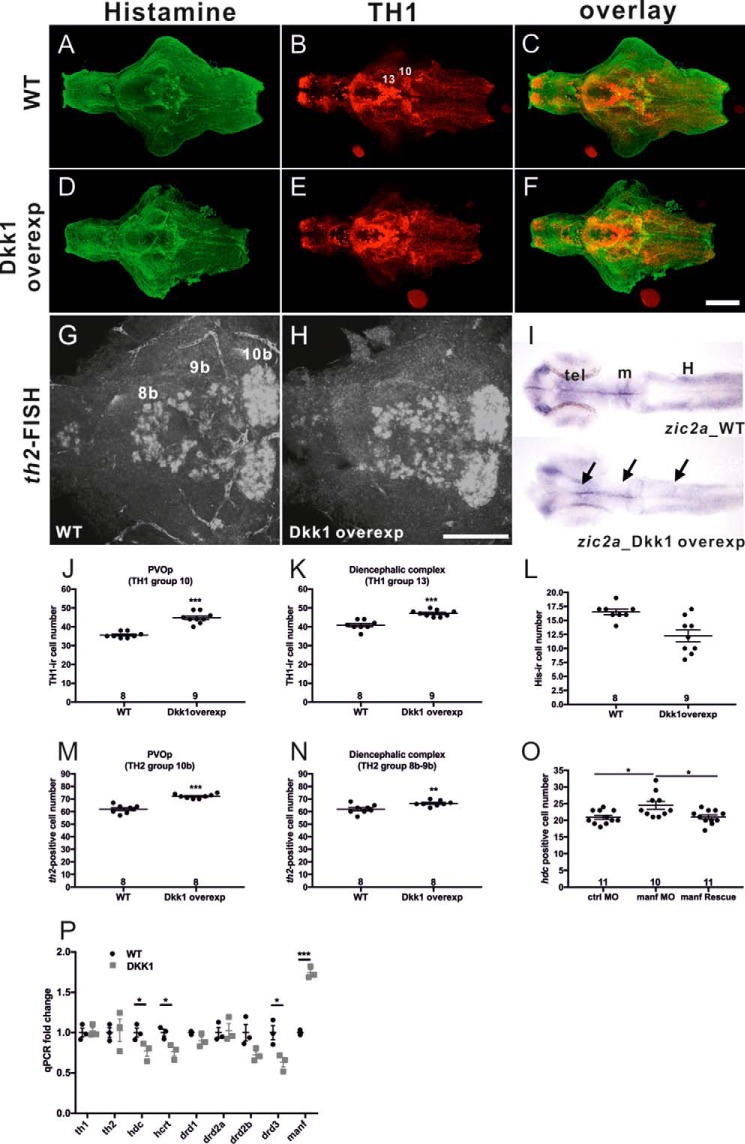
The Wnt signaling cascade is essential for hypothalamic progenitor differentiation (31), and overexpression of the Wnt antagonist dickkopf 1 (dkk1) mRNA elevates the number of dopaminergic th1-expressing neurons in 2-dpf zebrafish brains (32) To study whether Wnt signaling is involved in the regulation of histaminergic neuron development, his-ir and TH1-ir cells were counted following double staining of histamine and TH1 in 5-dpf fish brains after DKK1 mRNA injections in the yolk at the one-cell stage. The inhibition effect on Wnt signaling was verified by testing the expression of the Wnt downstream targets zic2a and zic5 (33). A decreased signal of zic2a (Fig. 7I) and zic5 (data not shown) in the telencephalon, midbrain, and hindbrain was detected in the DKK1 mRNA-injected group. We next found that TH1-ir cells were robustly increased in the PVOp and diencephalic complex in TH1 cell populations 10 and 13 (Fig. 7, B, E, J, and K; p < 0.0001, Student's t test), which supports the result of Russek-Blum et al. (32). Moreover, a significant increase in th2-containing cell numbers was detected in TH2 cell populations 10b and 8b-9b (Fig. 7, G and H; p < 0.0001 and p = 0.0096, Student's t test). These cell populations are relevant for histaminergic neurons because these dopaminergic neurons are surrounded by histaminergic neurons (Fig. 1F). In contrast to the effect on TH1 populations, histaminergic neurons were significantly decreased in number in PVOp (Fig. 7, A, D, and L; p = 0.0033, Student's t test), the only site where histaminergic neurons are found in the zebrafish brain. An overlay image of histamine-ir and TH1-ir is shown in Fig. 7, C and F.
FIGURE 7.
Dynamic effects of Wnt signaling on dopaminergic and histaminergic neurons. The images of His-ir and TH1-ir cells are depicted in the wild-type group (A–C) and the DKK1 mRNA overexpression group (D–F). Images of th2-FISH distributions are shown in G and H. I, overexpression of Dkk1 mRNA down-regulates zic2a expression in the telencephalon (tel), midbrain (m), and hindbrain (H) regions compared with the WT. Comparisons of TH1-ir cell numbers between WT and DKK1 mRNA overexpression brains in PVOp (TH1 group 10) and in the diencephalic complex (TH1 group 13) are shown in J and K, respectively. Quantification of His-ir cell numbers is shown in L. Quantification of th2 expression cell numbers in PVOp (TH2 group 10b) and in the diencephalic complex (TH2 groups 8b and 9b)) are shown in M and N, respectively. Quantification of hdc-positive cell numbers in the ctrl MO, manf MO, and manf rescue morphant brain is demonstrated in O. P, levels of mRNA expression by qPCR (n = 3). -Fold changes were calculated relative to the average expression of wild-type groups. TH1 and TH2 group numbers are based on Ref. 61. TH1-labeled cells are shown in red. His-ir positive cells are shown in green. Arrows indicate regions where zic2a expression is reduced. The number of brains analyzed (n) and the mean value of the cell number are shown in the columns. *, p < 0.05; **, p < 0.01; ***, p < 0.001; Student's t test or one-way ANOVA with Tukey's test. Scale bars = 100 μm.
