Abstract
GRK2, a G protein-coupled receptor kinase, plays a critical role in cardiac physiology. Adrenergic receptors are the primary target for GRK2 activity in the heart; phosphorylation by GRK2 leads to desensitization of these receptors. As such, levels of GRK2 activity in the heart directly correlate with cardiac contractile function. Furthermore, increased expression of GRK2 after cardiac insult exacerbates injury and speeds progression to heart failure. Despite the importance of this kinase in both the physiology and pathophysiology of the heart, relatively little is known about the role of GRK2 in skeletal muscle function and disease. In this study we generated a novel skeletal muscle-specific GRK2 knock-out (KO) mouse (MLC-Cre:GRK2fl/fl) to gain a better understanding of the role of GRK2 in skeletal muscle physiology. In isolated muscle mechanics testing, GRK2 ablation caused a significant decrease in the specific force of contraction of the fast-twitch extensor digitorum longus muscle yet had no effect on the slow-twitch soleus muscle. Despite these effects in isolated muscle, exercise capacity was not altered in MLC-Cre:GRK2fl/fl mice compared with wild-type controls. Skeletal muscle hypertrophy stimulated by clenbuterol, a β2-adrenergic receptor (β2AR) agonist, was significantly enhanced in MLC-Cre:GRK2fl/fl mice; mechanistically, this seems to be due to increased clenbuterol-stimulated pro-hypertrophic Akt signaling in the GRK2 KO skeletal muscle. In summary, our study provides the first insights into the role of GRK2 in skeletal muscle physiology and points to a role for GRK2 as a modulator of contractile properties in skeletal muscle as well as β2AR-induced hypertrophy.
Keywords: adrenergic receptor, Akt PKB, G protein-coupled receptor (GPCR), mouse, muscle hypertrophy, muscle physiology
Introduction
G protein-coupled receptors (GPCRs)2 comprise the largest family of membrane proteins in the genome. GPCRs regulate diverse processes throughout the body by responding to a vast array of extracellular stimuli including hormones, neurotransmitters, and photons of light (1). Equally important to the stimulation and activation of GPCRs is the desensitization and “shutting-off” of the receptor. This task is primarily conducted by the GPCR kinase (GRK) family of proteins (2, 3). GRK2 is ubiquitously expressed throughout the tissues of the body, perhaps most notably in the heart where its regulation of adrenergic receptors (ARs) is critical to physiological heart function. Cardiac overexpression of GRK2 in mice suppresses contractility, whereas cardiac overexpression of the GRK2 inhibitor βARKct (a C-terminal peptide that competes with GRK2 binding to Gβγ) enhances contractile function (4). Catecholamine overdrive during heart failure drives increased GRK2 expression in the cardiomyocyte, ultimately leading to excessive desensitization of βARs, loss of receptor density, and a drop in inotropic reserve (5–7). Indeed, myocardial inhibition or deletion of GRK2 can prevent and even reverse heart failure in numerous animal models (4, 8–13).
Although we have a firm understanding of the role of GRK2 in the physiology and pathophysiology of the heart, relatively little is known about the function of this kinase in skeletal muscle. Many of the prominent effects of GRK2 in the heart are mediated by regulation of the β2AR (2, 14). This receptor is also expressed in skeletal muscle and modulates various aspects of skeletal muscle physiology. βAR agonists have long been known to induce hypertrophy of skeletal muscle and have been studied as a potential therapeutic for muscle wasting diseases (15). In particular, clenbuterol administration has been shown to induce skeletal muscle hypertrophy via a β2AR-dependent mechanism (16). In addition, as is the case in the heart, β2AR agonists can modulate the contractile properties of skeletal muscle (17).
Given the aforementioned roles of the β2AR in skeletal muscle and the fact that GRK2 is a regulator of the β2AR, we sought to determine the genetic requirement for GRK2 in skeletal muscle physiology. Specifically, using skeletal muscle-specific GRK2 knock-out (KO) mice, we assessed exercise performance and contractile properties of isolated muscles. Furthermore we investigated whether GRK2 ablation in skeletal muscle would enhance the pro-hypertrophic effects of a β2AR agonist.
Results
Generation of a Skeletal Muscle-specific GRK2 KO Mouse
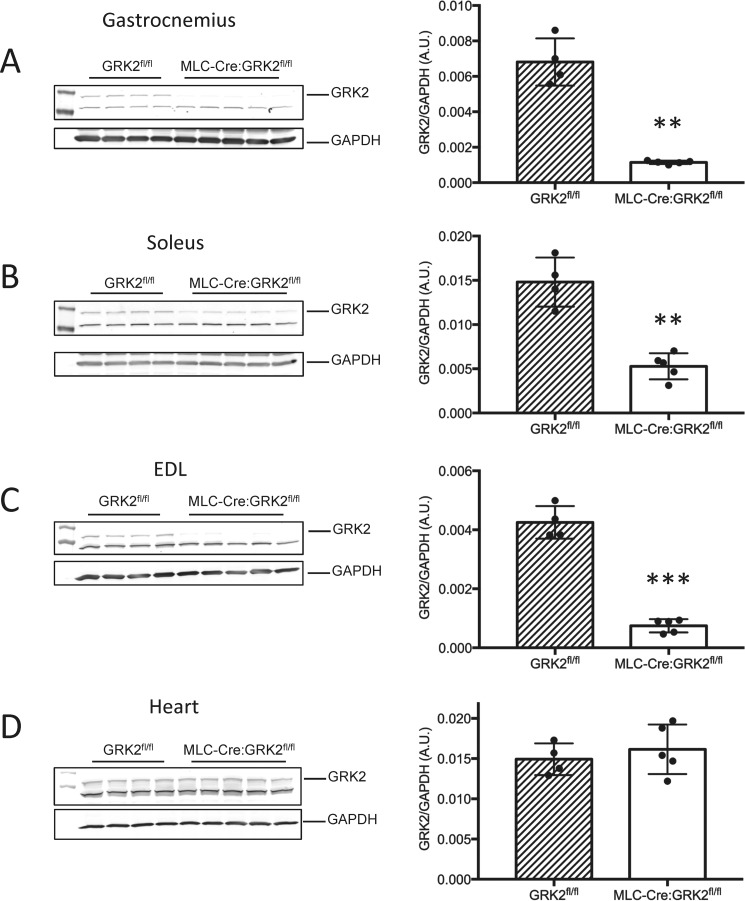
To study the role of GRK2 in skeletal muscle function and contractility, we generated a skeletal muscle-specific GRK2 KO mouse by crossing mice with loxP sites flanking exons 3–6 of GRK2 (GRK2fl/fl) (18) with MLC-Cre mice. The MLC-Cre mice express Cre-recombinase under control of the myosin light chain 1f (MLC1f) promoter (19). The MLC1f locus is active only in type II fast-twitch fibers. In agreement with others, we found the greatest levels of target gene knockdown in the gastrocnemius and EDL muscles (∼83 and 82% knockdown of GRK2 respectively), both of which contain a high proportion of type II fibers (Fig. 1, A and B) (20, 21). In contrast, the soleus muscle, which contains fewer type II fibers and a higher proportion of type I slow fibers showed ∼65% GRK2 knockdown, reflecting lower MLC1f promoter activity in this muscle (Fig. 1C). To convince ourselves of the skeletal muscle specificity of the MLC-Cre:GRK2fl/fl mice, we assessed GRK2 levels in heart tissue lysate by Western blot and found no change with respect to GRK2fl/fl animals (Fig. 1D).
FIGURE 1.
