Abstract
We recently discovered a structurally novel class of endogenous lipids, branched palmitic acid esters of hydroxy stearic acids (PAHSAs), with beneficial metabolic and anti-inflammatory effects. We tested whether PAHSAs protect against colitis, which is a chronic inflammatory disease driven predominantly by defects in the innate mucosal barrier and adaptive immune system. There is an unmet clinical need for safe and well tolerated oral therapeutics with direct anti-inflammatory effects. Wild-type mice were pretreated orally with vehicle or 5-PAHSA (10 mg/kg) and 9-PAHSA (5 mg/kg) once daily for 3 days, followed by 10 days of either 0% or 2% dextran sulfate sodium water with continued vehicle or PAHSA treatment. The colon was collected for histopathology, gene expression, and flow cytometry. Intestinal crypt fractions were prepared for ex vivo bactericidal assays. Bone marrow-derived dendritic cells pretreated with vehicle or PAHSA and splenic CD4+ T cells from syngeneic mice were co-cultured to assess antigen presentation and T cell activation in response to LPS. PAHSA treatment prevented weight loss, improved colitis scores (stool consistency, hematochezia, and mouse appearance), and augmented intestinal crypt Paneth cell bactericidal potency via a mechanism that may involve GPR120. In vitro, PAHSAs attenuated dendritic cell activation and subsequent T cell proliferation and Th1 polarization. The anti-inflammatory effects of PAHSAs in vivo resulted in reduced colonic T cell activation and pro-inflammatory cytokine and chemokine expression. These anti-inflammatory effects appear to be partially GPR120-dependent. We conclude that PAHSA treatment regulates innate and adaptive immune responses to prevent mucosal damage and protect against colitis. Thus, PAHSAs may be a novel treatment for colitis and related inflammation-driven diseases.
Keywords: colitis, defensin, innate immunity, lipid, T helper cells, anti-inflammatory lipids, branched fatty acid esters of hydroxy fatty acids, immune regulation, Paneth cell, ulcerative colitis
Introduction
Lipids have been a rich source of chemical matter for the development of new medicines (1). Endogenous small-molecule metabolites are another class of natural products made within the human body that have important biological activities (2). For example, the discovery and synthesis of prostaglandins revealed their diverse biological roles as autocrine and paracrine mediators in many tissues. This led to their development as therapeutic agents for a variety of indications ranging from vasodilatation to glaucoma treatment (3–6). More recently, compounds that target the sphingosine 1 receptors are emerging as novel therapeutics for autoimmune diseases (7). Thus, the discovery and characterization of bioactive lipids is of fundamental interest with broad impact. We recently discovered a structurally novel class of lipids, branched fatty acid esters of hydroxy fatty acids (FAHFAs),6 with anti-diabetic and anti-inflammatory properties (8). These lipids are products of endogenous synthesis in mammalian tissues and are also present in food. There are more than 16 FAHFA families, and each family is distinguished by having a different fatty acid and hydroxy fatty acid composition. Furthermore, within a single family, there are multiple isomers that are defined by the position of the ester bond. One FAHFA, 9-palmitic-acid-hydroxy-stearic-acids (9-PAHSA), attenuates pro-inflammatory cytokine production by adipose tissue macrophages from insulin-resistant obese mice. In addition, 9-PAHSA inhibits LPS-induced dendritic cell maturation in vitro (8). Because PAHSAs have direct anti-inflammatory effects on immune cells, we hypothesized that PAHSAs may also have protective effects against colitis, which is an immune-mediated disease.
Ulcerative colitis (UC) is a chronic, relapsing inflammatory condition affecting the colon. UC disease prevalence is highest in North America and Europe, and its incidence is rising in Asian countries adopting a more Westernized diet (9–11). Clinical presentation of UC includes intestinal ulceration, occult diarrhea, tenesmus, and lower abdominal pain (12). Although the etiology of colitis is unclear, the pathogenic mechanism is multifactorial, involving interaction between genetic predisposition and the environment to chronically trigger the host immune system (13). The development of UC has been strongly linked to impairments in gut homeostasis, which is normally maintained by the innate mucosal barrier (intact epithelium, Paneth cell-derived antimicrobial peptides, and the luminal mucosal layer) and the adaptive (acquired) immune response (14–16). The integrity of the mucosal barrier depends on an intact epithelium and luminal mucosal layer and antimicrobial peptides secreted by Paneth cells in the intestinal crypts. The main therapeutic goal for colitis is to abate inflammation and thereby induce remission.
Despite advances in effective biologic therapies for colitis, such as immunomodulators and immunosuppressants (anti-TNFα, anti-integrins, corticosteroids) and antibiotics, life-long treatment with these agents has systemic immunosuppressive effects, and 20% of UC patients will develop colorectal cancer unless they undergo surgical bowel resection (17). UC onset often occurs in women in their child-bearing years, making immune-directed biologics unsafe for therapy (18–20). Therefore, it is important to identify oral biologics possessing anti-inflammatory properties that are well tolerated with limited side effects. We report here that chronic oral treatment with PAHSAs delays the onset and attenuates the severity of DSS-induced colitis in wild-type mice by augmenting Paneth cell function to improve bactericidal potency and by reducing the activation and proliferation of pro-inflammatory T cells. The Paneth cell effects may be mediated through the G protein-coupled receptor GPR120, and the anti-inflammatory effects on T cells appear to be partially mediated by this pathway.
Results
PAHSAs Protect Mice from DSS-induced Colitis
To investigate the therapeutic potential of PAHSAs for colonic inflammation, wild-type mice were pretreated with vehicle or PAHSAs for 3 days, followed by concomitant treatment with 2% DSS water for 10 days (Fig. 1A). DSS treatment results in intestinal inflammation and the formation of colitic lesions, resembling human ulcerative colitis pathology (21).
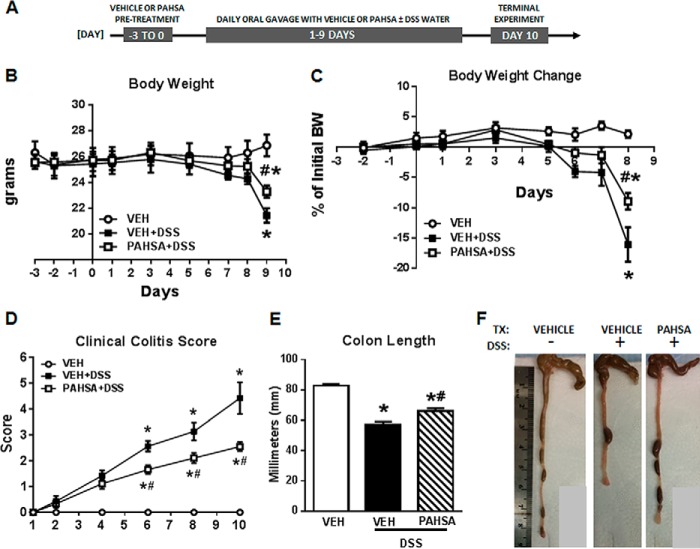
FIGURE 1.
PAHSAs protect mice from DSS-induced colitis. A, schematic of the experimental treatment time course. B and C, body weight (BW) and body weight change in 8- to 10-week-old male C57Bl6 mice pretreated with either vehicle (VEH) or 5- + 9-PAHSAs by oral gavage for 3 days, followed by either 0% or 2% DSS water with continued vehicle or 5- + 9-PAHSA treatment for an additional 10 days. D, clinical colitis score measured daily throughout the 10 days of simultaneous DSS water and vehicle or PAHSA treatment. E and F, colon length was measured on day 10. TX, treatment. Data are means ± S.E. and representative of three independent cohorts (n = 8–12 mice/group). *, p < 0.05 versus vehicle; #, p < 0.05 versus vehicle + 2% DSS; repeated measures two-way ANOVA.