We further used qPCR to examine the expression levels of representative genes of the histaminergic, hypocretin, and dopamine systems, including receptors and neurotrophic factors important for the dopaminergic system. The th1 and th2 transcripts in the larval heads were not altered, although the TH1 and th2-containing cell numbers in cell groups 10/10b and 13/8–9b were increased by about 36% and 15%, respectively, following DKK1 overexpression. The reason for the evident discrepancy between the cell counting result and the qPCR may be that only two populations adjacent to histaminergic neurons were counted in the brain, but the qPCR was done on the whole head, so that the total change could simply be too subtle to be detected in the overall th expression. A significant reduction of drd3 mRNA was also detected (Fig. 7P, p = 0.0258), and the expression level of hdc and hcrt transcripts declined significantly (Fig. 7P, p = 0.0368 and p = 0.026, respectively), confirming the reduction of the His-ir and hcrt neurons. Mesencephalic astrocyte-derived neurotrophic factor (MANF) is recognized as a dopaminergic neurotrophic factor (34, 35). Remarkably, a significant increase in manf transcripts was found in the DKK1 overexpression group (Fig. 7P, p < 0.001). To study whether MANF affects histaminergic neuron development, we knocked down manf expression by MOs and compared the number of hdc-expressing cells between control, MO-injected, and mRNA-rescued morphant groups. We found that the number of hdc-containing cells was significantly higher in the MANF-deficient morphants than in the control (ctrl) MO group and manf mRNA rescue morphants (Fig. 7O, F(2, 29) = 6.104, p = 0.0061). MANF deficiency thus not only causes the reduction in th1 and th2-containing cells (35) but also leads to a significant increase in hdc-expressing cell numbers found in this study. Moreover, DKK1 overexpression associated with a robust increase in manf transcripts and TH1-containing cells and a reduction in histaminergic cell number suggests that MANF has an impact on histaminergic system development.
Discussion
Here we report that dopaminergic signaling controls the numbers of histaminergic and hypocretin neurons in the vertebrate CNS. Using differential knockdown of the zebrafish th genes th1 and th2, we showed that TH2 acts as the mammalian tyrosine hydroxylase in dopamine synthesis and is involved in the regulation of histaminergic and hcrt neuron numbers. We confirmed our findings using experimental manipulations of dopaminergic signaling, including the use of dopamine agonists and Wnt antagonism.
The anatomy of the catecholaminergic systems in both larval and adult zebrafish brain has been well studied by tract tracing (18), in situ hybridization (27), and immunohistochemistry using tyrosine hydroxylase (TH) and dopamine antibodies (15, 17, 19, 21, 27, 36–39). In the embryonic zebrafish brain, th1 and th2 show a complementary expression pattern, and th1 expression is more widespread than th2 (13, 14). th1 shares higher amino acid sequence similarity with mammalian th genes, and comparative analysis of TH1-ir populations between zebrafish and mammals has revealed many conserved features and some differences (17–19).
Ren et al. (25) used the Tg(ETvmat2:GFP) transgenic line having GFP expression in vmat2 neurons as a reference marker to indirectly show that th2 and 5-HT were co-localized in the ventral diencephalon and the caudal hypothalamus. The conclusion is contradictory to our current findings and several other reports, which confirm the fact that zebrafish th2 has tyrosine hydroxylase activity in dopamine synthesis (39, 40). In addition, VMAT2 is found in all aminergic neurons, including dopaminergic, histaminergic, noradrenergic, and serotonergic neurons (28, 41). Based on the conserved structure and functional domain comparison of tyrosine hydroxylase among species (22), the co-existence of dopaminergic cell markers with th2 (14, 15, 27), the intensive dopamine immunoreactivity in neurons which express th2 (22, 27, 40), and the functional assays provided in this study strongly indicate that TH2 has tyrosine hydroxylase activity and contributes to the synthesis of dopamine. Moreover, no specific co-localization exists among TH2-ir, histaminergic, and serotonergic markers in the caudal hypothalamus in the 5-dpf and adult zebrafish brain (22). In this study, we provide high-resolution images with multiple labels using Tg(f.TH:egfp) transgenic fish having GFP expressed in th2-expressing cells to prove that th2- and serotonin-containing cells are distinct in the caudal hypothalamus. We also measured endogenous brain catecholamine and 5-HT levels by HPLC. The 5-HT level and tph1a mRNA expression were not affected in th2MO1 + 2 morphants, whereas dopamine levels declined, and the effects were rescued by th2 mRNA co-injection. On the other hand, in the study by Ren et al. (25), neither endogenous tph1a activity and expression level nor dopamine concentration in th2 morphants were analyzed. Additionally, we also analyzed and reported the primary structure of zebrafish TH1 and TH2 (22). In brief, TH2 possesses the arginine residues needed for feedback inhibition by dopamine, and the Leu, Trp, and Asp residues required for substrate (tyrosine) binding are conserved. Importantly, the Leu residue Leu-294 is present in zebrafish TH1 and TH2, whereas, in tryptophan hydroxylase, this is replaced by a Tyr residue. Our findings strongly support that TH2 can function as an active tyrosine hydroxylase, a conclusion that could be further verified by studying a stable th2 mutant fish when one becomes available.
