Abstract
AIM
To identify a small, clinically applicable immunohistochemistry (IHC) panel that could be combined with magnetic resonance imaging (MRI)-detected extramural vascular invasion (EMVI) for assessment of prognosis concerning the non-advanced rectal cancer patients prior to operation.
METHODS
About 329 patients with pathologically confirmed rectal carcinoma (RC) were screened in this research, all of whom had been examined via an MRI and were treatment-naïve from July 2011 to July 2014. The candidate proteins that were reported to be altered by RC were examined in tissues by IHC. All chosen samples were adopted from the fundamental cores of histopathologically confirmed carcinomas during the initial surgeries.
RESULTS
Of the three proteins that were tested, c-MYC, PCNA and TIMP1 were detected with relatively significant expression in tumors, 35.9%, 23.7% and 58.7% respectively. The expression of the three proteins were closely connected with prognosis (P = 0.032, 0.003, 0.021). The patients could be classified into different outcome groups according to an IHC panel (P < 0.01) via these three proteins. Taking into consideration known survival covariates, especially EMVI, the IHC panel served as an independent prognostic factor. The EMVI combined with the IHC panel could categorize patients into different prognostic groups with distinction (P < 0.01).
CONCLUSION
These studies argue that this three-protein panel of c-MYC, PCNA, coupled with TIMP1 combined with MRI-detected EMVI could offer extra prognostic details for preoperative treatment of RC.
Keywords: Rectal cancer, Magnetic resonance imaging, Prognosis, Immunohistochemistry, Extramural vascular invasion
Core tip: Magnetic resonance imaging (MRI) to determine the stage of rectal cancer has been shown to be accurate and reproducible. The patients who showed MRI-detected extramural vascular invasion (EMVI) positivity had worse survival outcomes than those with EMVI negativity. However, the survival rates of patients with an EMVI-positive histology varies dramatically, which suggests that this factor does not relate to each individual tumor in terms of the biology or molecular features. The ideal staging system evolves from the consideration of them and the correlation of the prognosis with patient-specific tumor biomarkers. In this study, we hypothesized that the combination of imaging evidence of EMVI and biomolecular factors could provide additional predictive value in clinical practice, especially for non-advanced cancer patients.
INTRODUCTION
The transanal approach may be used to effectively excise rectal carcinoma (RC), which is usually found in the lower part of the rectum. Transanal excision of RCs, however, may be associated with poor locoregional control[1,2] .Therefore, accuracy of preoperative staging is vital in modern rectal cancer management. Magnetic resonance imaging (MRI) to determine the stage of rectal cancer has been shown to be accurate and reproducible[3,4]. MRI enables planning for primary tumor resection.
Recent development in MRI provides the distinguishment of extramural vascular invasion (EMVI) prior to surgery being performed[5]. EMVI is described as blood vessels invaded by malignant cells surpassing the muscularis propria of rectal cancer[6]. Chand et al[7] showed that EMVI leads to worse survival outcomes[6]. However, the survival rates of patients with an EMVI-positive histology varies dramatically, which suggests that this factor does not relate to each individual tumor in terms of the biology or molecular features.
The ideal staging system will evolve from the consideration of them and the correlation of the prognosis with patient-specific tumor biomarkers[8,9]. Based on previous studies, we hypothesized that the combination of imaging evidence of EMVI and biomolecular factors could provide additional predictive value in clinical practice, especially for non-advanced cancer patients.
In this study, three candidate proteins were screened in paraffin-embedded tissue samples via immunohistochemistry (IHC). When combined with MRI-detected EMVI, this panel could help clinicians determine the prognosis for patients with RC without regional lymph node involvement and distant metastasis.
MATERIALS AND METHODS
Patients and tissue specimens
This study was approved by the institutional review board of Harbin Medical University, and consent was obtained from all of the patients in written form. Patients with pathologically confirmed rectal adenocarcinoma who were treatment-naïve were screened in this research, and were examined through preoperative rectal MRI at the Second Afflict Hospital of Harbin Medical University from July 2011 to July 2014. Patients who were found to have a synchronous regional lymph node and/or distant metastases at the initial staging were excluded from the study. All chosen samples were adopted from the crucial cores of histopathologically confirmed carcinomas during the initial surgeries. Two pathologists performed diagnosis on all of the lesions and the findings were reviewed by an expert colorectal cancer pathologist independently; additionally, the stage of tumor was determined according to the system of the International Union Against Cancer.