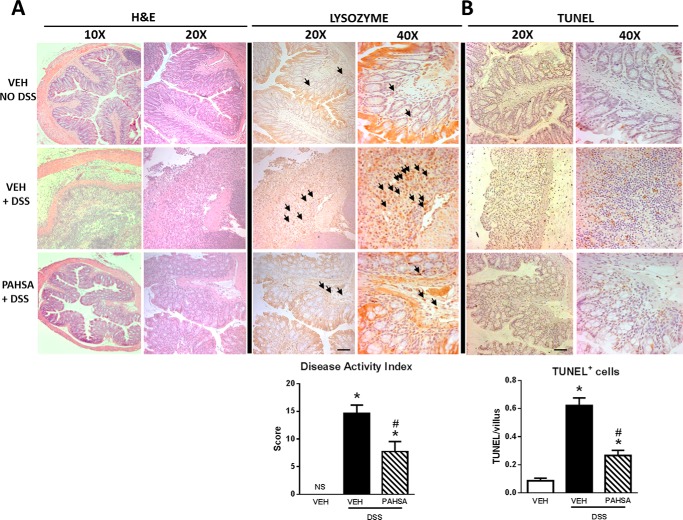
Combination oral treatment with 5-PAHSA and 9-PAHSA (5- + 9-PAHSAs) in wild-type mice that were drinking DSS water attenuated body weight loss by 50% compared with DSS-vehicle mice (Fig. 1, B and C). Additionally, the clinical colitis score (Fig. 1D) for DSS-vehicle mice was 60% higher than that of DSS-PAHSA mice. DSS water treatment markedly reduced colon length in vehicle-treated mice compared with control mice on regular water (without DSS). However, PAHSA treatment reduced the effect of DSS water treatment on colon length (Fig. 1, E and F). In addition, PAHSA treatment reduced colitis disease activity index scores determined by histopathology. DSS-vehicle mice showed the typical histopathology of colitis, which consists of increased crypt abscesses, mucosal inflammation as scored by leukocyte infiltration, and enlargement of the muscularis propria with loss of colonic epithelia and crypt structure. PAHSA treatment resulted in a reduction in all of these histopathology parameters (Fig. 2A). In addition, lysozyme staining in immune cells (neutrophils and monocytes) was increased in DSS-vehicle mice and reduced with PAHSA treatment (Fig. 2A). TUNEL-positive staining in colonocytes indicates apoptosis. Similar to the effect on disease activity index scores, DSS treatment increased the number of TUNEL-positive cells 5-fold compared with vehicle-regular water mice, and PAHSA treatment reduced this by more than 50% (Fig. 2B). Together, these results support a role for oral PAHSA treatment in attenuating the severity of colitis in mice.
FIGURE 2.
PAHSAs attenuate colitis disease severity. A, the disease activity index was measured by histopathological analysis of H&E-stained full-thickness colon in 8- to 10-week-old male C57Bl6 mice pretreated with either vehicle (VEH) or 5- + 9-PAHSAs by oral gavage for 3 days, followed by either 0% or 2% DSS water with continued vehicle or 5- + 9-PAHSA treatment for an additional 10 days. Identical colon sections were stained for lysozyme. B, apoptosis in the colon was measured by TUNEL staining in colon sections. For all microscopy imaging, five to eight histological sections were analyzed per animal, with n = 5–8 mice/group for all experiments, and all images were taken at ×10, ×20, and ×40 magnification. Arrows indicate positively stained cells. Scale bars = 100 μm. Data are means ± S.E. *, p < 0.05 versus vehicle; #, p < 0.05 versus vehicle + 2% DSS; determined by two-way ANOVA.
PAHSAs Improve Paneth Cell Bactericidal Function
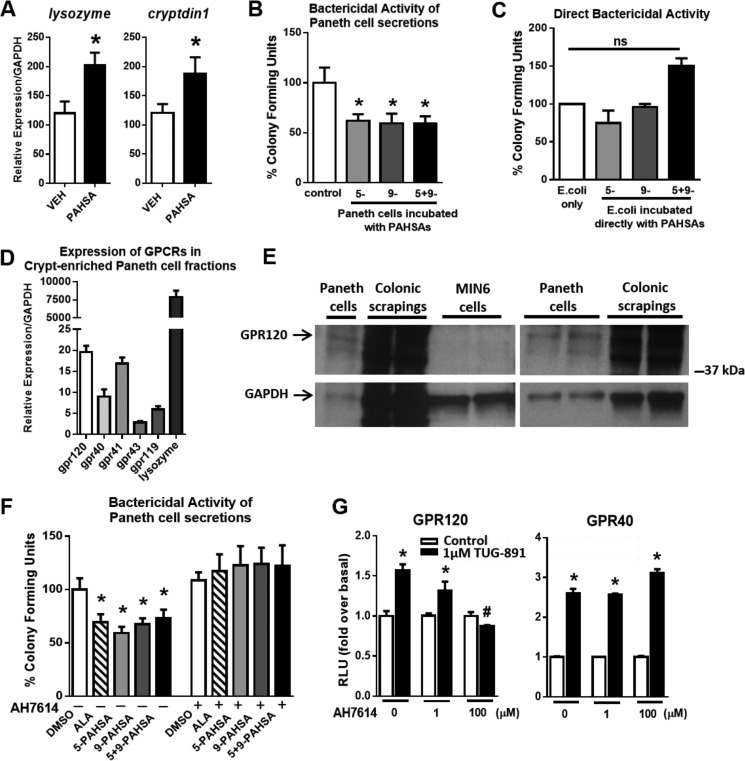
Expression of the antimicrobial peptides lysozyme and cryptdin1 in the ileum were increased 50% in PAHSA-treated mice compared with vehicle-treated mice (Fig. 3A). Because the mouse colon lacks Paneth cells (unlike the human colon), we used mouse small intestine that was Paneth cell-enriched as a model for innate gut immunity to measure bactericidal function. Because lysozyme is produced and secreted specifically by Paneth cells in the gut, we speculated that an important factor in the protective effects of PAHSAs may be augmented Paneth cell function. Acute stimulation of crypt-enriched fractions of Paneth cells with 5-, 9-, or 5- + 9-PAHSAs resulted in a 40% increase in bactericidal potency, as evidenced by the decreased number of colony-forming units of Escherichia coli (Fig. 3B). By contrast, PAHSAs do not have a direct effect on bacteria (Fig. 3C) but potently induce the secretion of antimicrobial peptides from Paneth cells (Fig. 3B). Because PAHSAs activate GPR120 (8), and some gut-secretory cells express GPR120 (22), we next investigated whether Paneth cells express GPR120 and whether this receptor mediates the effects of PAHSAs to enhance Paneth cell bactericidal activity. We found that gpr120 is expressed in crypt-enriched fractions of Paneth cells, and its expression level is similar to gpr41 and appears higher than several other GPCRs, gpr40, gpr43, and gpr119 (Fig. 3D). Lysozyme was used as a control gene marker to confirm that the crypt preparations were enriched with Paneth cells. Furthermore, GPR120 protein is expressed in crypt-enriched Paneth cell fractions. Colonic scrapings were used as a positive control, and Min6 cells were used as a negative control for GPR120 protein (Fig. 3E, two representative blots are shown). We next sought to determine the contribution of GPR120 in mediating PAHSA effects on bactericidal activity in Paneth cells. Because the Paneth cell bactericidal assay has a short incubation period, this precluded the use of genetic knockdown techniques. Therefore, we used AH7614 to pharmacologically antagonize GPR120 in Paneth cells. GPR120 inhibition with AH7614 in the presence of 5-, 9-, or 5- + 9-PAHSAs in the crypt-enriched Paneth cell assay completely blocked the bactericidal effect of PAHSAs on decreasing the number of colony-forming units of E. coli (Fig. 3F). To address the specificity of AH7614 for GPR120, we studied the effects of AH7614 on the activation of GPR120 compared with GPR40 (another GPCR that is also activated by long-chain fatty acids (23)) using an SRE-luciferase reporter assay (Fig. 3G). TUG-891, an agonist for GPR120 and GPR40, activated both GPR120 and GPR40 as expected. However, AH7614 specifically inhibited GPR120 activation and not GPR40 activation, even at high concentrations (100 μm). These data indicate that AH7614 has selectivity for inhibition of GPR120.