The hypothalamus is responsible for regulating metabolic processes, sleep, and circadian cycles, in which dopamine is also involved (42). Here we first reported that loss of th2 expression caused a significant increase in histaminergic and hypocretin cells as well as histamine level in larval brains, suggesting that th2-expressing neurons affect the development of the adjacent neurons, possibly by direct innervation through synaptic neurotransmission or paracrine actions. This provides evidence that dopamine actively limits the number of histaminergic neurons and fibers, which is in agreement with findings reported in post-mortem brains with Parkinson's disease. The density of histaminergic fibers in the substantia nigra pars compacta is increased (8), and an increase in histamine level is restricted to brain areas that are affected by lack of dopamine (7).
There is good evidence of dopaminergic regulation of histamine neurons from rodent studies (43). This study showed that rodent histamine neurons are excited by l-DOPA and both dopamine D1 and D2 receptor agonists and that they express both D1- and D2-type dopamine receptors. These results are in full agreement with our results on zebrafish. There is also a growing body of evidence indicating that histamine receptor 3 directly interacts with dopaminergic neurotransmission and forms heterodimers with dopamine receptors in the rodent dorsal striatal target neurons. In zebrafish, it is still unclear whether similar interactions occur in the hypothalamus, where histamine receptor 3 and dopamine receptors are found.
To our knowledge, the developmental regulation of hdc-expressing neurons is largely unknown, except that γ-secretase activity regulates histamine cell numbers through notch1 activity (44). We found that the dopamine-mediated reduction in hdc neuron number was specific because no effects on the serotonergic system or slc6a3-containing cells in preoptic area, posterior tuberculum, and caudal hypothalamus area were found. It is useful to point out that slc6a3 is an important transporter in dopaminergic neurons but not a reliable marker of these cells because slc6a3 develops late in the caudal hypothalamus area (27, 45). In addition to dopamine signaling, Wnt signaling and MANF, known to regulate dopaminergic system development, were also found to affect histaminergic neuron development in this study, although it is unclear whether the regulation is direct or occurs through the imbalance of dopamine signaling. The changes in histaminergic neurons were verified using several methods, including hdc ISH, histamine immunocytochemistry, HPLC, and qPCR, which render the results reliable. Notably, knockdown of th2 was followed by an increase in both histaminergic and hcrt neurons. We have shown previously that histamine regulates the number of developing hcrt neurons in a bidirectional manner through the H1 receptor (20). The most likely mechanism is that dopamine or histamine, for histaminergic and hcrt neurons, respectively, can act as paracrine factors regulating neuronal specification during embryonic neurogenesis.
Previous studies have shown that dopamine can affect cell proliferation and differentiation in the embryonic mouse telencephalon (46) and in the adult subventricular zone (47, 48). In zebrafish, suppression of D2 receptor signaling through the Akt pathway reduces the number of GABAergic neurons. In this study, dopamine receptor antagonists did not alone affect cell numbers. However, they normalized the changes caused by the agonists. The effects on target neurons of l-DOPA could also be in part mediated by l-DOPA, which is produced by TH and which alone also has an effect, as seen in Fig. 6. l-DOPA excites histaminergic neurons in the rodent brain (43). Our finding of dopaminergic regulation of neurotransmitter identity specification is consistent with two potential mechanisms. First, th2 deficiency may disturb dopaminergic control of hypothalamic neurogenesis (31, 49). Altered neurogenesis ratios of different neuron populations could lead to an increase in hcrt-expressing neurons. Second, th2 deficiency could affect transmitter specification. This could, in turn, give rise to remodeling of the neurotransmitter circuits so that an increase in histamine leads to increased hcrt-expressing neurons (20). These findings may have implications in mechanisms underlying changes observed in the brains of patients suffering from Parkinson's disease and schizophrenia because both the histaminergic and dopaminergic systems are abnormal in these disease states (7, 10). The abnormally high numbers of histamine neurons in narcoleptic patients (50–52) and notch1-mediated increase in histamine neurons in adult presenilin1-deficient zebrafish (44) suggest that histamine neuron specification also occurs in adult vertebrates (53).
Experimental Procedures
Zebrafish Strain and Maintenance
Zebrafish were obtained from our breeding line maintained in the laboratory for more than a decade (17, 19, 29, 54). Fish were raised at 28 °C and staged in hours post-fertilization or days post-fertilization as described previously (55). The Tg(f.TH:egfp) transgenic line was reported previously (Tg(f.TH.A:egfp)zc56) by Fujimoto et al. (16); the Zebrafish Model Organism Database nomenclature is Tg(Tru.Th:EGFP)zc56. The permits for the experiments were obtained from the Office of the Regional Government of Southern Finland in agreement with the ethical guidelines of the European convention.
Characterization of Rabbit TH2 Antibody
The TH2 antibodies used in this study were produced against a recombinant protein containing a 150-amino acid N-terminal fragment of TH2 tagged by GST at the N terminus (22). The crude antiserum (TH2 169C) reacted with both forms of zebrafish TH (TH1 and TH2). The TH2 antiserum can be used in combination with a monoclonal antibody targeting TH1 to allow identification of single-stained TH2 cells and double-stained TH1 cells. The full characterization of the antibodies is described in detail elsewhere (22).
Western Blotting
Western blotting analysis was performed as described previously (22). Briefly, 5-dpf zebrafish larvae were collected and manually homogenized on ice in 0.05 m Tris-HCl buffer (pH 7.5) containing Complete Mini protease inhibitors (Roche) and 0.3 mm PMSF. 40 μg of proteins was loaded on each lane, followed by blotting onto PVDF membranes, and the membranes were stained with ProAct membrane stain (M282–1L, Amresco Inc.) as the loading control. Rabbit TH2 antiserum was preadsorbed on PFA-fixed 1-dpf embryos, and primary and secondary antibodies were diluted 1:3000. The intensities of the Western blotting bands were measured by ImageJ 1.49c.