Skeletal muscle-specific knockdown of GRK2 in MLC-Cre:GRK2fl/fl mice. GRK2 protein levels were measured via Western blot in lysates from gastrocnemius (A), soleus (B), EDL (C), and heart (D). GAPDH levels were assessed as a loading control. Bars depict the mean signal intensity of GRK2 normalized to GAPDH, and error bars represent S.D. **, p < 0.01; ***, p < 0.001 between GRK2fl/fl and MLC-Cre:GRK2fl/fl animals; n = 4 and 5 per group, respectively. A.U., arbitrary units.
Skeletal Muscle GRK2 Knockdown Does Not Impair Exercise Performance
GRK2 activity levels in the heart correspond directly with contractile function (4). To assess whether GRK2 ablation in the skeletal muscle has a direct functional impact, we challenged GRK2fl/fl and MLC-Cre:GRK2fl/fl mice with involuntary treadmill running to exhaustion. After a weeklong acclimatization period, mice were run using a protocol of increasing speed over time until they were unable to continue despite receiving a mild electrical shock from a platform at the rear of the treadmill. Using this protocol, we found no difference in time taken to reach exhaustion between GRK2fl/fl and MLC-Cre:GRK2fl/fl mice (13.33 min and 12.52 min, respectively) (Fig. 2A). Likewise we found no difference in the maximum speed attained during the treadmill protocol with GRK2fl/fl mice reaching 25.56 m/min and MLC-Cre:GRK2fl/fl mice reaching an average of 24.0 m/min (Fig. 2B). The average total distance run was also calculated for both groups, and again we observed no difference between GRK2fl/fl and MLC-Cre:GRK2fl/fl mice, covering 267.84 m and 250.39 m, respectively (Fig. 2C). Finally, we also kept a tally of the number of times each mouse engaged the shock grid at the rear of the treadmill and once again found no difference between groups with GRK2fl/fl mice engaging the grid an average of 2.75 times/min and MLC-Cre:GRK2fl/fl mice averaging 2.89 times/min (Fig. 2D). In conclusion we find no effect of muscle GRK2 ablation on exercise capacity in four separate parameters of treadmill performance.
FIGURE 2.
Skeletal muscle GRK2 deletion does not impair exercise capacity. A–D, treadmill exercise performance. After an acclimatization period, mice were run on a treadmill with increasing speed until exhaustion was reached (engaging the shock grid for 5 s). The mean time to exhaustion (A), maximum speed reached (B), distance covered (C), and frequency per minute of shock grid engagement (D) were calculated from individual performances. Bars depict mean values, and error bars represent S.D., n = 6- 9 mice per group.
Skeletal Muscle GRK2 Knockdown Differentially Modulates ex Vivo Mechanics of Isolated Soleus and Extensor Digitorum Longus (EDL) Muscles
To obtain a more detailed characterization of skeletal muscle function in the absence of GRK2, we next studied the ex vivo mechanics of soleus and EDL muscles isolated from GRK2fl/fl and MLC-Cre:GRK2fl/fl mice. Whole muscle mechanical measurements using a force transducer provide a highly sensitive and reproducible assessment of muscle function. Muscle fiber cross-sectional area (CSA), twitch, and tetanus did not differ between GRK2fl/fl and MLC-Cre:GRK2fl/fl groups in either soleus or EDL muscle (Fig. 3, A–C). We did, however, find a statistically significant decrease in EDL muscle-specific force of contraction in MLC-Cre:GRK2fl/fl compared with GRK2fl/fl mice (17.5 ± 0.54 N/cm2 and 19.9 ± 0.69 N/cm2, respectively) (Fig. 3D). By contrast, we found an opposite trend in slow-twitch soleus muscle in which there was a marginal, albeit non-significant, increase in specific force in the MLC-Cre:GRK2fl/fl compared with GRK2fl/fl mice (18.66 ± 0.57 newtons/cm2 and 17.49 ± 0.58 newtons/cm2 respectively) (Fig. 3D). In addition, EDL muscle twitch:tetanus ratio was significantly higher in MLC-Cre:GRK2fl/fl compared with GRK2fl/fl mice (0.3 ± 0.004 and 0.26 ± 0.01, respectively), whereas no difference was detected between groups in the soleus (Fig. 3E).
FIGURE 3.
Contractile properties of soleus and EDL muscles from GRK2fl/fl and MLC-Cre:GRK2fl/fl animals. A–E, ex vivo muscle mechanics of soleus and EDL muscles isolated from GRK2fl/fl and MLC-Cre:GRK2fl/fl mice. Fiber cross-sectional area (CSA) (A), twitch (B), tetanus (C), specific force (D), and twitch:tetanus (E) are graphically represented (n = 6 mice per group). *, p < 0.05; ***, p < 0.001 by Student's t test. F, force frequency of soleus muscle isolated from GRK2fl/fl and MLC-Cre:GRK2fl/fl. Force frequency measurements were obtained at 10, 20, 30, 50, 70, 90, and 100 Hz (n = 6 mice per genotype). G, percent fatigability of soleus muscle isolated from GRK2fl/fl and MLC-Cre:GRK2fl/fl mice after a fatigue protocol of 10 min (n = 6 mice per genotype). All graphs depict mean values and S.D.
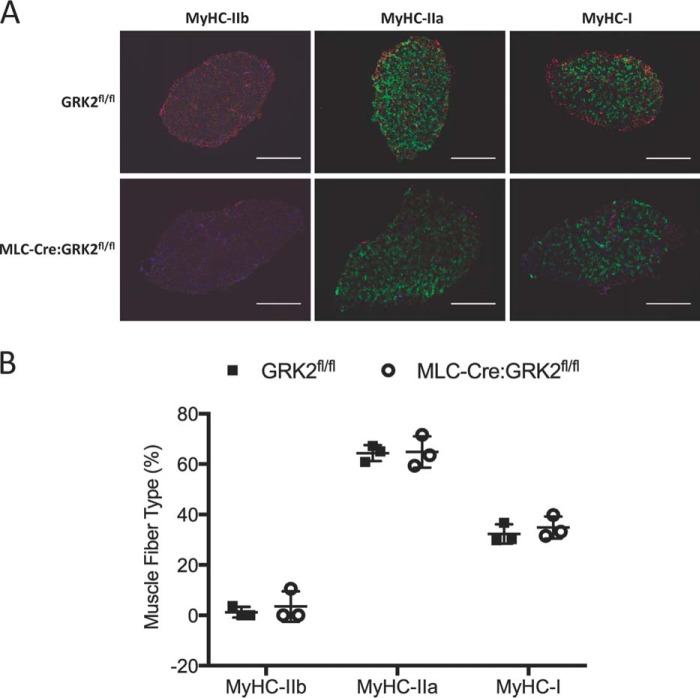
We next made force frequency measurements in the soleus of GRK2fl/fl and MLC-Cre:GRK2fl/fl mice to detect any physiological shift in the fiber type composition (Fig. 3F). We were unable to detect any difference between the force frequency profiles of soleus muscle from GRK2fl/fl and MLC-Cre:GRK2fl/fl mice. Similarly, we found no difference in the percent fatigability of soleus muscles from these mice during a 10-min fatigue protocol (Fig. 3G). Both the force frequency and fatigability results suggest the ablation of GRK2 in skeletal muscle has little effect on fiber type composition. Indeed, fiber type composition assessed via immunostaining was identical in GRK2fl/fl and MLC-Cre:GRK2fl/fl animals (Fig. 4, A and B). We found a close correlation between the percentage of fast-twitch fibers in the soleus (∼67%) and the degree of GRK2 knockdown (∼65%; Fig. 1B), confirming the fidelity of the MLC1f driven Cre used to generate the knock-out mice. Overall, these results suggest that GRK2 deletion may differentially alter specific force of contraction in the fast-twitch EDL versus slow-twitch soleus muscles without causing a fiber type switch.