FIGURE 3.
PAHSAs improve Paneth cell bactericidal function. A, 24-week-old male C57Bl6 mice were treated with vehicle (VEH) or 5- + 9-PAHSAs by oral gavage for 28 days. Colon was collected on day 28, and gene expression for the Paneth cell markers lysozyme and cryptdin1 was measured. n = 4 mice/group. B, Paneth cell-enriched crypt fractions were acutely treated with DMSO in PIPES buffer (control) or 5-PAHSA (20 μm), 9-PAHSA (20 μm), or 5- + 9-PAHSAs (10 μm each) for 30 min ex vivo, and Paneth cell secretions were collected for a bactericidal killing assay against E. coli. n = 3 mice/group with triplicate wells per treatment condition. C, PAHSAs were directly incubated with E. coli to determine whether PAHSAs have direct bactericidal activity. n = 3 wells per treatment condition. D, gene expression for GPCRs and lysozyme in crypt-enriched Paneth cell fractions from normal untreated mice. n = 4–6 mice/group. E, two representative Western blots for GPR120 in Paneth cell fractions, colonic mucosal scrapings (positive control), and MIN6 cells (negative control). F, Paneth cell-enriched crypt fractions were acutely treated with DMSO, α-linolenic acid (20 μm), 5-PAHSA (20 μm), 9-PAHSA (20 μm), or 5- + 9-PAHSAs (10 μm each) in the presence or absence of AH7614 (100 μm), and Paneth cell secretions were collected for a bactericidal killing assay against E. coli. n = 3–4 mice/group with duplicate wells for each treatment condition. G, GPCR activation assays using HEK 293T cells transiently transfected with mGPR40 or mGPR120. Cells were treated with DMEM-0.5% FBS or 1 μm TUG-891 with 0, 1, or 100 μm AH7614, and relative luciferase activity was measured. n = 3. *, p < 0.05 versus vehicle control, DMSO, or same treatment with AH7614; #, p < 0.05 versus all other samples with 1 μm TUG-891; determined by Student's t-test or ANOVA. ns, not significant. Data are means ± S.E.
T Cells Mediate the Anti-inflammatory Effects of PAHSAs
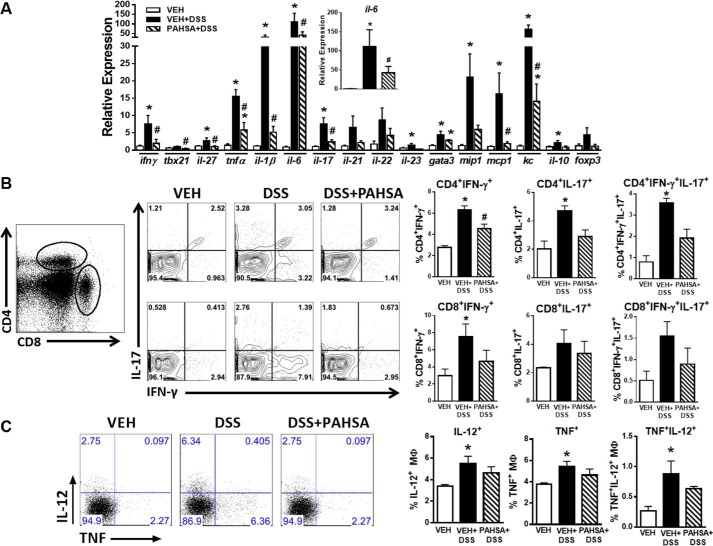
Complementary to disease activity index scores, treatment with DSS water induced the expression of pro-inflammatory cytokines, chemokines, and transcription factors compared with vehicle-treated mice on regular water (Fig. 4A). Remarkably, expression of the majority of these DSS-inducible genes was attenuated 2.5- to 6-fold by PAHSA treatment (Fig. 4A). Expression of pro-inflammatory genes specific to T cell responses, particularly Th1-related transcripts (ifn-γ and tbx21) and genes related to Th17 (il-17 and il-23), were attenuated by PAHSA treatment. Moreover, PAHSAs markedly reduced the expression of pro-inflammatory cytokines (tnfα, il-6, and il-1β) and chemokines (mip-1, mcp-1, and kc). PAHSAs also reduced the expression of the anti-inflammatory cytokine IL-10 to levels comparable with the vehicle group (Fig. 4A). Because T cells play a key role in DSS-induced colitis (21), we next investigated whether recruitment and/or activation of T cells may be affected by PAHSA treatment in mice. PAHSAs reduced the percentage of both colonic CD4+ and CD8+ T cells positive for IFN-γ+, IL-17+, or IFN- γ+IL-17+ compared with T cells in colons of DSS-vehicle mice (Fig. 4B). The activation status of macrophages (expression of IL-12+ and TNF+) in the lamina propria was not affected by PAHSA treatment (Fig. 4C), indicating that it is unlikely that macrophage polarization (M1 and M2) is changed. Together, these data indicate that oral PAHSA treatment attenuates DSS-induced gut inflammation by reducing chemokine and cytokine production and subsequent activation of pro-inflammatory T cells independent of macrophage polarization and/or activation.
FIGURE 4.
PAHSA treatment reduces colonic inflammation. A, 8- to 10-week-old male C57Bl6 mice pretreated with either vehicle (VEH) or 5- + 9-PAHSAs by oral gavage for 3 days, followed by either 0% or 2% DSS water with continued vehicle or 5- + 9-PAHSA treatment for an additional 10 days. Colon was collected for gene expression of pro-inflammatory chemokines and cytokines specific to T cell responses. n = 4–6 mice/group. B, lamina propria CD4+ and CD8+ T cells were isolated, and the percentage of IFN-γ+, IL-17+, or IFN-γ+IL-17+ cells was measured by flow cytometry. C, lamina propria macrophages were isolated, and the percentage of IL-12+ and TNF+ cells was measured by flow cytometry. Data are means ± S.E. n = 5–8 mice/group. *, p < 0.05 versus vehicle; #, p < 0.05 versus vehicle + 2% DSS; determined by ANOVA.