HPLC
The heads of 5-dpf larvae were analyzed following removal of the eyes and trunks on ice. Fifteen dissected heads were grouped and lysed in 150 μl of 2% perchloric acid with sonication. After centrifugation, 10 μl of supernatant was assessed for monoamine concentration by HPLC. Three individual groups per treatment condition were measured as a blinded experiment. The detection details are described in Sallinen et al. (17, 29).
RNA Isolation and cDNA Synthesis
For quantitative real-time PCR analysis, total RNA was extracted from 15 pooled fish heads collected at 5 dpf (RNeasy mini kit, Qiagen, Valencia, CA). To synthesize cDNA, 2 μg of total RNA was reverse-transcribed using SuperScriptTM III reverse transcriptase (Invitrogen) according to instructions provided by the manufacturer. The primers for cloning tph1a, zic2a, and zic5 were as follows: tph1a, 5′-CCATGAACCTCGGAATGACTT-3′ and 5′-CCTGAAACGTGGTGATGATGCA-3′; zic2a, 5′-GGATGTGATCGACGCTTTGC-3′ and 5′-AAATGCCCCTGTTTAGCCCA-3′; and zic5, 5′-ACAATAGCGTTGAGCGTGGA-3′ and 5′-ATTTCCTGTCGCAGCCATCA-3′.
MO Design and Use and mRNA Injections
Antisense MOs (Gene Tools LLC, Philomath, OR) were designed to target the splice donor sites of exon 2 and exon 4 of th1 (th1 MO1, 5′-ATTATGTTAGCCTACCTCGAAAACC-3′; th1 MO2, 5′-TAATCCAGCACTTACTGGGTGATCC-3′), the splice donor sites of exon 3 and exon 7 of th2 (th2 MO1, 5′-CTGTTGTTCACTTACAGGGTGATCC-3′; th2 MO2, 5′-TTATGCATTGTACGTACGGTTCAGG-3′), and splice donor sites of exon2 and exon3 of manf (manf MO1, 5′-GACGGGTACTTACAAATCGGTTTTC-3′; manf MO2, 5′-TGCAAACAACTCACCGTATTTGAGT-3′). The working concentration was determined by injecting serially diluted MOs. The injection dose of th2 MO1 or th2 MO2 was 8 ng. The combination doses of 3.5 ng each of th1 MO1 and th1 MO2 (th1 MO1 + 2), 4 ng each of th2 MO1 and th2 MO2 (th2 MO1 + 2), and 4 ng each of manf splice-blocking MOs (manf MOs) were found to produce the most effective inhibition (22, 35). A standard control MO (ctrl MO, 5′-CCTCTTACCTCAGTTACAATTTATA-3′) purchased directly from Gene-Tools was injected at 8 ng/embryo. The th2 full-length open reading frame cDNA constructs were prepared by RT-PCR using Phusion High-Fidelity PCR Master Mix (Finnzymes, Espoo, Finland). The primers for th2 cloning were th2F-5′-ATAAGAATGGAATTCCCACCATGAAGTCGGACAGTATAGCGCAG and th2R-5′-ATAAGAATGGGATCCATTATTTCTGTCCCAGTCTCCCCAAG. The PCR amplicons were cloned into the pGEM-T Easy vector (Promega, Madison, WI) and verified by sequencing. The insert sequence with no mutations was subcloned into the expression vector pMC containing untranslated repeats and polyadenylation, which can enhance mRNA stability and translation efficiency. pMC was kindly given by Dr. Thomas Czerny (56). The plasmid was linearized with ApaI for mRNA synthesis. Capped full-length open reading frame transcripts were generated by the mMESSAGE mMACHINE kit (Ambion, Austin, TX) using T7 RNA polymerase. For the mRNA rescue experiment, 500 pg of th2 mRNA with th2MO1 + 2 was co-injected into embryos at the one-cell stage. The MOs were thoroughly characterized for targeting and specificity (22). The th1 MO and th2 MO abolished TH1 immunoreactivity and the TH2 signal in immunocytochemistry, respectively (22). In this study, all essential th2 morphant phenotypes were rescued by th2 mRNA co-injection.