FIGURE 4.
Fiber type composition of soleus muscle is not altered by GRK2 ablation. A, muscle fiber type immunostaining of soleus cross-sections from GRK2fl/fl and MLC-Cre:GRK2fl/fl mice. Separate myosin heavy chain (MyHC) isoforms were immunostained in sequential sections. Scale = 100 μm. B, graphs depict the mean percentage contribution of each fiber type to the make-up of the entire muscle section, and error bars represent S.D.; n = 3 mice per group.
Skeletal Muscle GRK2 Knockdown Does Not Alter Ca2+ Transients in Isolated Myotubes
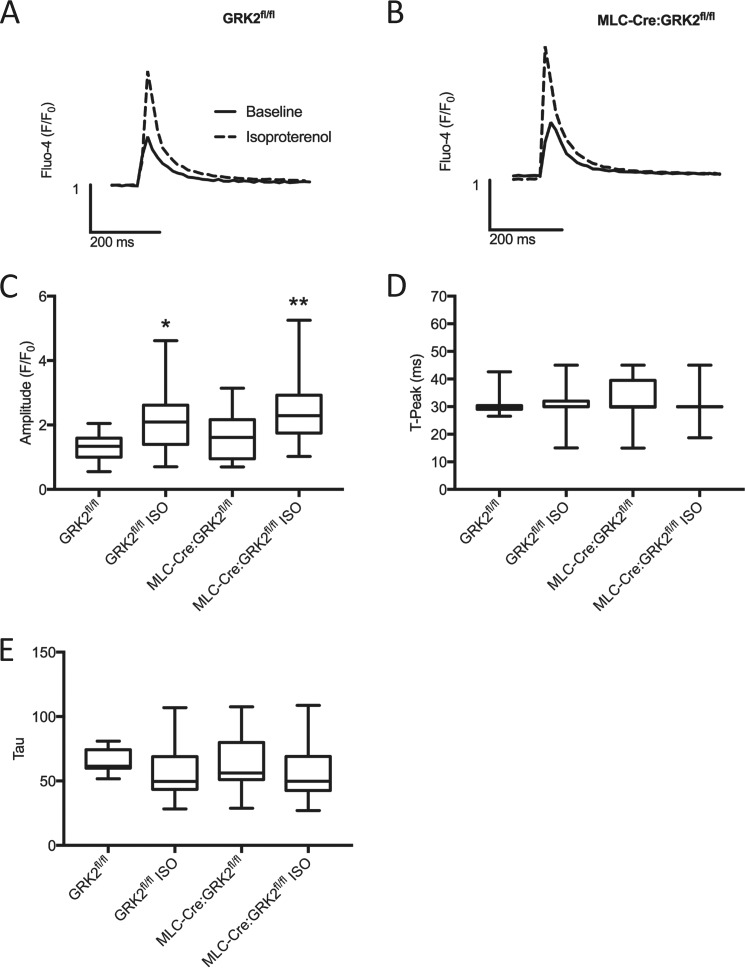
In the heart, βAR signaling modulates sarcolemmal calcium regulation, which in turn influences inotropy and chronotropy. We, therefore, next asked whether deletion of GRK2, a key regulator of βARs, could alter Ca2+ transients in skeletal muscle and thus explain the altered contractility observed in GRK2 ablated EDL muscle (Fig. 3D). We isolated single skeletal muscle myotubes via enzymatic digestion of the fast-twitch flexor digitorum brevis (FDB) muscle. Myotubes, loaded with the fluorescent Ca2+ indicator Fluo-4, were electrically paced at 0.2 Hz, and cytosolic Ca2+ transients were assessed at baseline and, after isoproterenol stimulation (100 nm, 5 min), via fluorescence microscopy.
Baseline Ca2+ transients from GRK2fl/fl and MLC-Cre:GRK2fl/fl myotubes produced almost identical traces (Fig. 5, A and B). Isoproterenol stimulation caused a significant increase in Ca2+ transient amplitude in both GRK2fl/fl and MLC-Cre:GRK2fl/fl myotubes compared with their respective non-stimulated control groups (Fig. 5, A–C). We found no statistical significance between the Ca2+ transient amplitudes of the isoproterenol-treated groups; however, MLC-Cre:GRK2fl/fl myotubes trended higher than GRK2fl/fl. Further analysis found no significant difference between groups in the time to peak (Fig. 5D) or in the time rate of decay of Ca2+ transients (Fig. 5E). From these results, we conclude that neither baseline nor isoproterenol-pretreated Ca2+ transients are significantly influenced by GRK2 deletion in skeletal muscle myotubes.
FIGURE 5.
Cytosolic Ca2+ transients in isolated mouse myotubes. Myotubes were isolated and loaded with the cytosolic Ca2+ sensor, Fluo-4 AM. Cytosolic Ca2+ was assessed in myotubes paced at 0.2 Hz in the presence or absence of isoproterenol stimulation (100 nm, 5 min). A and B, representative traces of cytosolic Ca2+ transients from GRK2fl/fl and MLC-Cre:GRK2fl/fl mice respectively. C, amplitude of Ca2+ transients. D, time to peak of cytosolic Ca2+ transients. E, time rate of decay. Boxes show the median and the 25th and 75th percentile values; whiskers depict minimum and maximum values. *, p < 0.05; **, p < 0.01 by ANOVA; control versus isoproterenol (ISO)-treated (same genotype). n = 11–56 myotubes per group.
Clenbuterol-stimulated Skeletal Muscle Hypertrophy Is Enhanced by Skeletal Muscle-specific GRK2 Knockdown
Previous studies have identified clenbuterol administration as an effective way to stimulate muscle hypertrophy and inhibit atrophy (15). Furthermore, this effect of clenbuterol is β2AR-dependent (16). Given that GRK2-mediated phosphorylation is instrumental in β2AR desensitization, we next asked whether ablation of GRK2 in the skeletal muscle would enhance clenbuterol-induced hypertrophy. GRK2fl/fl and MLC-Cre:GRK2fl/fl mice were administered clenbuterol or PBS continuously for 14 days via subcutaneous osmotic minipump. The effect of clenbuterol on body and muscle mass is shown in Table 1.
TABLE 1.
Clenbuterol stimulated skeletal muscle hypertrophy is enhanced by skeletal muscle specific GRK2 knockdown
Data (mean ± S.E.) shown for 10–11 mice per group. BW, body weight; TA, tibialis anterior.
| Measurement | GRK2fl/fl |
MLC-Cre:GRK2fl/fl |
||||
|---|---|---|---|---|---|---|
| PBS | Clenbuterol | -Fold | PBS | Clenbuterol | -Fold | |
| BW gain (g) | 1.03 ± 0.25 | 2.17 ± 0.16a | 2.11 ± 0.15 | 0.82 ± 0.3 | 2.33 ± 0.14a | 2.83 ± 0.17c |
| EDL weight (mg/g BW) | 0.39 ± 0.01 | 0.39 ± 0.02 | 1.0 ± 0.05 | 0.38 ± 0.01 | 0.42 ± 0.01 | 1.11 ± 0.03 |
| TA weight (mg/g BW) | 1.87 ± 0.04 | 2.02 ± 0.05 | 1.08 ± 0.03 | 1.87 ± 0.05 | 2.15 ± 0.04a | 1.15 ± 0.02 |
| Soleus weight (mg/g BW) | 0.32 ± 0.01 | 0.37 ± 0.01a | 1.15 ± 0.03 | 0.32 ± 0.01 | 0.41 ± 0.01a,b | 1.31 ± 0.02c |
| Gastroc weight (mg/g BW) | 2.83 ± 0.06 | 2.91 ± 0.09 | 1.03 ± 0.03 | 2.74 ± 0.06 | 3.17 ± 0.08a | 1.16 ± 0.03c |
a p < 0.01 by ANOVA; PBS vs. clenbuterol (same genotype).
b p < 0.01 by ANOVA; GRK2fl/fl clenbuterol vs. MLC-Cre:GRK2fl/fl.
c p < 0.01 by Student's t test; -fold GRK2fl/fl vs. -fold MLC-Cre:GRK2fl/fl.