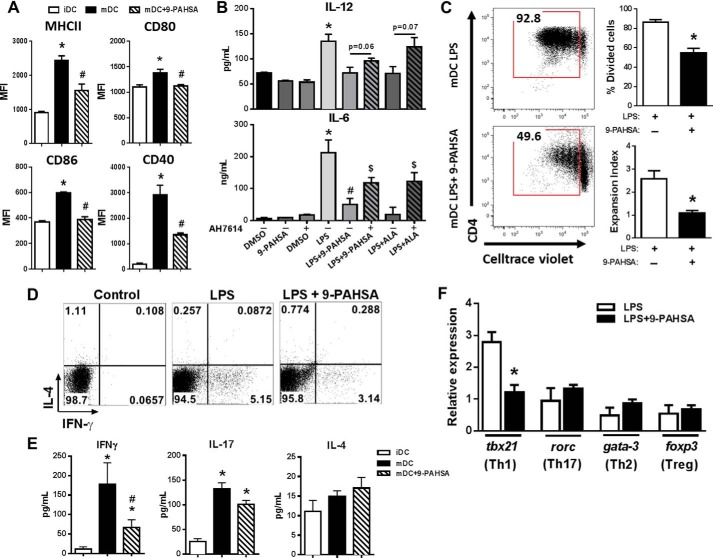
9-PAHSA Attenuates Dendritic Cell-dependent Pro-inflammatory T Cell Activation
We reported previously that 9-PAHSA treatment in vitro reduces dendritic cell (DC) activation (8). Thus, we hypothesized that 9-PAHSA may have direct effects on DC maturation to inhibit the activation and subsequent expansion of pro-inflammatory T cells. Fig. 5A shows that stimulation with LPS alone induced the activation of bone marrow-derived DCs, resulting in increased levels of MHCII, CD80, CD86, and CD40. However, pretreatment of bone marrow-derived DCs with 9-PAHSA followed by stimulation with LPS reduced levels of MHCII and co-stimulatory molecules (Fig. 5A). Because GPR120 mediates some anti-inflammatory effects (24), we next investigated whether GPR120 mediates the effects of 9-PAHSA on DCs. Using three genetic techniques to knock down GPR120 in BMDCs (GPR120 KO mice, siRNA, and CRISPR-Cas9), we consistently saw a reduced response to LPS in GPR120 knockdown BMDCs compared with control BMDCs. For example, TNFα secretion and activation of co-stimulatory molecules in response to LPS stimulation was at least 50% reduced in GPR120 knockdown BMDCs (data not shown). Therefore, we used AH7614, the selective antagonist to GPR120, to address the involvement of GPR120 in mediating the anti-inflammatory effects of PAHSAs. DCs were pretreated with the GPR120 inhibitor prior to LPS and 9-PAHSA treatment, and the pro-inflammatory cytokines IL-12 and IL-6 were measured in the secretions. 9-PAHSA effects to reduce IL-6 secretion but not IL-12 secretion were attenuated by GPR120 blockade, indicating that at least some of the anti-inflammatory effects of 9-PAHSA are GPR120-dependent (Fig. 5B). Next we investigated whether the reduction of these key DC-derived molecules would result in reduced proliferation and/or polarization of CD4+ T cells. We incubated LPS-activated bone marrow-derived DCs in the presence or absence of 9-PAHSA with splenic syngeneic CD4+ T cells from wild-type mice and found that 9-PAHSA attenuated DC-induced CD4+ T cell proliferation (Fig. 5C).
FIGURE 5.
9-PAHSA attenuates dendritic cell-dependent pro-inflammatory T cell activation. BMDCs were generated from 8- to 10-week-old male C57Bl6 mice. A, LPS-activation of BMDCs was confirmed by increased expression of MHCII, CD40, CD80, and CD86, determined by flow cytometry. Levels of co-stimulatory molecules were also measured following co-treatment of BMDCs with LPS and 9-PAHSA (20 μm). Immature DCs (iDC), mature DCs (mDC), and mean fluorescence intensity (MFI). B, BMDCs were pretreated with the GPR120 inhibitor AH7614 (100 μm) prior to LPS and 9-PAHSA treatment, and cytokine (IL-6 and IL-12) levels were measured 24 h after LPS treatment by ELISA. ALA (20 μm) was used as a positive control. *, p < 0.05 versus DMSO, 9-PAHSA, and DMSO + AH7614; #, p < 0.05 versus LPS; $, p < 0.05 versus LPS + the same lipid treatment without AH7614. C, LPS-activated BMDCs treated with vehicle or 9-PAHSA were co-cultured with bead-purified splenic syngeneic cell trace-labeled CD4+ T cells to determine CD4+ T cell proliferation. D, intracellular staining of CD4+ T cells for IL-4 and IFN-γ after 5 days of co-culture. The dot plot shows combined data from three mice. E, IFN-γ, IL-17, and IL-4 secretion was measured from co-culture assays of BMDCs and T cells. F, mRNA expression of transcription factors that determine CD4+ T cell polarization. Data are means ± S.E. and represent three experiments performed in triplicate, each using pools of bone marrow cells from two to six mice. *, p < 0.05 versus iDC or LPS control; #, p < 0.05 versus mDC; determined by Student's t test or ANOVA.
We established co-culture assays using bone-marrow derived DCs and splenic syngeneic CD4+ T cells to determine whether PAHSAs could attenuate pro-inflammatory CD4+ T cell polarization. DCs treated with LPS alone increased the percentage of CD4+ T cells positive for IFN-γ with no change in CD4+ T cells positive for IL-4. The increased percentage of CD4+ T cells positive for IFN-γ was attenuated with 9-PAHSA treatment of DCs (Fig. 5D). This indicates that PAHSA treatment affects the capacity of DCs to induce Th1 polarization, resulting in reduced IFN-γ production and secretion. To confirm Th1 polarization, we measured the secretion of IFN-γ, IL-17, and IL-4 from activated DCs. 9-PAHSA treatment of activated DCs reduced the amount of secreted IFN-γ from CD4+ T cells with no change in IL-4 and a tendency to reduce IL-17 levels (Fig. 5E). To further confirm Th1 polarization, we measured the gene expression of specific T cell lineage transcription factors. 9-PAHSA treatment of DCs reduced the expression of tbx21 but not of other CD4+ T cell-related transcription factors (rorc, gata-3, and foxp3), indicating specificity for Th1 cells (Fig. 5F). Thus, PAHSAs abrogate the adaptive immune system by inhibiting DC-driven Th1 cell polarization (Fig. 5, D–F).
Discussion
Effective treatment of UC is difficult to achieve, and complete protection from disease relapse generally does not occur without surgery. Most current therapies induce only temporary remission and are accompanied by many adverse side effects. Up to 40% of patients suffering from UC eventually require surgery (25). However, patients with fulminant UC often choose to pursue aggressive immunosuppressive therapy to avoid proctocolectomy and permanent ostomy despite the fact that immunotherapy increases the risk for infections and colorectal cancer (26). Therefore, new oral biologics with safer immunomodulatory properties need to be identified to achieve remission without compromising the host immune system.
The goal for patients with UC is to optimize medical therapy to achieve prolonged disease remission with minimal side effects. First-line therapies for UC include aminosalicylates and glucocorticoids, and when these fail, biological therapies such as azathioprine and infliximab that target the adaptive immune system are prescribed. But these are not well tolerated and result in immunocompromised states with systemic side effects, which is particularly problematic because UC patients require persistent life-long biologic therapy. Some naturally occurring long-chain fatty acids have been reported to reduce inflammation in murine colitis models (27, 28). For example, chronic feeding with an oleic acid-rich diet (C18 LC-PUFA) in mice treated with DSS delayed diarrhea and rectal bleeding but did not improve colonic histopathology compared with control mice (29). Reports are mixed regarding the efficacy of omega-3 fatty acids, specifically eicosapentaenoic acid and docosahexaenoic acid, in delaying or reducing the severity of colitis (30, 31). Some reports actually show exacerbation of disease activity in rodents and humans with chronic docosahexaenoic acid treatment (32–34). Therefore, there is an unmet clinical need for the medical treatment of UC.
Overactivity of the adaptive immune system plays a large role in the progression of gut inflammation in UC, resulting in excess chemokine and cytokine production and increased adaptive immune cell (T cell) activation (35, 36). Th1 cells are a subtype of CD4+ T cells, which are polarized by IL-12 secreted from antigen-presenting cells (dendritic cells, macrophages). Th1 cells produce IFN-γ, and these cells have been implicated in the pathogenesis of inflammation-driven diseases, including UC (37). Other studies have identified Th17 cells as another subset of pro-inflammatory CD4+ T cells also implicated in the pathogenesis of colitis (38, 39). Th17 cells are maintained by IL-23 and produce IL-17 (40). Transfer of Th17 cells into mice with a fully functional innate immune system potently induces colitis (41). Thus, Th1/Th17 cell responses play a major role in the onset of colitis.