MRI protocol
The MRI examination of patients with RC was carried out using a 3.0 T Philips Achieva TX (Philips Medical Systems, Holland) system with an 8-channel body phased array coil. Each rectal magnetic resonance examination was performed as follows: To decrease the colonic motility, an intramuscular injection of 20 mg of scopolamine butylbromide (Buscopan; Boehringer Ingelheim, Germany) was administered prior to the MRI. Approximately 100-120 mL of saline was rectally administered using an enema syringe. High-resolution magnetic resonance images were obtained, including high-resolution oblique axial T2-weighted turbo spin-echo, oblique coronal T2-weighted turbo spin-echo and sagittal T2-weighted turbo spin-echo coupled with the following parameters: TE (echo time) 81 ms-185 ms, TR (repetition time) 3900-5600 ms, thickness of 3 mm, spacing of 3 mm, matrix of 256 × 256 to 320 × 320, field of view of 250 × 250 to 199 × 199, and echo train length of 17-35.
Image analysis
All magnetic resonance images required at least three abdominal radiologists to analyze the images separately with no clinical information about the patients. The MRI analysis involved tumor morphology, the presence of EMVI, circumferential resection margin, lymph node involvement and tumor stage. The EMVI grading score was adopted from the system proposed by Smith et al[10]. According to this scoring system, scores of 0 to 2 were defined as EMVI-negative disease, and scores of 3 and 4 corresponded to EMVI-positive disease. A score of 3 EMVI included a tumor with a vein that did not change its contour and may have only slightly expanded the vessel. A score of 4 EMVI described an irregularly expanded vein, which indicated the vessel wall was invaded. To achieve a consensus agreement, EMVI negative cases were re-evaluated together by three abdominal radiologists.
IHC
To select the study samples, we employed a usual histological categorization according to the WHO classification of tumors. A 3-tiered histological grading system played an important role in this research. The tumor-node-metastasis stage was evaluated, based on the guideline of the 2002 International Union Against Cancer classification. Monoclonal mouse anti-human c-MYC, PCNA and TIMP-1 antibodies were introduced from Santa Cruz Biotechnology (United States). The slides were deparaffinized, rehydrated, immersed in 3% hydrogen peroxide solution for 10 min, heated in citrate buffer (pH 6.0) for 25 min at 95 °C, and cooled for 60 min at room temperature. The slides were washed with PBS (pH 7.4) between each incubation step. Then, the slides were incubated separately with the primary antibodies. Immunoperoxidase staining was performed using the 2-step Envision Method (DAKO, Denmark) abiding by the manufacturer’s instructions, and visualized with 3,3 9-diaminobenzidine tetrachloride (Sigma, United States). Negative controls were slides with no primary antibodies.
Cytoplasm and membrane staining were mensurated for the TIMP1 antibody, and c-MYC and PCNA staining showed nuclear localization. Two pathologists counted the positive cells. The scores of the results according to the immunohistochemical staining were obtained with the examiners being completely ignorant of any information related to the patient’s clinical data. When it comes to clinicopathological association, the researchers applied a 4-tiered scoring system (negative to 3+), taking into consideration the proportion of positive cells and staining intensity as represented before[11].
Statistical analysis
The statistical analyses were carried out via the SPSS software program (standard version 19.0; SPSS, United States). The distinctions between groups were determined according to the Mann-Whitney U test or the Kruskal-Wallis H test. The correlation among categorical data underwent statistical analysis using the χ2 test. Kaplan-Meier curves resulted from the log-rank test for survival analyses. The clinical end point in the research, the period from cancer confirmed to death starting at RC or last contact, was overall survival. Multivariate Cox proportional hazards regression analysis served in the detection of independent prognosticators, which had a conspicuous influence on patient survival. A difference was regarded to be significant under the condition that the P-value was less than 0.05.
RESULTS
Characteristics of the study population
A total of 329 RC specimens were collected from patients who underwent an operation. The median patient age was 62 years (range, 21-82 years), and the subjects included 122 (37.1%) females and 207 (62.9%) males. All patients underwent curative resection with tumor-free margins. Among all patients, 99 underwent adjuvant chemotherapy. All tumors were histologically diagnosed, graded, and grouped as lowly and moderately differentiated (n = 319) or highly differentiated (n = 10). The follow-up interval ranged from 1 to 58 mo from the date of diagnosis.
MRI findings
There were 53 cases (16.1%) of EMVI-positive RCs on the initial preoperative MRI scans. Of them, 30 patients (56.6%) had an EMVI score of 3, and 23 patients (43.4%) had an EMVI score of 4. Score 3 MRI-detected EMVI only occurred in small vessels. Score 4 was only observed in medium and large vessels, which was consistent with previous findings[12].