Chronic clenbuterol treatment (14 days) caused a significant increase in body weight in both GRK2fl/fl and MLC-Cre:GRK2fl/fl mice (2.17 ± 0.16 g and 2.33 ± 0.14 g, respectively) although -fold change in body weight, comparing PBS to clenbuterol treatment was significantly greater in MLC-Cre:GRK2fl/fl compared with GRK2fl/fl mice (2.83 ± 0.17-fold and 2.11 ± 0.15-fold, respectively). Clenbuterol treatment caused a significant increase in the soleus weight of both GRK2fl/fl and MLC-Cre:GRK2fl/fl mice (0.37 ± 0.01 g and 0.41 ± 0.01 g, respectively). However, both the absolute weight gain and -fold change in soleus weight comparing PBS to clenbuterol treatment were significantly greater in MLC-Cre:GRK2fl/fl compared with GRK2fl/fl mice (1.31 ± 0.02-fold and 1.15 ± 0.03-fold, respectively). In addition, clenbuterol treatment caused a significant increase in TA and gastrocnemius weight relative to PBS treatment in MLC-Cre:GRK2fl/fl mice, whereas the same muscles in GRK2fl/fl mice trended toward a clenbuterol-stimulated increase in mass yet did not reach significance. As with the soleus, -fold change in gastrocnemius weight comparing PBS to clenbuterol treatment was significantly greater in MLC-Cre:GRK2fl/fl compared with GRK2fl/fl mice (1.16 ± 0.03-fold and 1.03 ± 0.03-fold, respectively).
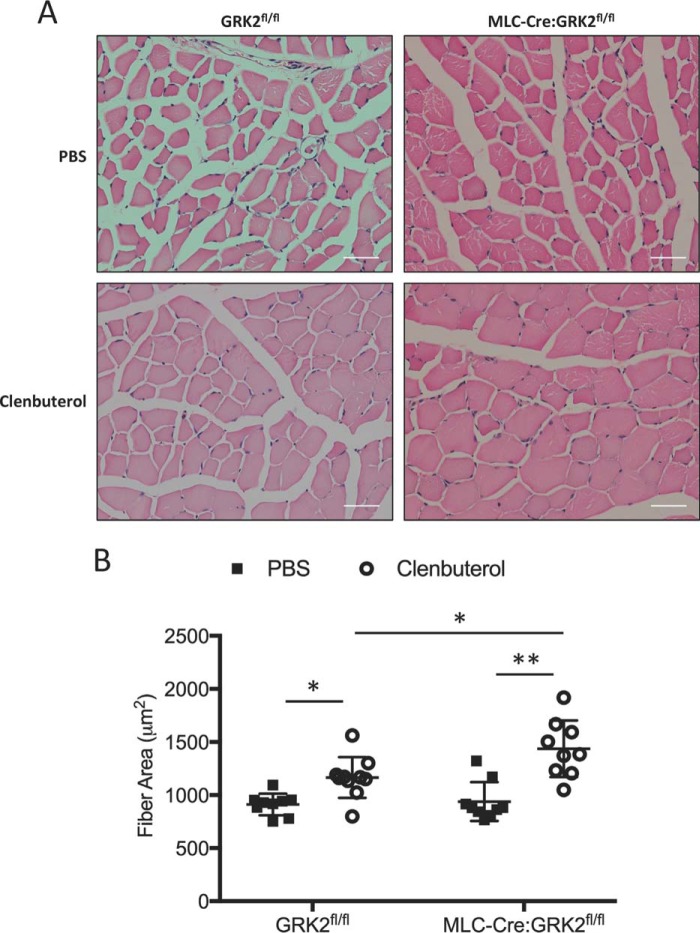
To support the muscle weight data, we analyzed fiber cross-sectional area of TA muscle from these mice (Fig. 6, A and B). Clenbuterol treatment significantly increased the cross-sectional area of both GRK2fl/fl and MLC-Cre:GRK2fl/fl muscle fibers compared with PBS-treated groups. Importantly, the clenbuterol stimulated increase in fiber cross-sectional area was greater in MLC-Cre:GRK2fl/fl compared with GRK2fl/fl mice (1436.29 ± 88.70 μm2 versus 1166.57 ± 64.27 μm2, respectively). Overall, we found GRK2 ablation increases clenbuterol-induced hypertrophy of skeletal muscle.
FIGURE 6.
Clenbuterol administration causes significant increase in muscle fiber area of MLC-Cre:GRK2fl/fl mice. A, hematoxylin and eosin staining of tibialis anterior cross-sections from GRK2fl/fl and MLC-Cre:GRK2fl/fl mice after 2 weeks of continuous PBS or clenbuterol (3 mg/kg per day) administration via osmotic minipump. Scale = 50 μm. B, graphs depict the mean fiber cross-sectional area, and error bars represent S.D. *, p < 0.05; **, p < 0.01 by ANOVA; n = 9–10 mice per group.
Pro-hypertrophic Akt Signaling Is Elevated in Clenbuterol-treated MLC-Cre:GRK2fl/fl Mice
β2-Adrenergic agonists are known to stimulate hypertrophy, in part via activation of the pro-hypertrophic Akt signaling pathway; we, therefore, hypothesized that increased Akt signaling contributes to the enhanced hypertrophy response in clenbuterol-treated MLC-Cre:GRK2fl/fl mice.