This work illustrates the beneficial effects of treatment with the recently discovered class of lipids, PAHSAs, on DSS-induced colitis. PAHSAs reduce the percentage of CD4+ T-cells in the colon and attenuate the expression of IL-17 and IL-23. Moreover, PAHSAs decrease the expression of the Th1 cell-specific transcription factor tbx21 and secretion of IFN-γ, and these effects may be partially mediated through GPR120. This is based on the effects of the GPR120 inhibitor AH7614 to attenuate these immune responses. We expected that genetic knockdown of GPR120 would also augment the pro-inflammatory response to LPS. However, using three genetic approaches (GPR120 KO mice, siRNA knockdown, and CRISPR-Cas9), we observed that reduction of GPR120 in BMDCs consistently attenuated the response to LPS even before PAHSAs were added (data not shown). The difference in the effect of pharmacologic inhibition versus genetic reduction in GPR120 may indicate that GPR120 plays a role in dendritic cell development or early priming for immune responses. Furthermore, reduction in the GPR120 protein may affect signaling scaffolds or complexes, which would result in different effects than enzymatic inhibition of GPR120 activity. Together, our results show that PAHSAs protect mice from experimental colitis by limiting the colonic pro-inflammatory response, which may be through direct inhibition of dendritic cell activation and subsequent Th1 cell polarization. These effects are partially dependent on GPR120 and could be through pathways downstream of toll-like receptor-4 similar to the effects of omega-3 fatty acids (24) or to non-toll-like receptor signaling.
Although DSS-induced colitis in mice is a commonly used preclinical model for testing new compounds for UC, there are limitations to this model of acute chemical injury that may not fully simulate the chronically inflamed environment in human UC. Therefore, in light of the positive results from this study, further investigation using other autoimmune UC models is warranted to predict the therapeutic value of PAHSAs in UC.
The innate immune system also plays a role in UC, which involves compromise of the gut mucosal barrier (42). This is comprised of mucins from goblet cells and antimicrobial peptides called defensins from Paneth cells. Together, secretions from goblet cells and Paneth cells act as a biomolecular shield to protect the gut. Paneth cells are localized to the intestinal crypts and mediate bactericidal activity in the gut lumen. Paneth cell degranulation is an innate immune response that mediates enteric mucosal defense by killing foreign pathogens in the intestinal lumen (43). Paneth cell metaplasia and hyperplasia are stimulated by intestinal inflammation and are signatures of UC (44). However, although Paneth cells are present in the human colon, they are found only in the mouse small intestine. Our findings demonstrate that PAHSAs can induce Paneth cell degranulation of antimicrobial peptides; this may occur through a GPR120-dependent mechanism, and this likely contributes to protecting mice from severe DSS-induced colitis. To further illustrate the potential importance of Paneth cells and their secreted products, human genome-wide association studies have identified single-nucleotide polymorphisms in defensins that increase the risk for UC and other inflammatory bowel diseases (45, 46).
The mechanism for action of PAHSAs in UC is of interest. Chronic treatment with PAHSAs for 6 months is safe and well tolerated, with no compromise in renal or hepatic function. Furthermore, PAHSAs improve metabolic status in mice (data not shown), reinforcing the safety and potential beneficial value of long-term use of these novel lipids. Some metabolic effects of PAHSAs are mediated through GPR120, a known long-chain fatty acid lipid sensor (8). Pro-inflammatory BMDCs and some gut secretory cells express GPR120 (22, 24). Our data show that, in colitis, beneficial PAHSAs may act directly on Paneth cells in the gut to enhance antimicrobial activity and on BMDCs to abrogate T cell activation, which may be partially mediated by GPR120.
In conclusion, we have uncovered a new effective and safe therapeutic modality for colitis, i.e. oral administration of the structurally novel class of endogenous natural products, the PAHSAs. These signaling lipids protect against colitis by altering the innate and adaptive immune responses in the gut. This work broadens the already impressive set of beneficial biologic activities ascribed to these lipids (8). Based on this work, PAHSAs may be an important primary or adjunct therapy for UC and other gut-related inflammatory diseases.
Experimental Procedures
Animals
Male C57Bl/6J wild-type mice were obtained from The Jackson Laboratory at 8–10 weeks of age. Mice were randomized to treatment group based on body weight at 8–10 or 24 weeks of age. Mice had ad libitum access to chow (Formulab 5008) and water. Mice were housed singly at Beth Israel Deaconess Medical Center with a 14:10-h light-dark cycle. Mice were sacrificed by decapitation for serum collection, and tissues were harvested, snap-frozen in liquid nitrogen, and stored at −80 °C for further processing. Fresh tissues were collected for immunological assays or placed in 10% formalin for histology. All aspects of animal care were in accordance with federal guidelines and approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center and Harvard Medical School.
Experimental Treatments and Monitoring
8- to 10-week-old mice were treated once daily by oral gavage with vehicle (50% PEG-400, 0.5% Tween 80, and 49.5% distilled water) or 5-PAHSA (10 mg/kg) and 9-PAHSA (5 mg/kg) starting 3 days prior to dextran sodium sulfate (DSS, Sigma) treatment. DSS was dissolved in sterile water to a concentration of 2%. Vehicle or PAHSA treatment continued concurrent with either sterile water or 2% DSS-water for 10 days. 24-week-old mice were treated once daily by oral gavage with vehicle (50% PEG-400, 0.5% Tween 80, and 49.5% distilled water) or 5-PAHSA (10 mg/kg) and 9-PAHSA (5 mg/kg) with sterile water for 28 days. The GPR120 agonists α-linolenic acid (Sigma-Aldrich, 20 μm) and TUG-891 (Tocris, 1 μm), and the GPR120 antagonist AH7614 (Tocris, 1 and 100 μm) were used for ex vivo and in vitro studies.
Qualitative Measures of Experimental Colitis
Body weight and clinical colitis score (ratings of 0–4 based on stool consistency, rectal bleeding, and mouse appearance) (47) were measured daily throughout the experimental treatment period and used as an indirect measure of colitis severity. All mice were sacrificed by decapitation by day 10, when body weight loss in the control DSS group was equal to or greater than 20% of the starting body weight or when the maximum clinical colitis score of 7 was reached. Fresh colon was harvested for length measurement and stored at −80 °C until further processing.
Histopathological and Immunohistochemical Analyses of Mouse Colon Tissue
1 cm of the proximal colon was collected and fixed in 10% formalin for histological analysis following paraffin embedding and 10-μm sectioning for hematoxylin and eosin processing (Beth Israel Deaconess Medical Center Histology Core). Histopathology scoring was performed blind based on the following combined criteria: inflammation, extent of injury, crypt damage, and percentage of affected tissue, examined in five to eight transverse gut sections per animal for a combined score of 0–20 (48). Colon sections were stained for lysozyme (Novus Biologicals LLC). Colon sections were stained for TUNEL (EMD Millipore kit) to detect cell apoptosis. For all histological analyses, a minimum of five images were taken at magnifications of ×10, ×20, and ×40 with a light microscope, and the number of TUNEL-positive cells were measured on at least three villi per image for quantification.