Quantitative Real-time PCR (qPCR)
qPCR was performed in the LightCycler 480 instrument (Roche) using LightCycler® 480 SYBR Green I Master Mix (Roche). Primers for amplification were designed by Primer-BLAST (NCBI). Two housekeeping genes, β-actin and ribosomal protein L13a (rpl13a), were used as reference controls. All primer sets were confirmed to amplify only a single product of the correct size. Sequences of primers were as follows: β-actin, 5′-CGAGCAGGAGATGGGAACC-3′ and 5′-CAACGGAAACGCTCATTGC-3′; rpl13a, 5′-AGAGAAAGCGCATGGTTGTCC-3′ and 5′-GCCTGGTACTTCCAGCCAACTT-3′; p53, 5′-ATGAGGAGATCTTTACCCTGCAG-3′ and 5′ TGAGGCAGGCACCACATC-3′; Δ113p53, 5′-ATATCCTGGCGAACATTTGGAGGG-3′ and 5-CCTCCTGGTCTTGTAATGTCAC-3′; th1, 5′-GACGGAAGATGATCGGAGACA and 5′-CCGCCATGTTCCGATTTCT-3′; th2, 5′-CTCCAGAAGAGAATGCCACATG and 5′-ACGTTCACTCTCCAGCTGAGTG-3′; hdc, 5′-TTCATGCGTCCTCTCCTGC-3′ and 5′-CCCCAGGCATGATGATGTTC-3′; hcrt, 5′-TCTACGAGATGCTGTGCCGAG and 5′-CGTTTGCCAAGAGTGAGAATC-3′; drd1, 5′-TGCCATGGAAAGCCGCCACG-3′ and 5′-TGGCCCAGTAGCGGTCCACA-3′; drd2a, 5′-ACCTCCATCGCCTGAAGCTGGT-3′ and 5′-TTGCCGGTGGGGGAGACCTG; drd2b, 5′-GGTTCTACGCAAGCGGCGGA-3′ and 5′-GGCAGGTACACCCCCGTTGG-3′; drd3, 5′-CCACGGTTTGGGTCCTCGCC-3′ and 5′-AGGGTCACCGCAAACGGCAA-3′; and manf, 5′-AGATGGAGAGTGTGAAGTCTGTGTG-3′ and 5′-CAATTGAGTCGCTGTCAAACTTG-3′tj;1. Cycling parameters were as follows: 95 °C for 5 min and 45 cycles of the following: 95 °C for 10 s, 60 °C for 15 s, and 72 °C for 20 s. Fluorescence changes were monitored with SYBR Green after every cycle. Dissociation curve analysis was performed (0.1 °C/s increase from 60 °C to 95 °C with continuous fluorescence readings) at the end of cycles to ensure that only a single amplicon was obtained. All reactions were performed in duplicates and three individual replicates. Results were evaluated with LightCycler 480 software. The data were calculated by the comparative method using Ct values of β-actin and rpl13a, respectively, as the reference control (57). Because the gene expression changes showed the same trend when normalized to different housekeeping genes (data not shown), the results referring to β-actin are shown in this study.
WISH
Whole-mount in situ hybridization (WISH) was performed on 4% paraformaldehyde (PFA)-fixed 5-dpf dissected brains as described earlier (13). Antisense and sense digoxigenin (DIG)-labeled RNA probes were generated using the DIG RNA labeling kit (Roche Diagnostics) following the instructions of the manufacturer. The WISH procedure followed the protocol of Thisse and Thisse (58). Prehybridization and hybridization were conducted at 65 °C for all riboprobes. In situ hybridization signals were detected with sheep anti-digoxigenin-AP Fab fragments (1:10,000, Roche Diagnostics). Color staining was carried out with chromogen substrates (nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate). For th2 fluorescent in situ hybridization (FISH), samples were incubated in 2% H2O2 in methanol for 20 min to inactivate endogenous peroxidase activity. A th2-DIG-labeled probe was used for hybridization. To visualize the hybridized probe, samples were incubated in peroxidase-conjugated anti-DIG antibody (1:500, Roche, 11207733910) followed by the bench-made carboxyfluorescein tyramide reaction (59).
Immunocytochemistry
Immunostaining was performed on 2% PFA or 4% 1-ethyl-3 (3-dimethylaminopropyl)-carbodiimide-fixed zebrafish. For 5-dpf fixed larvae, brains were dissected to enhance antigen presentation and improve image quality. Antibody incubations were carried out with 4% normal goat serum and 1% dimethyl sulfoxide in 0.3% Triton X-100/phosphate-buffered saline for 16 h at 4 °C with gentle agitation. Primary antibodies were chicken anti-green fluorescent protein (1:1000, A10263, Invitrogen), rabbit anti-histamine 19C (1:10,000 (20, 60)), rabbit anti-TH2 antibody (1:2000 (22)), rabbit anti-serotonin antibody (1:1000, S5545, Sigma), rat anti-serotonin antibody (1:250, MAB352, EMD Millipore), and anti-tyrosine hydroxylase monoclonal mouse antibody (1:1000, product no. 22941, Immunostar, Hudson, WI). The specificities of the histamine, commercial mouse monoclonal TH, and serotonin antibodies have been verified previously (19). The following secondary antibodies were applied: Alexa Fluor® 488, 568, or 647 goat anti-chicken, anti-mouse, or anti-rabbit IgG (1:1000, Invitrogen).
Imaging
Bright-field images were taken with a Leica DM IRB inverted microscope with a DFC 480 charge-coupled device camera, and z stacks were processed with Leica Application Suite software and Corel DRAW X3 software (13). Immunofluorescence samples were examined using a Leica TCS SP2 AOBS confocal microscope. For excitation, an argon laser (488 nm), green diode laser (561 nm), and red HeNe laser (633 nm) were used. Emission was detected at 500–550 nm, 560–620 nm, and 630–680 nm, respectively. Cross-talk between the channels and background noise was eliminated with sequential scanning and frame-averaging as described earlier (17). Stacks of images taken at 0.2- to 1.2-μm intervals were compiled, and the maximum intensity projection algorithm was used to produce final images with Leica confocal software and Imaris imaging software version 6.0 (Bitplane AG, Zurich, Switzerland). Cell numbers were counted in each 1.0-μm optical slice using ImageJ 1.46r software (National Institutes of Health, Bethesda, MD), and all cell counts were performed by an investigator blinded to the sample type.
Pharmacological Treatments
24-hpf wild-type or morphant embryos were manually dechorionated, and 20 embryos/group were raised in 6-well plates containing 3 ml of E3 medium (5 mm NaCl, 0.17 mm KCl, 0.33 mm CaCl2, and 0.33 mm MgSO4 with or without drug additions (l-DOPA, D9628; SKF38398, S101; quinpirole, Q102; haloperidol, H1512; SCH23390, D054; Sigma-Aldrich, St. Louis, MO)). The incubation medium was replaced daily until 5 dpf. For the serotonin measurement control, 4-dpf fish were exposed to 100 μm p-chlorophenylalanine (25920, Sigma-Aldrich) for 24 h.