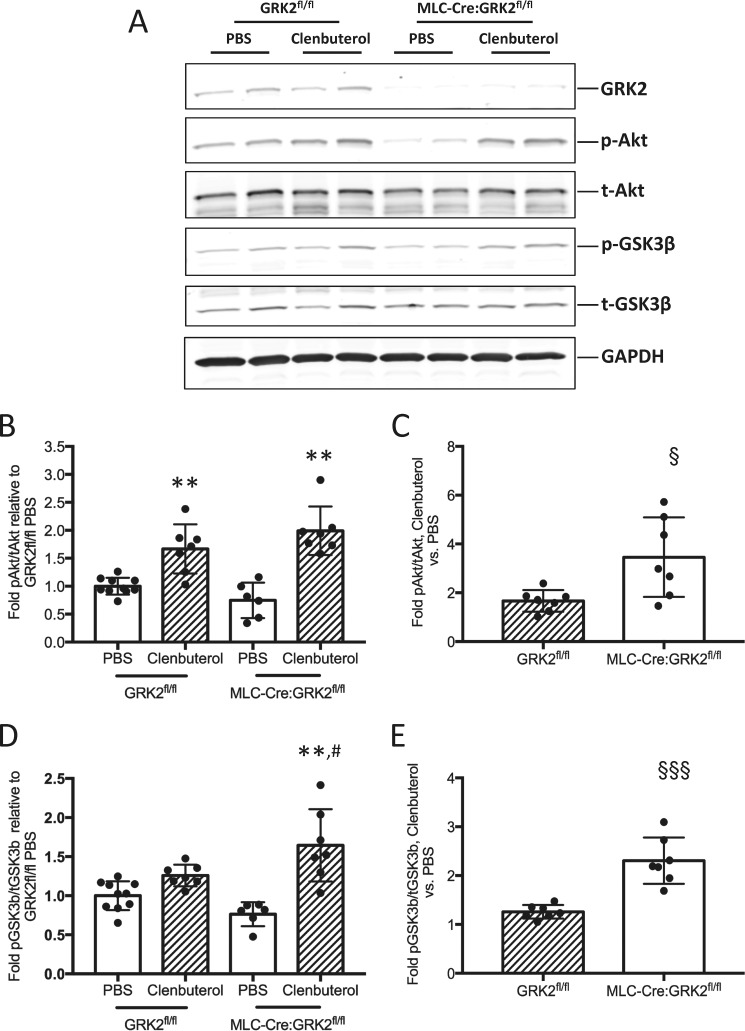
We examined Akt signaling in soleus muscle harvested from mice after 4 h of acute clenbuterol stimulation (1 mg/ml). p-Akt levels were significantly increased in both GRK2fl/fl and MLC-Cre:GRK2fl/fl soleus muscle after clenbuterol stimulus (Fig. 7, A and B); however, -fold increase in p-Akt in clenbuterol-treated mice relative to PBS controls was significantly greater in MLC-Cre:GRK2fl/fl mice (Fig. 7C). Levels of p-GSK3β, a downstream target of Akt, were likewise significantly increased in clenbuterol-treated MLC-Cre:GRK2fl/fl mice compared with PBS controls (Fig. 7, A and D). p-GSK3β levels trended higher in clenbuterol-treated GRK2fl/fl mice yet fell short of statistical significance (Fig. 7, A and D). As with p-Akt, -fold increase in p-GSK3β levels in clenbuterol-treated mice relative to PBS controls was significantly greater in MLC-Cre:GRK2fl/fl mice (Fig. 7E). In conclusion, clenbuterol-stimulated Akt signaling is enhanced in GRK2-ablated skeletal muscle, which may in part explain the pro-hypertrophic phenotype in these mice.
FIGURE 7.
Enhanced Akt signaling in clenbuterol treated MLC-Cre:GRK2fl/fl mice. A, Western blot analysis of p-Akt (Ser-473) and p-GSK3β (S9) levels in soleus muscle isolated from GRK2fl/fl and MLC-Cre:GRK2fl/fl mice 4 h after a single subcutaneous injection of PBS or clenbuterol (1 mg/kg). B, bars depict -fold change in signal intensity of p-Akt/t-Akt relative to the GRK2fl/fl PBS treated group; error bars represent S.D. **, p < 0.01 by ANOVA; PBS versus clenbuterol (same genotype). C, bars depict -fold increase in signal intensity of p-Akt/t-Akt in clenbuterol versus PBS-treated animals; error bars represent S.D. §, p < 0.05 by Student's t test. D, bars depict -fold change in signal intensity of p-GSK3β/t-GSK3β relative to the GRK2fl/fl PBS-treated group; error bars represent S.D. **, p < 0.01 by ANOVA; PBS versus Clenbuterol (same genotype); #, p < 0.05 by ANOVA; GRK2fl/fl clenbuterol versus MLC-Cre:GRK2fl/fl clenbuterol. E, bars depict -fold increase in signal intensity of p-GSK3β/t-GSK3β in clenbuterol versus PBS-treated animals; error bars represent S.D. §§§p < 0.001 by Student's t test. n = 6–10 mice per group.
Discussion
Research over the last few decades has identified dichotomous roles for GRK2 in physiology and pathophysiology of the heart. GRK2 activity is instrumental in the physiological homeostasis of the heart by phosphorylating and thereby desensitizing activated GPCRs, predominantly βARs (2). As a consequence, GRK2 activity levels in the heart correlate with contractile function (4, 22). However, after cardiac insult, increased GRK2 expression directly contributes to injury and progression to heart failure via excessive βAR desensitization and increased myocyte apoptosis (5–7, 23).
Given the well characterized roles of GRK2 in heart function and disease, in this study we sought insight into the role of GRK2 in skeletal muscle function. Although not identical in nature, skeletal muscle and cardiac muscle still share many features, including many of the same mechanisms and proteins required for excitation-contraction coupling. To examine the role of GRK2 in skeletal muscle we generated a skeletal muscle-specific GRK2 KO mouse. To our knowledge this is the first study to directly investigate the role of GRK2 in skeletal muscle function.
We studied the contractile properties of isolated soleus and EDL muscle and found that skeletal muscle GRK2 KO caused a decrease in specific force of contraction in the fast-twitch EDL muscle. Furthermore the twitch:tetanus ratio was significantly elevated in GRK2-ablated EDL. In contrast, we found no significant changes in the slow-twitch soleus mechanics from these animals; in fact, contrary to the EDL, the specific force trended higher in the soleus of skeletal muscle GRK2 KO mice. The EDL mechanics results were somewhat unexpected, as deletion of GRK2 increases isoproterenol-stimulated contractility and calcium transients in isolated cardiomyocytes (24). In this study we found no difference in baseline Ca2+ transients in GRK2 KO and wild-type (WT) myotubes. Isoproterenol pretreatment increased Ca2+ transient amplitude in both GRK2 KO and WT myotubes, and although GRK2 KO amplitude values trended higher than those of WT cells, we ultimately found no statistical difference between isoproterenol-treated groups. This would indicate that GRK2 KO alters EDL contractility independently of Ca2+ in the skeletal muscle. In GRK2-ablated cardiomyocytes, Raake et al. (24) found that elevated Ca2+ transient amplitudes were caused, at least in part, by enhanced phosphorylation of the L-type Ca2+ channel (LTCC) by protein kinase A (PKA), a downstream effector of βAR activation. Phosphorylation of the LTCC increases Ca2+ influx through the channel, which in turn heightens calcium-induced calcium release through the ryanodine receptor (RyR). This GRK2 dependent effect is irrelevant in skeletal muscle, since RyR opening is regulated by direct physical interaction with the LTCC and not by calcium-induced calcium release. Furthermore, Raake et al. (24) found that phosphorylation of phospholamban, a key regulator of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA)-mediated Ca2+ re-uptake into the sarcoplasmic reticulum, was decreased in GRK2-ablated cardiomyocytes, resulting in faster Ca2+ transient decay rates. Phospholamban is not expressed in type-II skeletal muscle fibers; hence, any effect of GRK2 deletion on phospholamban phosphorylation is not relevant to this cell type. Given these key distinctions in Ca2+ handling machinery, it is perhaps not surprising that isoproterenol-mediated changes in the Ca2+ transients of GRK2 ablated cardiomyocytes are largely absent in the skeletal muscle myotube.
In the heart, βAR stimulation generates an increase in inotropy and chronotropy (25). Research into the effects of βAR stimulation on skeletal muscle contraction has yet to reach a consensus; results in this tissue seem to vary based upon species, muscle type, and even dose of agonist (17). This may also help to explain why GRK2 deletion does not have the same effect on contractile properties in the EDL as it does in the heart. It is interesting to note, however, that similar to the heart the soleus trended toward increased contractility after GRK2 ablation. In contrast to the fast-twitch EDL, the soleus is largely composed of mitochondria-rich, type I muscle fibers, which are similar in nature to the slow oxidative fibers constituting the heart. It is, therefore, possible that the effects of GRK2 deletion on skeletal muscle contractile properties are dependent upon fiber type.