Gene Expression Analysis
Total RNA from colon tissue was extracted by TRIzol with additional lithium chloride treatment and cDNA synthesis performed by random hexamers and SuperScript III (Invitrogen). Real-time quantitative PCR was performed on an ABI Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA) with TaqMan Universal PCR Master Mix and TaqMan gene expression assays (Applied Biosystems) for the following genes: il-6, ifn-γ, il-23, il-22, gata3, il-21, il-17, il-10, foxp3, il-1β, il-27, tbx21, mip1, mcp1, tnfα, kc, lysozyme, cryptdin1, gpr120, gpr40, gpr41, gpr43, gpr119, tgf-β1, tgf-β2, and tgf-β3 (Applied Biosystems). TATA box binding protein, tbp, was used as the housekeeping gene for full-thickness colon, as its intestinal expression was unaltered independent of mouse genotype or treatment, and gapdh was used for crypt-enriched Paneth cell fractions. Relative quantification of transcript levels was performed by the 2−ΔΔCt method using Ct values obtained from PCR amplification kinetics measured by the ABI PRISM SDS 2.1 software.
Western Blotting Analysis
Lysates were prepared from crypt-enriched Paneth cell fractions and mucosal scrapings from colon of normal untreated wild-type mice. 30 μg of total protein lysates were subjected to immunoblotting by SDS-PAGE with the indicated antibodies (rabbit GPR120, catalog no. NBP1–00858, Novus Biologicals; GAPDH, catalog no. 6C5, Santa Cruz Biotechnology).
Crypt Cell Preparations and Paneth Cell Secretion Assays
The small intestine was removed from 8-week-old male C57Bl6 mice following decapitation. Gut contents were flushed with cold MgCl2-Ca2+-free PBS (PBS%), and the tissue was opened longitudinally to expose the lumen and was cut into 5-mm pieces. Tissues were rinsed quickly in 2 mm EDTA + PBS% at room temperature and transferred into 30 mm EDTA + PBS% at room temperature for 15 min, everted, and shaken vigorously to dissociate the villi. Supernatant was transferred and incubated twice more in 30 mm EDTA + PBS% 15 min each, everted, and shaken. Crypt-enriched supernatants from fractions 2–4 were pooled, strained (70 μm), and centrifuged at 850 × g for 5 min at room temperature. The supernatant was removed, and the crypt pellet was resuspended in isotonic PIPES buffer (iPIPES; 10 mm PIPES (pH 7.4) and 137 mm NaCl. For each experimental condition, ∼200 crypts were resuspended in iPIPES buffer and treated with 5- or 9-PAHSA (20 μm), or 5- + 9-PAHSAs (10 μm of each) in the presence or absence of AH7614 (100 μm) for 30 min at 37 °C. Following incubation, Paneth cell secretions (supernatants) were collected following centrifugation at 750 × g and stored at −80 °C until further use.
Bactericidal Activity Assays
Bactericidal assays were performed on secretions collected from crypts exposed to DMSO in PIPES or 5-, 9-, or 5- + 9-PAHSAs (20 μm) with or without AH7614 using XL1 E. coli. Briefly, 5 × 106 E. coli cells in their exponential growing phase were deposited by centrifugation, resuspended in 50 μl of iPIPES buffer, combined with 50 μl of secretions, and incubated for 1 h at 37 °C. To determine the bactericidal activity of Paneth cell secretions, the secretion-E. coli mixture was diluted 1:100 in iPIPES and plated onto lysogeny broth (LB) plates at 37 °C for overnight incubation. The number of colony-forming units was quantified, and all treatment groups were normalized to DMSO-PIPES control as a measure of bactericidal cell-killing efficiency. To determine the direct bactericidal activity of PAHSAs against E. coli, 20 μm of 5-, 9-, or 5- + 9-PAHSAs was incubated with E. coli for 1 h at 37 °C, and the mixture was diluted 1:100 in iPIPES and plated onto LB plates at 37 °C for overnight incubation, followed by colony-forming unit counting.
SRE-Luciferase Reporter Assay
HEK293T cells were trypsinized and plated at 105 cells/well per 24-well poly-l-lysine-coated (50 μg/ml) plate. Cells were incubated overnight prior to transfection with 0.5 μg of DNA (20 ng of CMVβgal, 50 ng of SRE-luciferase, 250 ng of mGPR40/mGPR120-pcDNA3.1+, and 180 ng of pcDNA3.1+) with 1.2 μl of Lipofectamine 2000 per well. Cells were transfected overnight with mGPR40 (Origene, MC212962) or mGPR120 (R&D Systems, RDC0803) and washed twice with DMEM prior to treatment with control (DMEM-0.5% FBS) or 1 μm TUG-891 with 0, 1, or 100 μm AH7614 for 18 h. Medium was aspirated, and cells were washed with PBS prior to lysing with 0.25 ml of luciferase lysis buffer. Lysate (50 μl) was transferred to measure luciferase. β-Galactosidase was measured as a control. Absorbance was read at 420 nm to calculate relative luciferase units.
Lamina Propria Cell Isolation and Flow Cytometry
8-to 10-week-old male C57Bl6 mice treated with vehicle or PAHSAs with 0% or 2% DSS water were sacrificed on day 10 to investigate the peak immune response to DSS-induced colitis. The large intestine was washed in Hanks' balanced salt solution, minced into small pieces, and placed in 10 ml of 3% FBS RPMI medium with 5 mm EDTA and 0.145 mg/ml DTT, and incubated with shaking for 20 min at 37 °C. Gut sections were washed twice with 10 ml of serum-free medium containing 2 mm EDTA. The supernatant containing intraepithelial lymphocytes was discarded. The remaining pieces were transferred to a new tube with 10 ml of serum-free medium containing 0.1 mg/ml Liberase (Roche) and 0.05% DNase (Sigma-Aldrich). After 30 min of incubation at 37 °C with stirring, the contents (liquid and intestinal pieces) were filtered through a 70-μm cell strainer. The cell suspension was centrifuged for 8 min at 1500 rpm at 4 °C, refiltered through a 40-μm cell strainer, and recentrifuged with the same settings. Cells were resuspended in RPMI medium containing 2% FBS.
Following isolation, lamina propria cells were stained for surface markers using the following fluorochrome-conjugated antibodies: F4/80, CD11b, CD45, CD3, CD4, and CD8 (BioLegend). For intracellular cytokine staining, cells were stained using antibodies for IFN-γ, IL-4, and IL-17, and the staining was performed with the Cytofix/Cytoperm kit (BD Biosciences) in accordance with the instructions of the manufacturer. Cell acquisition was performed on a BD LSRII FortessaTM flow cytometer (BD Biosciences) using FACSDiva software (BD Biosciences) at the Beth Israel Deaconess Medical Center Flow Cytometry Core, and the data were analyzed with FlowJo 9.5.3 software (Tree Star Inc.).
Generation and LPS Treatment of BMDCs
Mouse bone marrow cells were generated as described previously (49). GM-CSF (R&D Systems) at a concentration of 20 ng/ml was used for BMDC differentiation. Cells were incubated in 9-PAHSA for 10 min prior to LPS (100 ng/ml) stimulation. MHCII, CD40, CD80, and CD86 (BioLegend) were detected by flow cytometry as described previously (49). IFN-γ, IL-17, and IL-4 were measured by ELISA (BioLegend).
CD4+ T Cell Proliferation Assay
8-week-old male wild-type mice were used to generate BMDCs as described previously (49). BMDCs were pretreated with 9-PAHSA (20 μm) or with GPR120 inhibitor (100 μm) prior to LPS stimulation (100 ng/ml). BMDCs were co-cultured with cell trace violet-labeled, bead-purified splenic syngeneic CD4+ T cells as described previously (50). The expansion index was calculated with FlowJo 9.5.3 software.