Relationships between protein expression and clinicopathologic characteristics
For TIMP1, positive staining was found mainly in the cytoplasm and membrane of the neoplasm cells, and nuclear localization of c-MYC and PCNA was generally observed. Immunostaining of the three proteins was low in some samples but strong in others. Representative examples of IHC expression of TIMP1, c-MYC, and PCNA performed in the study cohorts are shown in Figure 1.
Figure 1.
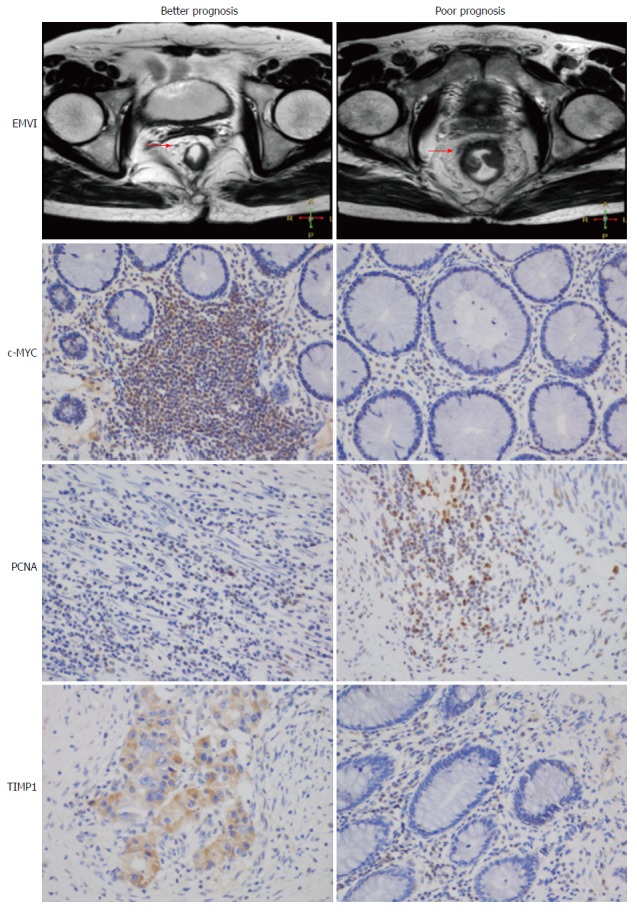
Representative examples of extramural vascular invasion and immunohistochemistry images in distinct prognostic groups. Overexpression of c-MYC and TIMP1 is a marker of better prognosis, whereas PCNA is a marker of poor prognosis, which was consistent with known biological roles.
There were no significant differences concerning age, sex, chemotherapy and tumor location. However, a significant association was detected between the expression levels of PCNA, TIMP1 and pT (P < 0.01; P = 0.045). Moreover, the overexpression of TIMP1 was associated with the histological grading and perineural invasion (P < 0.01; P < 0.01). Table 1 shows the relationships between the clinicopathological features and protein expression levels.
Table 1.
Relationship between expression of proteins and clinicopathologic parameters
| Variable | n | c-MYC expression | PCNA expression | TIMP1 expression | ||||||
| Strong (n = 118) | Low (n = 211) | P value1 | Strong (n = 78) | Low (n = 241) | P value1 | Strong (n = 193) | Low (n = 136) | P value1 | ||
| Sex | ||||||||||
| Male | 207 | 69 | 136 | 47 | 160 | 119 | 88 | |||
| Female | 122 | 49 | 73 | 0.236 | 31 | 81 | 0.324 | 74 | 48 | 0.573 |
| Age, median (range) | 62 (21-82) | |||||||||
| Histology grade, differentiation | ||||||||||
| Well | 10 | 4 | 6 | 2 | 8 | 5 | 5 | |||
| Moderate | 143 | 48 | 95 | 36 | 107 | 57 | 86 | |||
| Poor | 176 | 66 | 110 | 0.738 | 40 | 136 | 0.844 | 131 | 45 | < 0.01 |
| Pathologic T stage | ||||||||||
| T1 | 24 | 9 | 15 | 5 | 19 | 11 | 13 | |||
| T2 | 78 | 30 | 48 | 7 | 71 | 41 | 37 | |||
| T3 | 74 | 28 | 46 | 15 | 59 | 53 | 21 | |||
| T4 | 153 | 51 | 102 | 0.848 | 51 | 102 | < 0.01 | 88 | 65 | 0.045 |
| Lymphovascular invasion | ||||||||||
| Yes | 153 | 56 | 97 | 37 | 116 | 90 | 63 | |||
| No | 176 | 62 | 114 | 0.796 | 41 | 135 | 0.850 | 103 | 73 | 0.956 |
| Perineural invasion | ||||||||||
| Yes | 127 | 45 | 82 | 32 | 95 | 88 | 39 | |||
| No | 202 | 73 | 129 | 0.897 | 46 | 156 | 0.615 | 105 | 97 | < 0.01 |
| Chemotherapy | ||||||||||
| Yes | 99 | 43 | 56 | 24 | 75 | 58 | 41 | |||