GRK2 can translocate to the mitochondria, and although the consequences of this localization are not completely understood, our laboratory has shown that GRK2 disrupts mitochondrial function in the cardiomyocyte by altering substrate utilization for ATP production (26). In addition, our laboratory and others have found GRK2 to be a negative regulator of glucose uptake (27, 28). This may be of particular functional relevance to the glycolytic EDL muscle; it is possible that altering glucose homeostasis by GRK2 ablation in the skeletal muscle may result in a metabolic reprogramming, which in turn could affect energy production and muscle contractile properties as a consequence. Further studies examining this hypothesis are warranted.
We next conducted force frequency and fatigue tests on isolated soleus muscle to detect any physiological shift in the fiber type composition. Skeletal muscle GRK2 KO mice were indistinguishable from their WT counterparts in both tests, suggesting GRK2 expression in skeletal muscle has no effect on fiber-type composition. Immunostaining and quantification of muscle fibers in soleus sections confirmed this finding. β2AR agonists can promote slow-to-fast fiber-type switching in skeletal muscle (29, 30). Our data suggest that reducing the desensitization of β2ARs by GRK2 ablation alone is not sufficient to promote a fiber type shift in the absence of an exogenous β-agonist.
The lack of fiber type shift or difference in soleus fatigability between GRK2KO and WT mice helps to explain why we also see no difference in involuntary treadmill running to exhaustion in these animals. However, given the apparent difference in EDL-specific force generation between our mice, future studies may warrant additional experiments more suited to identifying differences in muscle strength, such as a grip strength test.
Chronic stimulation of βARs with β-agonists has long been known to cause a “repartitioning effect,” decreasing body fat while increasing skeletal muscle mass (15). This has led to extensive study of β-agonists as a potential therapeutic for muscle wasting diseases such as muscular dystrophy and cancer cachexia. The anabolic effect of clenbuterol on skeletal muscle is due to decreased protein degradation while simultaneously increasing protein synthesis (31, 16). The latter action seems to be primarily mediated by activation of Akt, a well characterized mediator of muscle hypertrophy (32). In our study we found that the anabolic effects of chronic clenbuterol administration (14 days) were potentiated in skeletal muscle by the deletion of GRK2, most likely due to decreased desensitization of activated β2AR. Furthermore, this appears to be mediated in part by enhanced Akt activation that can occur when GRK2 activity is lowered via knockdown in skeletal muscle cells, although other mechanisms including gene regulatory changes downstream of the β2AR cannot be ruled out. Interestingly, in cardiomyocytes, GRK2 deletion prevents hypertrophy after injury; however, this can be explained by the lower levels of cardiomyocyte apoptosis and increased retention of contractile mass after injury in GRK2 KO hearts, reducing the necessity for compensatory hypertrophy (24). Overall, we find that inhibition of GRK2 may increase the effectiveness of β2AR-mediated strategies to grow skeletal muscle mass, which could have translational significance.
In summary, our findings provide novel insight into the function of GRK2 in skeletal muscle. These results highlight a role for GRK2 as a modulator of contractile properties in skeletal muscle. Furthermore, our data show that GRK2 deletion in skeletal muscle potentiates the anabolic effect of clenbuterol administration, in part by enhancing Akt activity. These observations may prove useful in the understanding of skeletal muscle physiology and in developing more effective therapeutic strategies for the treatment of skeletal muscle wasting diseases.
Experimental Procedures
Mice
All animal studies were conducted with the approval of the Animal Care and Use Committee at Temple University. To obtain skeletal muscle-specific GRK2 KO mice (MLC-Cre:GRK2fl/fl), MLC-Cre mice expressing Cre-recombinase under control of the myosin light chain 1f (MLC1f) promoter (19) were crossed with GRK2fl/fl mice (18). GRK2fl/fl mice were used as WT controls for all experiments. All mice used were between 10 and 16 weeks of age.
Treadmill Exercise
Animals were acclimatized to treadmill running (Columbus Instruments) over 5 days starting with a 5-min session at a speed of 6 m/min on day 1, gradually increasing to a speed of 15 m/min by day 5. Mice were given two full days rest after acclimatization before undergoing the treadmill test to exhaustion. The test was conducted as follows: 3 min at 10 m/min, 3 min at 20 m/min, then increasing speed by 2 m/min every 3 min until a final speed of 28 m/min was reached. A 0% gradient was used for all treadmill running. Mice were considered to be exhausted when they engaged a platform at the rear of the treadmill for more than 5 s despite receiving a mild electric shock. Running time, maximum speed reached, distance covered, and number of times mice engaged the shock grid were recorded.
Muscle Functional Testing
Soleus and EDL muscles were subjected to isolated mechanical measurements using a previously described apparatus (Aurora Scientific, Ontario, Canada) (33) and bathed in Ringer's solution gas-equilibrated with 95% O2, 5% CO2. Optimum muscle length (Lo) was determined with iterative manual adjustments of length to achieve maximum twitch force with supramaximal stimulation.
Maximum isometric twitch was measured in the muscles followed after 20 s by maximum isometric tetanus during a 500-ms stimulation. The maximum twitch and tetanus were measured a total of 3 times with 5-min intervals between tests, and the individual maximum value was used. The soleus was subjected to additional functional measures. First, a force-frequency test was employed using stimulation frequencies of 10, 20, 30, 50, 70, 90, and 100 Hz. Force generation at 90 and 100 Hz did not differ in any muscle studied, and the force was normalized to the isometric tetanus at 100 Hz to generate force-frequency curves. Second, soleus muscles were subjected to a fatigue test, as previously described (34). Briefly, muscles were stimulated once per second for 10 min (200-μs pulse, 100 Hz, 330-ms duration) to determine resistance to fatigue. Upon completion of functional testing, muscles were blotted, weighed, and rapidly frozen for subsequent analysis. Specific force was determined based on the physiological cross-sectional area (PCSA) using the formula,
where m is muscle mass, Lo is muscle length, Lf/Lo is the ratio of fiber length to optimal muscle length, and density of muscle (ρ) = 1.06 g/cm3. Lf/Lo was 0.45 for EDL and 0.69 for soleus.
Immunoblotting
After SDS-PAGE and transfer to nitrocellulose membranes, primary antibody incubations were performed overnight at 4 °C. Rabbit-680 fluorescent secondary antibody was obtained from Li-Cor (#A21109), and mouse-800 fluorescent secondary antibody was obtained from Cell Signaling (#5257S). Membranes were scanned with the Odyssey infrared imaging system (LI-COR). Both GRK2 (#sc-562) and GAPDH (#sc-32233) primary antibodies used were from Santa Cruz. Phospho-Akt (Ser-473; #9271L), total-Akt (#2920S), and phospho-GSK3β (Ser-9; #9336S) were from Cell Signaling Technology. Total-GSK3β was from Invitrogen (#44-610).
Micro-osmotic Pumps
Chronic infusion of clenbuterol (clenbuterol hydrochloride, Sigma) was done using Alzet 14-day micro-osmotic pumps (model 1002, DURECT Corp.). Pumps were filled following the manufacturer's specifications with sterile PBS or clenbuterol (3 mg/kg per day) and inserted as previously described (35). Briefly, mice were anesthetized with isoflurane (2.5% v/v), and pumps were implanted subcutaneously through a subscapular incision, which was then closed using 4.0 silk suture (Ethicon). The contents of the pumps were delivered at a rate of 0.25 μl/h for 14 days.