Statistical Analysis
Data are represented as mean ± S.E. All data were analyzed by two-tailed Student's t test and/or ANOVA with Bonferroni's post-hoc test where appropriate using GraphPad Prism v5.0 (GraphPad Software, San Diego, CA). p < 0.05 was considered statistically significant.
Author Contributions
J. L., P. M. M. V., and A. C. conceived, designed, and performed the experiments. J. L. and P. M. M. V. analyzed the data from the experiments and made the figures. P. A. performed all of the transfection studies in BMDCs. C. J. D. performed the GPCR activation assays. O. P. designed and cloned sgRNAs for the CRISPR-Cas9 studies. C. V. and A. S. performed chemical synthesis of the lipids. B. B. K. and A. S. supervised the experimental plan. B. B. K., J. L., P. P. M. V., E. U. Y., and A. S. interpreted the experiments. B. B. K., J. L., and P. M. M. V. wrote the manuscript. B. B. K. and A. S. edited the manuscript.
Acknowledgments
We thank Dr. Aviv Regev from the Broad Institute for helpful advice and providing the Cas9 mice for the CRISPR-Cas9 BMDC studies and Dr. Kotryna Simonyte for experimental assistance.
This work was supported by NIDDK, National Institutes of Health Grants RO1 DK043051 and P30DK57521 (to B. B. K.) and RO1 DK106210 (to B. B. K. and A. S.), a grant from the JPB Foundation (to B. B. K.), Leona M. and Harry B. Helmsley Charitable Trust Grant 2012-PG-MED002 (to A. S.), Fundação de Amparo a Pesquisa do Estado de São Paulo 2014/02218-6 and 2011/15682-4 (to A. C.), and 5T32DK007516-31 (to B. B. K. and J. L.). B. B. K., A. S., and P. V. are inventors on a patent and patent applications related to the fatty acid hydroxy fatty acids. J. L. is an inventor on a patent application related to the lipids. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- FAHFA
- fatty acid ester of hydroxy fatty acid
- PAHSA
- palmitic acid ester of hydroxy stearic acid
- UC
- ulcerative colitis
- GPCR
- G protein-coupled receptor
- DC
- dendritic cell
- BMDC
- bone marrow-derived dendritic cell
- DSS
- dextran sodium sulfate
- ANOVA
- analysis of variance
- SRE
- serum response element.
References
- 1. Clardy J., and Walsh C. (2004) Lessons from natural molecules. Nature 432, 829–837 [DOI] [PubMed] [Google Scholar]
- 2. Funk C. D. (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294, 1871–1875 [DOI] [PubMed] [Google Scholar]
- 3. Corey E. J., Schaaf T. K., Huber W., Koelliker U., and Weinshenker N. M. (1970) Total synthesis of prostaglandins F2-α and E2 as the naturally occurring forms. J. Am. Chem. Soc. 92, 397–398 [DOI] [PubMed] [Google Scholar]
- 4. Higenbottam T., Butt A. Y., McMahon A., Westerbeck R., and Sharples L. (1998) Long-term intravenous prostaglandin (epoprostenol or iloprost) for treatment of severe pulmonary hypertension. Heart 80, 151–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lindén C., and Alm A. (1999) Prostaglandin analogues in the treatment of glaucoma. Drugs Aging 14, 387–398 [DOI] [PubMed] [Google Scholar]
- 6. Moncada S., and Vane J. R. (1978) Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol. Rev. 30, 293–331 [PubMed] [Google Scholar]
- 7. Gonzalez-Cabrera P. J., Cahalan S. M., Nguyen N., Sarkisyan G., Leaf N. B., Cameron M. D., Kago T., and Rosen H. (2012) S1P(1) receptor modulation with cyclical recovery from lymphopenia ameliorates mouse model of multiple sclerosis. Mol. Pharmacol. 81, 166–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yore M. M., Syed I., Moraes-Vieira P. M., Zhang T., Herman M. A., Homan E. A., Patel R. T., Lee J., Chen S., Peroni O. D., Dhaneshwar A. S., Hammarstedt A., Smith U., McGraw T. E., Saghatelian A., and Kahn B. B. (2014) Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159, 318–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kitahora T., Utsunomiya T., and Yokota A. (1995) Epidemiological study of ulcerative colitis in Japan: incidence and familial occurrence: the Epidemiology Group of the Research Committee of Inflammatory Bowel Disease in Japan. J. Gastroenterol. 30, 5–8 [PubMed] [Google Scholar]
- 10. Lakatos L., Mester G., Erdelyi Z., Balogh M., Szipocs I., Kamaras G., and Lakatos P. L. (2004) Striking elevation in incidence and prevalence of inflammatory bowel disease in a province of western Hungary between 1977–2001. World J. Gastroenterol. 10, 404–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Molodecky N. A., Soon I. S., Rabi D. M., Ghali W. A., Ferris M., Chernoff G., Benchimol E. I., Panaccione R., Ghosh S., Barkema H. W., and Kaplan G. G. (2012) Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54.e42; quiz e30 [DOI] [PubMed] [Google Scholar]
- 12. Feuerstein J. D., and Cheifetz A. S. (2014) Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clinic Proc. 89, 1553–1563 [DOI] [PubMed] [Google Scholar]
- 13. Danese S., Sans M., and Fiocchi C. (2004) Inflammatory bowel disease: the role of environmental factors. Autoimmun. Rev. 3, 394–400 [DOI] [PubMed] [Google Scholar]
- 14. Dignass A. U., Baumgart D. C., and Sturm A. (2004) Review article: the aetiopathogenesis of inflammatory bowel disease: immunology and repair mechanisms. Aliment. Pharmacol. Ther. 20, 9–17 [DOI] [PubMed] [Google Scholar]
- 15. Gersemann M., Wehkamp J., and Stange E. F. (2012) Innate immune dysfunction in inflammatory bowel disease. J. Internal Med. 271, 421–428 [DOI] [PubMed] [Google Scholar]
- 16. Pastorelli L., De Salvo C., Mercado J. R., Vecchi M., and Pizarro T. T. (2013) Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front. Immunol. 4, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eaden J. A., Abrams K. R., and Mayberry J. F. (2001) The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 48, 526–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Curdia and Goncalves T. (2015) Impact of the age of diagnosis on the natural history of ulcerative colitis. Rev. Esp. Enferm. Dig. 107, 614–621 [DOI] [PubMed] [Google Scholar]
- 19. Damas O. M., Deshpande A. R., Avalos D. J., and Abreu M. T. (2015) Treating inflammatory bowel disease in pregnancy: the issues we face today. J. Crohns Colitis 9, 928–936 [DOI] [PubMed] [Google Scholar]
- 20. Horst S., and Kane S. (2014) The use of biologic agents in pregnancy and breastfeeding. Gastroenterol. Clin. North Am. 43, 495–508 [DOI] [PubMed] [Google Scholar]
- 21. Castoldi A., Favero de Aguiar C., Moraes-Vieira P. M., and Olsen Saraiva Câmara N. (2015) They must hold tight: junction proteins, microbiota and immunity in intestinal mucosa. Curr. Protein Pept. Sci. 16, 655–671 [DOI] [PubMed] [Google Scholar]
- 22. Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., Sugimoto Y., Miyazaki S., and Tsujimoto G. (2005) Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 11, 90–94 [DOI] [PubMed] [Google Scholar]
- 23. Briscoe C. P., Tadayyon M., Andrews J. L., Benson W. G., Chambers J. K., Eilert M. M., Ellis C., Elshourbagy N. A., Goetz A. S., Minnick D. T., Murdock P. R., Sauls H. R. Jr., Shabon U., Spinage L. D., Strum J. C., et al. (2003) The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 278, 11303–11311 [DOI] [PubMed] [Google Scholar]