| No | 230 | 75 | 155 | 0.060 | 54 | 176 | 0.881 | 135 | 95 | 0.985 |
| EMVI | ||||||||||
| Positive | 53 | 18 | 35 | 13 | 40 | 32 | 21 | |||
| Negative | 276 | 100 | 176 | 0.752 | 65 | 211 | 0.878 | 161 | 115 | 0.782 |
| Location | ||||||||||
| Below peritoneal reflection | 120 | 41 | 79 | 31 | 89 | 71 | 49 | |||
| At peritoneal reflection | 43 | 22 | 21 | 4 | 39 | 25 | 18 | |||
| Above peritoneal reflection | 166 | 55 | 111 | 0.080 | 43 | 123 | 0.059 | 97 | 69 | 0.990 |
P value was obtained by χ2 test. EMVI: Extramural vascular invasion.
Independent prognostic value of three-protein IHC panel
Due to the fact that it illustrated that the three-protein panel and MRI-detected EMVI was of significant predictive value for survival in the long run, multivariate Cox regression analysis was implemented to decide whether these parameters supplied further information of prognosis independent of known clinicopathologic features which could affect the prognosis. As summarized in Table 2, each parameter had a prognostic significance independently (P < 0.05). Additionally, the three-protein panel displayed greater significance as an independent prognostic factor (HR = 2.110, 95%CI: 1.631-2.556, P < 0.01). The panel combined with the EMVI status could rank patients in a more accurate way (P < 0.01) (Figure 2).
Table 2.
Multivariate Cox regression analysis of factors predicting survival time of patients without regional lymph node and distant metastasis
| Variable | HR | 95%CI | P value |
| Sex (male vs female) | 1.312 | 0.784-2.159 | 0.354 |
| Pathologic T stage (T3-4 vs T1-2) | 1.226 | 0.817-2.657 | 0.012 |
| Lymphovascular invasion (yes vs no) | 1.413 | 0.716-1.971 | 0.241 |
| Perineural invasion (yes vs no) | 1.539 | 0.992-2.321 | 0.114 |
| EMVI (positive vs negative) | 3.071 | 2.784-5.754 | < 0.01 |
| c-MYC expression (low vs strong) | 1.138 | 1.003-2.421 | 0.032 |
| PCNA expression (strong vs low) | 2.582 | 1.748-4.373 | 0.003 |
| TIMP1 expression (low vs strong) | 2.643 | 1.869-5.821 | 0.021 |
| IHC panel (2-3 markers high vs 0-1 marker high) | 2.110 | 1.631-2.556 | < 0.01 |
EMVI: Extramural vascular invasion; IHC: Immunohistochemistry.
Figure 2.
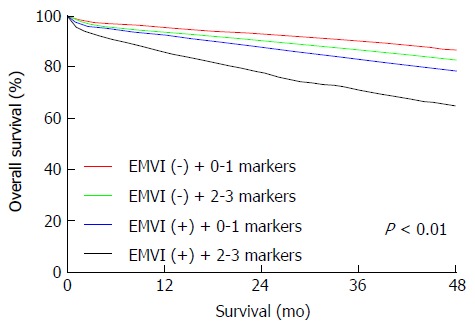
Immunohistochemistry panel combined with magnetic resonance imaging-detected extramural vascular invasion enhanced the accuracy of prognoses for patients with rectal carcinoma. Combination of EMVI and the IHC panel separated patients into distinct prognostic groups (P < 0.01). EMVI: Extramural vascular invasion; IHC: Immunohistochemistry.