Acute Clenbuterol Treatment
Acute clenbuterol stimulation of mice was achieved via a single, subcutaneous injection of clenbuterol (1 mg/kg) or sterile PBS (control). 4 h post-injection muscles were isolated and rapidly frozen for subsequent analysis.
Fiber Cross-sectional Area
TA muscles were paraffin-embedded, and 7-μm sections were cut at the center of the muscle. Muscle sections were stained with H&E; images were taken using a Nikon DS-Ri1 and quantified in a blind manner using ImageJ. 100 fibers across 5 separate fields were measured per mouse.
Fiber-type Immunohistochemistry
After harvest, soleus muscles were immediately embedded in Tissue-Tek OCT compound (Sakura Finetek), and 10-μm sections were cut using a cryotome. Sections were blocked in 5% BSA for 45 min before primary antibody incubation overnight at 4 °C in a humid chamber. All sections were incubated with laminin primary antibody (Thermo, #RB-082-A0) in addition to one of the following fiber-type primary antibodies: MyHCI, MyHCIIa, or MyHCIIb (all MyHC antibodies were from the Developmental Studies Hybridoma Bank, #BF-F3, SC-71, and BA-F8). Secondary antibodies (Life Technologies A21434 and A21202; ImmunoResearch Laboratories 715-545-140) were applied for 1 h at room temperature. Sections were treated with mounting media containing DAPI and coverslipped. Sections were visualized using a Nikon-Ti fluorescence microscope.
Isolation and Dissociation of FDB Muscle
Single skeletal muscle myotubes were obtained via enzymatic digestion of FDB muscles. Following dissection, isolated FDB muscles were placed in 35-mm dishes containing dissociation media composed of DMEM, 2% fetal bovine serum, and 2 mg/ml collagenase II (Worthington Biochemical Corp.) and incubated at 37 °C, 5% CO2 for 1.5–2 h. Next, muscles were transferred to a new 35-mm dish containing incubation media composed of DMEM, 2% fetal bovine serum, and 1% penicillin/streptomycin. The muscles were gently triturated with a sterile, wide-bore P1000 pipette until single myotubes were dissociated. Myotubes were then seeded onto 35-mm collagen-coated, glass-bottomed dishes (MatTek Corp.) at 50–60% confluence and allowed to settle and adhere overnight in a 37 °C, 5% CO2 incubator. Experiments were conducted the following day.
Myotube Cytosolic Ca2+ Transient Measurements
Isolated myotubes were loaded with 5 μm Fluo-4 AM (Invitrogen) for 20 min at room temperature. Loaded myotubes were placed in a 37 °C heated chamber on an inverted microscope stage. Myotubes were perfused with a normal physiological Tyrode's buffer (150 mm NaCl, 5.4 mm KCl, 1.2 mm MgCl2, 10 mm glucose, 2 mm sodium pyruvate, and 5 mm HEPES, pH 7.4) containing 2 mm Ca2+. Myotubes were paced at 0.2 Hz and continuously recorded for Ca2+ transients using a Zeiss Observer Z1 fluorescent microscope at 490/20ex and 535/50em. Ca2+ transients were measured at baseline and after pretreatment with isoproterenol (100 nm, 5 min). For intracellular Ca2+ fluorescence measurements, the F0 (or baseline) was measured as the average fluorescence of the myotube 100 ms before stimulation. The maximal Fluo-4 fluorescence (F) was measured for peak amplitude. Time to peak was calculated as the time from the beginning of the transient to peak amplitude. Tau was measured as the decay rate of the Ca2+ transient traces.
Statistics
All the values in the text and figures are presented as the mean ± S.E. Statistical significance was determined by Student's t test or ANOVA. p values of <0.05 were considered significant.
Author Contributions
B. P. W. designed and conducted most of the experiments, analyzed the results, and wrote the manuscript. M. C. W., T. S. L., and L. A. G. conducted some experiments and helped with analysis and interpretation of results. D. G. T. assisted in experimental design and analysis of results. J. W. E. conducted some experiments and assisted in experimental design and analysis of results. W. J. K. designed the experiments, analyzed and interpreted the results, and assisted in writing the manuscript.
Acknowledgments
We thank Dr. J Molkentin for the generous gift of the MLC-Cre mice. We also thank Drs. E. Barton, M. Liu, and the Physiological Assessment Core U54 AR052646 for assistance with isolated muscle mechanics.
This work was supported in part by National Institutes of Health Grant P01 HL091799. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- GPCR
- G protein-coupled receptor
- GRK
- GPCR kinase
- AR
- adrenergic receptor
- MLC
- myosin light chain
- EDL
- extensor digitorum longus
- FDB
- flexor digitorum brevis
- LTCC
- L-type Ca2+ channel
- ANOVA
- analysis of variance.
References
- 1. Katritch V., Cherezov V., and Stevens R. C. (2013) Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 53, 531–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sato P. Y., Chuprun J. K., Schwartz M., and Koch W. J. (2015) The evolving impact of G protein-coupled receptor kinases in cardiac health and disease. Physiol. Rev. 95, 377–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woodall M. C., Ciccarelli M., Woodall B. P., and Koch W. J. (2014) G protein-coupled receptor kinase 2, a link between myocardial contractile function and cardiac metabolism. Circ. Res. 114, 1661–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koch W. J., Rockman H. A., Samama P., Hamilton R. A., Bond R. A., Milano C. A., and Lefkowitz R. J. (1995) Cardiac Function in mice overexpressing the β-adrenergic receptor kinase or a beta ARK inhibitor. Science 268, 1350–1353 [DOI] [PubMed] [Google Scholar]
- 5. Iaccarino G., Tomhave E. D., Lefkowitz R. J., and Koch W. J. (1998) Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by β-adrenergic receptor stimulation and blockade. Circulation 98, 1783–1789 [DOI] [PubMed] [Google Scholar]
- 6. Petrofski J. A., and Koch W. J. (2003) The β-adrenergic receptor kinase in heart failure. J. Mol. Cell. Cardiol. 35, 1167–1174 [DOI] [PubMed] [Google Scholar]
- 7. Ungerer M., Böhm M., Elce J. S., Erdmann E., and Lohse M. J. (1993) Altered expression of β adrenergic receptor kinase and β 1-adrenergic receptors in the failing human heart. Circulation 87, 454–463 [DOI] [PubMed] [Google Scholar]
- 8. Rockman H. A., Chien K. R., Choi D. J., Iaccarino G., Hunter J. J., Ross J. Jr., Lefkowitz R. J., and Koch W. J. (1998) Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc. Natl. Acad. Sci. 95, 7000–7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akhter S. A., Eckhart A. D., Rockman H. A., Shotwell K., Lefkowitz R. J., and Koch W. J. (1999) In vivo inhibition of elevated myocardial β-adrenergic receptor kinase activity in hybrid transgenic mice restores normal β-adrenergic signaling and function. Circulation 100, 648–653 [DOI] [PubMed] [Google Scholar]