Statistical Analysis
Data analysis was performed by GraphPad Prism v.4.1 software (San Diego, CA). p Values were generated by one-way analysis of variance (ANOVA) for multiple comparisons using Tukey's multiple comparison test and Student's t test (unpaired test) for comparison of two groups. Data are presented as mean ± S.E. p <0.05 was considered statistically significant.
Author Contributions
Y. C. C. and P. P. conceived and designed the experiments. Y. C. C., S. S., S. R., and M. S. performed the experiments and analyzed the data. J. L. B. contributed the transgenic fish. Y. C. C. and P. P. wrote the manuscript with input from all authors.
Supplementary Material
Acknowledgments
We thank Henri Koivula and Reeta Huhtala for expert technical help.
This work was supported by the Academy of Finland and Sigrid Juselius Foundation (to P. P.) and DP2 MH100008 (to J. L. B.). The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Figure S1.
- MO
- morpholino oligonucleotide
- dpf
- day(s) post-fertilization
- ir
- immunoreactive
- his-ir
- histamine immunoreactivity
- IHC
- immunohistochemistry
- hpf
- hour(s) post-fertilization
- WISH
- whole-mount in situ hybridization
- ISH
- in situ hybridization
- hcrt
- hypocretin
- PVOp
- posterior part of the paraventricular organ
- qPCR
- quantitative PCR
- MANF
- mesencephalic astrocyte-derived neurotrophic factor
- PFA
- paraformaldehyde
- DIG
- digoxigenin
- ANOVA
- analysis of variance
- His
- histamine
- ctrl
- control
- l-DOPA
- l-3,4-dihydroxyphenylalanine
- TH
- tyrosine hydroxylase.
References
- 1. Beaulieu J. M., and Gainetdinov R. R. (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 63, 182–217 [DOI] [PubMed] [Google Scholar]
- 2. Todd R. D. (1992) Neural development is regulated by classical neurotransmitters: dopamine D2 receptor stimulation enhances neurite outgrowth. Biol. Psychiatry 31, 794–807 [DOI] [PubMed] [Google Scholar]
- 3. Schmidt U., Beyer C., Oestreicher A. B., Reisert I., Schilling K., and Pilgrim C. (1996) Activation of dopaminergic D1 receptors promotes morphogenesis of developing striatal neurons. Neuroscience 74, 453–460 [DOI] [PubMed] [Google Scholar]
- 4. Reimer M. M., Norris A., Ohnmacht J., Patani R., Zhong Z., Dias T. B., Kuscha V., Scott A. L., Chen Y. C., Rozov S., Frazer S. L., Wyatt C., Higashijima S., Patton E. E., Panula P., et al. (2013) Dopamine from the brain promotes spinal motor neuron generation during development and adult regeneration. Dev. Cell 25, 478–491 [DOI] [PubMed] [Google Scholar]
- 5. Panula P., and Nuutinen S. (2013) The histaminergic network in the brain: basic organization and role in disease. Nat. Rev. Neurosci. 14, 472–487 [DOI] [PubMed] [Google Scholar]
- 6. Haas H., and Panula P. (2003) The role of histamine and the tuberomamillary nucleus in the nervous system. Nat. Rev. Neurosci. 4, 121–130 [DOI] [PubMed] [Google Scholar]
- 7. Rinne J. O., Anichtchik O. V., Eriksson K. S., Kaslin J., Tuomisto L., Kalimo H., Röyttä M., and Panula P. (2002) Increased brain histamine levels in Parkinson's disease but not in multiple system atrophy. J. Neurochem. 81, 954–960 [DOI] [PubMed] [Google Scholar]
- 8. Anichtchik O. V., Rinne J. O., Kalimo H., and Panula P. (2000) An altered histaminergic innervation of the substantia nigra in Parkinson's disease. Exp. Neurol. 163, 20–30 [DOI] [PubMed] [Google Scholar]
- 9. Winterer G., and Weinberger D. R. (2004) Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 27, 683–690 [DOI] [PubMed] [Google Scholar]
- 10. Prell G. D., Green J. P., Kaufmann C. A., Khandelwal J. K., Morrishow A. M., Kirch D. G., Linnoila M., and Wyatt R. J. (1995) Histamine metabolites in cerebrospinal fluid of patients with chronic schizophrenia: their relationships to levels of other aminergic transmitters and ratings of symptoms. Schizophr. Res. 14, 93–104 [DOI] [PubMed] [Google Scholar]
- 11. Zhou Q. Y., and Palmiter R. D. (1995) Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83, 1197–1209 [DOI] [PubMed] [Google Scholar]
- 12. Kobayashi K., Morita S., Sawada H., Mizuguchi T., Yamada K., Nagatsu I., Hata T., Watanabe Y., Fujita K., and Nagatsu T. (1995) Targeted disruption of the tyrosine hydroxylase locus results in severe catecholamine depletion and perinatal lethality in mice. J. Biol. Chem. 270, 27235–27243 [DOI] [PubMed] [Google Scholar]
- 13. Chen Y. C., Priyadarshini M., and Panula P. (2009) Complementary developmental expression of the two tyrosine hydroxylase transcripts in zebrafish. Histochem. Cell Biol. 132, 375–381 [DOI] [PubMed] [Google Scholar]
- 14. Filippi A., Mahler J., Schweitzer J., and Driever W. (2010) Expression of the paralogous tyrosine hydroxylase encoding genes th1 and th2 reveals the full complement of dopaminergic and noradrenergic neurons in zebrafish larval and juvenile brain. J. Comp. Neurol. 518, 423–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamamoto K., Ruuskanen J. O., Wullimann M. F., and Vernier P. (2010) Two tyrosine hydroxylase genes in vertebrates: new dopaminergic territories revealed in the zebrafish brain. Mol. Cell. Neurosci. 43, 394–402 [DOI] [PubMed] [Google Scholar]