- 24. Oh D. Y., Talukdar S., Bae E. J., Imamura T., Morinaga H., Fan W., Li P., Lu W. J., Watkins S. M., and Olefsky J. M. (2010) GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Turner D., Walsh C. M., Steinhart A. H., and Griffiths A. M. (2007) Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin. Gastroenterol. Hepatol. 5, 103–110 [DOI] [PubMed] [Google Scholar]
- 26. Bewtra M., Kilambi V., Fairchild A. O., Siegel C. A., Lewis J. D., and Johnson F. R. (2014) Patient preferences for surgical versus medical therapy for ulcerative colitis. Inflamm. Bowel Dis. 20, 103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cho J. Y., Chi S. G., and Chun H. S. (2011) Oral administration of docosahexaenoic acid attenuates colitis induced by dextran sulfate sodium in mice. Mol. Nutr. Food Res. 55, 239–246 [DOI] [PubMed] [Google Scholar]
- 28. Ishida T., Yoshida M., Arita M., Nishitani Y., Nishiumi S., Masuda A., Mizuno S., Takagawa T., Morita Y., Kutsumi H., Inokuchi H., Serhan C. N., Blumberg R. S., and Azuma T. (2010) Resolvin E1, an endogenous lipid mediator derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis. Inflamm. Bowel Dis. 16, 87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen C., Shah Y. M., Morimura K., Krausz K. W., Miyazaki M., Richardson T. A., Morgan E. T., Ntambi J. M., Idle J. R., and Gonzalez F. J. (2008) Metabolomics reveals that hepatic stearoyl-CoA desaturase 1 downregulation exacerbates inflammation and acute colitis. Cell Metab. 7, 135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loeschke K., Ueberschaer B., Pietsch A., Gruber E., Ewe K., Wiebecke B., Heldwein W., and Lorenz R. (1996) n-3 fatty acids only delay early relapse of ulcerative colitis in remission. Digest. Dis. Sci. 41, 2087–2094 [DOI] [PubMed] [Google Scholar]
- 31. Almallah Y. Z., Richardson S., O'Hanrahan T., Mowat N. A., Brunt P. W., Sinclair T. S., Ewen S., Heys S. D., and Eremin O. (1998) Distal procto-colitis, natural cytotoxicity, and essential fatty acids. Am. J. Gastroenterol. 93, 804–809 [DOI] [PubMed] [Google Scholar]
- 32. Bosco N., Brahmbhatt V., Oliveira M., Martin F. P., Lichti P., Raymond F., Mansourian R., Metairon S., Pace-Asciak C., Bastic Schmid V., Rezzi S., Haller D., and Benyacoub J. (2013) Effects of increase in fish oil intake on intestinal eicosanoids and inflammation in a mouse model of colitis. Lipids Health Dis. 12, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Middleton S. J., Naylor S., Woolner J., and Hunter J. O. (2002) A double-blind, randomized, placebo-controlled trial of essential fatty acid supplementation in the maintenance of remission of ulcerative colitis. Aliment. Pharmacol. Ther. 16, 1131–1135 [DOI] [PubMed] [Google Scholar]
- 34. Woodworth H. L., McCaskey S. J., Duriancik D. M., Clinthorne J. F., Langohr I. M., Gardner E. M., and Fenton J. I. (2010) Dietary fish oil alters T lymphocyte cell populations and exacerbates disease in a mouse model of inflammatory colitis. Cancer Res. 70, 7960–7969 [DOI] [PubMed] [Google Scholar]
- 35. Sikiric P. (2014) From gut inflammation to gastrointestinal disorders current update on pathophysiology, molecular mechanism and pharmacological treatment modalities. Curr. Pharm. Des. 20, 1039–1040 [DOI] [PubMed] [Google Scholar]
- 36. Wallace K. L., Zheng L. B., Kanazawa Y., and Shih D. Q. (2014) Immunopathology of inflammatory bowel disease. World J. Gastroenterol. 20, 6–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simpson S. J., Holländer G. A., Mizoguchi E., Allen D., Bhan A. K., Wang B., and Terhorst C. (1997) Expression of pro-inflammatory cytokines by TCR α β+ and TCR γ δ+ T cells in an experimental model of colitis. Eur. J. Immunol. 27, 17–25 [DOI] [PubMed] [Google Scholar]
- 38. Takedatsu H., Michelsen K. S., Wei B., Landers C. J., Thomas L. S., Dhall D., Braun J., and Targan S. R. (2008) TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology 135, 552–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rovedatti L., Kudo T., Biancheri P., Sarra M., Knowles C. H., Rampton D. S., Corazza G. R., Monteleone G., Di Sabatino A., and Macdonald T. T. (2009) Differential regulation of interleukin 17 and interferon γ production in inflammatory bowel disease. Gut 58, 1629–1636 [DOI] [PubMed] [Google Scholar]
- 40. Geremia A., and Jewell D. P. (2012) The IL-23/IL-17 pathway in inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 6, 223–237 [DOI] [PubMed] [Google Scholar]
- 41. Feng T., Qin H., Wang L., Benveniste E. N., Elson C. O., and Cong Y. (2011) Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. J. Immunol. 186, 6313–6318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Müller C. A., Autenrieth I. B., and Peschel A. (2005) Innate defenses of the intestinal epithelial barrier. Cell. Mol. Life Sci. 62, 1297–1307 [DOI] [PubMed] [Google Scholar]
- 43. Ouellette A. J. (2011) Paneth cell α-defensins in enteric innate immunity. Cell. Mol. Life Sci. 68, 2215–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paterson J. C., and Watson S. H. (1961) Paneth cell metaplasia in ulcerative colitis. Am. J. Pathol. 38, 243–249 [PMC free article] [PubMed] [Google Scholar]
- 45. Franke A., Balschun T., Karlsen T. H., Sventoraityte J., Nikolaus S., Mayr G., Domingues F. S., Albrecht M., Nothnagel M., Ellinghaus D., Sina C., Onnie C. M., Weersma R. K., Stokkers P. C., Wijmenga C., et al. (2008) Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat. Genet. 40, 1319–1323 [DOI] [PubMed] [Google Scholar]
- 46. Julià A., Domènech E., Chaparro M., García-Sánchez V., Gomollón F., Panés J., Mañosa M., Barreiro-De Acosta M., Gutiérrez A., Garcia-Planella E., Aguas M., Muñoz F., Esteve M., Mendoza J. L., Vera M., et al. (2014) A genome-wide association study identifies a novel locus at 6q22.1 associated with ulcerative colitis. Hum. Mol. Genet. 23, 6927–6934 [DOI] [PubMed] [Google Scholar]
- 47. Maxwell J. R., Brown W. A., Smith C. L., Byrne F. R., and Viney J. L. (2009) Methods of inducing inflammatory bowel disease in mice. Curr. Protoc. Pharmacol. Chapter 5, Unit 5 58 [DOI] [PubMed] [Google Scholar]
- 48. Bressenot A., Salleron J., Bastien C., Danese S., Boulagnon-Rombi C., and Peyrin-Biroulet L. (2015) Comparing histological activity indexes in UC. Gut 64, 1412–1418 [DOI] [PubMed] [Google Scholar]
- 49. Moraes-Vieira P. M., Yore M. M., Dwyer P. M., Syed I., Aryal P., and Kahn B. B. (2014) RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 19, 512–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moraes-Vieira P. M., Larocca R. A., Bassi E. J., Peron J. P., Andrade-Oliveira V., Wasinski F., Araujo R., Thornley T., Quintana F. J., Basso A. S., Strom T. B., and Câmara N. O. (2014) Leptin deficiency impairs maturation of dendritic cells and enhances induction of regulatory T and Th17 cells. Eur. J. Immunol. 44, 794–806 [DOI] [PMC free article] [PubMed] [Google Scholar]