- 10. Harding V. B., Jones L. R., Lefkowitz R. J., Koch W. J., and Rockman H. A. (2001) Cardiac βARK1 inhibition prolongs survival and augments β blocker therapy in a mouse model of severe heart failure. Proc. Natl. Acad. Sci. 98, 5809–5814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah A. S., White D. C., Emani S., Kypson A. P., Lilly R. E., Wilson K., Glower D. D., Lefkowitz R. J., and Koch W. J. (2001) In vivo ventricular gene delivery of a β-adrenergic receptor kinase inhibitor to the failing heart reverses cardiac dysfunction. Circulation 103, 1311–1316 [DOI] [PubMed] [Google Scholar]
- 12. Rengo G., Lymperopoulos A., Zincarelli C., Donniacuo M., Soltys S., Rabinowitz J. E., and Koch W. J. (2009) Myocardial adeno-associated virus serotype 6: βARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation 119, 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raake P. W., Schlegel P., Ksienzyk J., Reinkober J., Barthelmes J., Schinkel S., Pleger S., Mier W., Haberkorn U., Koch W. J., Katus H. A., Most P., Müller O. J. (2013) AAV6 βARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur. Heart J. 34, 1437–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brinks H., Boucher M., Gao E., Chuprun J. K., Pesant S., Raake P. W., Huang Z. M., Wang X., Qiu G., Gumpert A., Harris D. M., Eckhart A. D., Most P., and Koch W. J. (2010) Level of G protein-coupled receptor kinase-2 determines myocardial ischemia/reperfusion injury via pro- and anti-apoptotic mechanisms. Circ. Res. 107, 1140–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lynch G. S., and Ryall J. G. (2008) Role of β-Adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol. Rev. 88, 729–767 [DOI] [PubMed] [Google Scholar]
- 16. Hinkle R. T., Hodge K. M., Cody D. B., Sheldon R. J., Kobilka B. K., and Isfort R. J. (2002) Skeletal muscle hypertrophy and anti-atrophy effects of clenbuterol are mediated by the β2-adrenergic receptor. Muscle Nerve 25, 729–734 [DOI] [PubMed] [Google Scholar]
- 17. Cairns S. P., and Borrani F. (2015) β-Adrenergic modulation of skeletal muscle contraction: key role of excitation-contraction coupling. J. Physiol. 593, 4713–4727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matkovich S. J., Diwan A., Klanke J. L., Hammer D. J., Marreez Y., Odley A. M., Brunskill E. W., Koch W. J., Schwartz R. J., and Dorn G. W. (2006) Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and β-adrenergic signaling. Circ. Res. 99, 996–1003 [DOI] [PubMed] [Google Scholar]
- 19. Bothe G. W., Haspel J. A., Smith C. L., Wiener H. H., and Burden S. J. (2000) Selective expression of Cre recombinase in skeletal muscle fibers. Genesis 26, 165–166 [PubMed] [Google Scholar]
- 20. Parsons S. A., Millay D. P., Wilkins B. J., Bueno O. F., Tsika G. L., Neilson J. R., Liberatore C. M., Yutzey K. E., Crabtree G. R., Tsika R. W., and Molkentin J. D. (2004) Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy. J. Biol. Chem. 279, 26192–26200 [DOI] [PubMed] [Google Scholar]
- 21. Zechner C., Lai L., Zechner J. F., Geng T., Yan Z., Rumsey J. W., Collia D., Chen Z., Wozniak D. F., Leone T. C., and Kelly D. P. (2010) Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab. 12, 633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rockman H. A., Choi D. J., Akhter S. A., Jaber M., Giros B., Lefkowitz R. J., Caron M. G., and Koch W. J. (1998) Control of myocardial contractile function by the level of β-adrenergic receptor kinase 1 in gene-targeted mice. J. Biol. Chem. 273, 18180–18184 [DOI] [PubMed] [Google Scholar]
- 23. Chen M., Sato P. Y., Chuprun J. K., Peroutka R. J., Otis N. J., Ibetti J., Pan S., Sheu S. S., Gao E., and Koch W. J. (2013) Prodeath signaling of G protein-coupled receptor kinase 2 in cardiac myocytes after ischemic stress occurs via extracellular signal-regulated kinase-dependent heat shock protein 90-mediated mitochondrial targeting. Circ. Res. 112, 1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raake P. W., Zhang X., Vinge L. E., Brinks H., Gao E., Jaleel N., Li Y., Tang M., Most P., Dorn G. W. 2nd, Houser S. R., Katus H. A., Chen X., and Koch W. J. (2012) Cardiac G-protein-coupled receptor kinase 2 ablation induces a novel Ca2+ handling phenotype resistant to adverse alterations and remodeling after myocardial infarction. Circulation 125, 2108–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woo A. Y., and Xiao R. P. (2012) β-adrenergic receptor subtype signaling in heart: from bench to bedside. Acta Pharmacologica Sinica 33, 335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sato P. Y., Chuprun J. K., Ibetti J., Cannavo A., Drosatos K., Elrod J. W., and Koch W. J. (2015) GRK2 compromises cardiomyocyte mitochondrial function by diminishing fatty acid-mediated oxygen consumption and increasing superoxide levels. J. Mol. Cell Cardiol. 89, 360–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ciccarelli M., Chuprun J. K., Rengo G., Gao E., Wei Z., Peroutka R. J., Gold J. I., Gumpert A., Chen M., Otis N. J., Dorn G. W. 2nd, Trimarco B., Iaccarino G., and Koch W. J. (2011) G protein coupled receptor kinase 2 activity impairs cardiac glucose uptake and promotes insulin resistance after myocardial ischemia. Circulation 123, 1953–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garcia-Guerra L., Nieto-Vazquez I., Vila-Bedmar R., Jurado-Pueyo M., Zalba G., Díez J., Murga C., Fernández-Veledo S., Mayor F. Jr., and Lorenzo M. (2010) G protein-coupled receptor kinase 2 plays a relevant role in insulin resistance and obesity. Diabetes 59, 2407–2417 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Maltin C. A., Delday M. I., and Reeds P. J. (1986) The effect of a growth promoting drug, clenbuterol, on fiber frequency and area in hind limb muscles from young male rats. Biosci. Rep. 6, 293–299 [DOI] [PubMed] [Google Scholar]
- 30. Zeman R. J., Ludemann R., Easton T. G., and Etlinger J. D. (1988) Slow to fast alterations in skeletal muscle fibers caused by clenbuterol, a β-receptor agonist. Am. J. Physiol. 254, E726–E732 [DOI] [PubMed] [Google Scholar]
- 31. Benson D. W., Foley-Nelson T., Chance W. T., Zhang F. S., James J. H., and Fischer J. E. (1991) Decreased myofibrillar protein breakdown following treatment with clenbuterol. J. Surg. Res. 50, 1–5 [DOI] [PubMed] [Google Scholar]
- 32. Kline W. O., Panaro F. J., Yang H., and Bodine S. C. (2007) Rapamycin inhibits the growth and muscle-sparing effects of clenbuterol. J. Appl. Physiol. 102, 740–747 [DOI] [PubMed] [Google Scholar]
- 33. Barton E. R., Morris L., Kawana M., Bish L. T., and Toursel T. (2005) Systemic administration of l-arginine benefits mdx skeletal muscle function. Muscle Nerve 32, 751–760 [DOI] [PubMed] [Google Scholar]
- 34. Selsby J. T., Morine K. J., Pendrak K., Barton E. R., and Sweeney H. L. (2012) Rescue of dystrophic skeletal muscle by PGC-1α involves a fast to slow fiber type shift in the mdx mouse. PLoS ONE. 7, e30063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schumacher S. M., Gao E., Zhu W., Chen X., Chuprun J. K., Feldman A. M., Tesmer J. J., and Koch W. J. (2015) Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Sci. Transl. Med. 7, 277ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]