- 16. Fujimoto E., Stevenson T. J., Chien C. B., and Bonkowsky J. L. (2011) Identification of a dopaminergic enhancer indicates complexity in vertebrate dopamine neuron phenotype specification. Dev. Biol. 352, 393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sallinen V., Torkko V., Sundvik M., Reenilä I., Khrustalyov D., Kaslin J., and Panula P. (2009) MPTP and MPP+ target specific aminergic cell populations in larval zebrafish. J. Neurochem. 108, 719–731 [DOI] [PubMed] [Google Scholar]
- 18. Rink E., and Wullimann M. F. (2002) Connections of the ventral telencephalon and tyrosine hydroxylase distribution in the zebrafish brain (Danio rerio) lead to identification of an ascending dopaminergic system in a teleost. Brain Res. Bull. 57, 385–387 [DOI] [PubMed] [Google Scholar]
- 19. Kaslin J., and Panula P. (2001) Comparative anatomy of the histaminergic and other aminergic systems in zebrafish (Danio rerio). J. Comp. Neurol. 440, 342–377 [DOI] [PubMed] [Google Scholar]
- 20. Sundvik M., Kudo H., Toivonen P., Rozov S., Chen Y. C., and Panula P. (2011) The histaminergic system regulates wakefulness and orexin/hypocretin neuron development via histamine receptor H1 in zebrafish. FASEB J. 25, 4338–4347 [DOI] [PubMed] [Google Scholar]
- 21. McLean D. L., and Fetcho J. R. (2004) Relationship of tyrosine hydroxylase and serotonin immunoreactivity to sensorimotor circuitry in larval zebrafish. J. Comp. Neurol. 480, 57–71 [DOI] [PubMed] [Google Scholar]
- 22. Semenova S. A., Chen Y. C., Zhao X., Rauvala H., and Panula P. (2014) The tyrosine hydroxylase 2 (TH2) system in zebrafish brain and stress activation of hypothalamic cells. Histochem. Cell Biol. 142, 619–633 [DOI] [PubMed] [Google Scholar]
- 23. Candy J., and Collet C. (2005) Two tyrosine hydroxylase genes in teleosts. Biochim. Biophys. Acta 1727, 35–44 [DOI] [PubMed] [Google Scholar]
- 24. Yamamoto K., and Vernier P. (2011) The evolution of dopamine systems in chordates. Front. Neuroanat. 5, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ren G., Li S., Zhong H., and Lin S. (2013) Zebrafish tyrosine hydroxylase 2 gene encodes tryptophan hydroxylase. J. Biol. Chem. 288, 22451–22459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robu M. E., Larson J. D., Nasevicius A., Beiraghi S., Brenner C., Farber S. A., and Ekker S. C. (2007) p53 activation by knockdown technologies. PLoS Genet. 3, e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamamoto K., Ruuskanen J. O., Wullimann M. F., and Vernier P. (2011) Differential expression of dopaminergic cell markers in the adult zebrafish forebrain. J. Comp. Neurol. 519, 576–598 [DOI] [PubMed] [Google Scholar]
- 28. Eiden L. E., and Weihe E. (2011) VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann. N.Y. Acad. Sci. 1216, 86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sallinen V., Sundvik M., Reenilä I., Peitsaro N., Khrustalyov D., Anichtchik O., Toleikyte G., Kaslin J., and Panula P. (2009) Hyperserotonergic phenotype after monoamine oxidase inhibition in larval zebrafish. J. Neurochem. 109, 403–415 [DOI] [PubMed] [Google Scholar]
- 30. Irons T. D., Kelly P. E., Hunter D. L., Macphail R. C., and Padilla S. (2013) Acute administration of dopaminergic drugs has differential effects on locomotion in larval zebrafish. Pharmacol. Biochem. Behav. 103, 792–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang X., Kopinke D., Lin J., McPherson A. D., Duncan R. N., Otsuna H., Moro E., Hoshijima K., Grunwald D. J., Argenton F., Chien C. B., Murtaugh L. C., and Dorsky R. I. (2012) Wnt signaling regulates postembryonic hypothalamic progenitor differentiation. Dev. Cell 23, 624–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Russek-Blum N., Gutnick A., Nabel-Rosen H., Blechman J., Staudt N., Dorsky R. I., Houart C., and Levkowitz G. (2008) Dopaminergic neuronal cluster size is determined during early forebrain patterning. Development 135, 3401–3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nyholm M. K., Wu S. F., Dorsky R. I., and Grinblat Y. (2007) The zebrafish zic2a-zic5 gene pair acts downstream of canonical Wnt signaling to control cell proliferation in the developing tectum. Development 134, 735–746 [DOI] [PubMed] [Google Scholar]
- 34. Lindholm P., and Saarma M. (2010) Novel CDNF/MANF family of neurotrophic factors. Dev. Neurobiol. 70, 360–371 [DOI] [PubMed] [Google Scholar]
- 35. Chen Y. C., Sundvik M., Rozov S., Priyadarshini M., and Panula P. (2012) MANF regulates dopaminergic neuron development in larval zebrafish. Dev. Biol. 370, 237–249 [DOI] [PubMed] [Google Scholar]
- 36. Rink E., and Wullimann M. F. (2002) Development of the catecholaminergic system in the early zebrafish brain: an immunohistochemical study. Brain Res. Dev. Brain Res. 137, 89–100 [DOI] [PubMed] [Google Scholar]
- 37. Rink E., and Wullimann M. F. (2004) Connections of the ventral telencephalon (subpallium) in the zebrafish (Danio rerio). Brain Res. 1011, 206–220 [DOI] [PubMed] [Google Scholar]
- 38. Schweitzer J., and Driever W. (2009) Development of the dopamine systems in zebrafish. Adv. Exp. Med. Biol. 651, 1–14 [DOI] [PubMed] [Google Scholar]
- 39. McPherson A. D., Barrios J. P., Luks-Morgan S. J., Manfredi J. P., Bonkowsky J. L., Douglass A. D., and Dorsky R. I. (2016) Motor behavior mediated by continuously generated dopaminergic neurons in the zebrafish hypothalamus recovers after cell ablation. Curr. Biol. 26, 263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mu Y., Li X. Q., Zhang B., and Du J. L. (2012) Visual input modulates audiomotor function via hypothalamic dopaminergic neurons through a cooperative mechanism. Neuron 75, 688–699 [DOI] [PubMed] [Google Scholar]
- 41. Kukko-Lukjanov T. K., and Panula P. (2003) Subcellular distribution of histamine, GABA and galanin in tuberomamillary neurons in vitro. J. Chem. Neuroanat. 25, 279–292 [DOI] [PubMed] [Google Scholar]
- 42. Machluf Y., Gutnick A., and Levkowitz G. (2011) Development of the zebrafish hypothalamus. Ann. N.Y. Acad. Sci. 1220, 93–105 [DOI] [PubMed] [Google Scholar]
- 43. Yanovsky Y., Li S., Klyuch B. P., Yao Q., Blandina P., Passani M. B., Lin J. S., Haas H. L., and Sergeeva O. A. (2011) L-Dopa activates histaminergic neurons. J. Physiol. 589, 1349–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sundvik M., Chen Y. C., and Panula P. (2013) Presenilin1 regulates histamine neuron development and behavior in zebrafish, Danio rerio. J. Neurosci. 33, 1589–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Holzschuh J., Ryu S., Aberger F., and Driever W. (2001) Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mech. Dev. 101, 237–243 [DOI] [PubMed] [Google Scholar]
- 46. Popolo M., McCarthy D. M., and Bhide P. G. (2004) Influence of dopamine on precursor cell proliferation and differentiation in the embryonic mouse telencephalon. Dev. Neurosci. 26, 229–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Freundlieb N., François C., Tandé D., Oertel W. H., Hirsch E. C., and Höglinger G. U. (2006) Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J. Neurosci. 26, 2321–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O'Keeffe G. C., Barker R. A., and Caldwell M. A. (2009) Dopaminergic modulation of neurogenesis in the subventricular zone of the adult brain. Cell Cycle 8, 2888–2894 [DOI] [PubMed] [Google Scholar]
- 49. Maggi R., Zasso J., and Conti L. (2014) Neurodevelopmental origin and adult neurogenesis of the neuroendocrine hypothalamus. Front. Cell. Neurosci. 8, 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Valko P. O., Gavrilov Y. V., Yamamoto M., Reddy H., Haybaeck J., Mignot E., Baumann C. R., and Scammell T. E. (2013) Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann. Neurol. 74, 794–804 [DOI] [PubMed] [Google Scholar]
- 51. John J., Thannickal T. C., McGregor R., Ramanathan L., Ohtsu H., Nishino S., Sakai N., Yamanaka A., Stone C., Cornford M., and Siegel J. M. (2013) Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann. Neurol. 74, 786–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sundvik M., and Panula P. (2015) Interactions of the orexin/hypocretin neurons and the histaminergic system. Acta Physiol. 213, 321–333 [DOI] [PubMed] [Google Scholar]
- 53. Panula P., Sundvik M., and Karlstedt K. (2014) Developmental roles of brain histamine. Trends Neurosci. 37, 159–168 [DOI] [PubMed] [Google Scholar]
- 54. Kaslin J., Nystedt J. M., Ostergård M., Peitsaro N., and Panula P. (2004) The orexin/hypocretin system in zebrafish is connected to the aminergic and cholinergic systems. J. Neurosci. 24, 2678–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., and Schilling T. F. (1995) Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- 56. Fink M., Flekna G., Ludwig A., Heimbucher T., and Czerny T. (2006) Improved translation efficiency of injected mRNA during early embryonic development. Dev. Dyn. 235, 3370–3378 [DOI] [PubMed] [Google Scholar]
- 57. Livak K. J., and Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔ C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 58. Thisse C., and Thisse B. (2008) High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59–69 [DOI] [PubMed] [Google Scholar]
- 59. Lauter G., Söll I., and Hauptmann G. (2014) Sensitive whole-mount fluorescent in situ hybridization in zebrafish using enhanced tyramide signal amplification. Methods Mol. Biol. 1082, 175–185 [DOI] [PubMed] [Google Scholar]
- 60. Panula P., Airaksinen M. S., Pirvola U., and Kotilainen E. (1990) A histamine-containing neuronal system in human brain. Neuroscience 34, 127–132 [DOI] [PubMed] [Google Scholar]
- 61. Sallinen V., Kolehmainen J., Priyadarshini M., Toleikyte G., Chen Y. C., and Panula P. (2010) Dopaminergic cell damage and vulnerability to MPTP in Pink1 knockdown zebrafish. Neurobiol. Dis. 40, 93–101 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.