DISCUSSION
Nowadays, local excision has won growing popularity as a strategy for treating RC in that it removes the necessity for colostomy, lowers the threatening perioperative risks for patients, and ensures optimistic functional outcomes[13,14]. This surgical procedure, however, requires a thorough analysis of its oncologic risks since standard resection protocols used to eliminate the rectum with its surrounding lymph nodes have gained favorable oncologic results, and are considered as the gold standard[15]. Hence, proper preoperative staging is necessary. However, staging remains challenging owing to the variable accuracy and limitations of imaging. Patient-specific tumor biomarkers could function as a useful aid to present clinicopathologic parameters for risk stratification. This study identified a three-protein panel for the prognostic assessment of non-advanced RC patients that could supplement current diagnostic systems.
The c-MYC proto-oncogene encodes a transcription factor that has a vital effect on cell proliferation, differentiation, apoptosis, metabolism, and survival[16,17]. In recent study on adenocarcinoma of the colorectum, patients with a c-MYCGCN gain had low rates of survival compared to those without the gain, which was found to be a poor independent prognostic factor[18]. However, the correlation between expression of c-MYC in colorectal cancer and prognosis was contradicted in previous studies[19-21]. The conflicting results presumably occurred as c-MYC expression is controlled by a complicated regulatory pathway, engaging multi-interactive activities with other molecules[22]. In the present study, we clarified that c-MYC overexpression determined by IHC solely was significantly linked with higher rates of survival in colorectal cancer patients when evaluated by multivariate analysis.
PCNA, which is a non-histamine nuclear protein, has been confirmed to connect with the degree of malignancy, vascular infiltration, distant metastasis and survival[23-25]. TIMPs have been proposed to be important inhibitors of tumor invasion of the extracellular matrix based on in vitro and animal studies[26,27]. Pellegrini et al[28] put forward that, from the phase of pre-invasive node to the phase of invasion, detections of serum levels of CEA and TIMP1 in colorectal cancer patients simultaneously could serve as markers for disease progression in prognosis and diagnosis.
It has been established that patients may experience a drastically increased risk of distant metastasis, given pathological EMVI at the moment of primary resection[29]. The high-resolution MRI provides the preoperative identification of EMVI[30]; preoperative MRI-detected EMVI status for rectal cancer is a crucial procedure, especially in the screening of patients for neoadjuvant treatment[31]. A microRNA panel has been shown to have considerable clinical value in the early diagnosis of colorectal cancer[32]. Through the current research, we identified a three-protein prognostic panel independent of clinicopathological features. What’s more important, the three-protein panel combined with MRI-detected EMVI could rank patients into subgroups with diverse prognoses (P < 0.01), which may benefit clinical intervention.
In conclusion, we have identified a three-protein panel in a considerable number of rectal tissue specimens that determines prognosis with accuracy independent of clinical prognostic parameters, and it has magnificent clinical value for surgical decision-making for treating RC.
ACKNOWLEDGMENTS
The authors thank the patients and families who participated in the study.
COMMENTS
Background
Non-advanced rectal cancer (RC) can be treated using various strategies. Accuracy of preoperative staging is vital for modern management of RC. To accurately classify prognostic risks, this study set out to identify a small, clinically applicable immunohistochemistry (IHC) panel that could be combined with magnetic resonance imaging (MRI)-detected extramural vascular invasion (EMVI) for preoperative prognostic assessment of RC patients.
Research frontiers
MRI is important for the local staging of rectal cancer, the patients who showed MRI-detected EMVI positivity had worse survival outcomes than the EMVI-negative cases. Few prior reports contain analyses of the combination of imaging evidence of EMVI and biomolecular factors in RC. The results of this study suggest that the three-protein panel of c-MYC, PCNA and TIMP1 combined with MRI-detected EMVI could provide additional prognostic information for preoperative treatment of RC.
Innovations and breakthroughs
In this study, c-MYC, PCNA and TIMP1 were detected with high expression in 35.9%, 23.7% and 58.7% of tumors, respectively. Significant associations were found between the expression of these proteins and the prognosis (P = 0.032, 0.003, 0.021). Applying these three proteins as an IHC panel could be used to classify patients into different subgroups (P < 0.01). Upon adjustment for known survival covariates, including EMVI, the IHC panel remained an independent prognostic factor. The combination of EMVI and the IHC panel could separate patients into distinct prognostic groups (P < 0.01).
Applications
These findings suggest that the three-protein panel of c-MYC, PCNA and TIMP1 combined with MRI-detected EMVI could provide additional prognostic information for preoperative treatment of RC.
Terminology
EMVI is defined as the presence of malignant cells within the blood vessels beyond the muscularis propria of rectal cancer.
Peer-review
This is an excellent study about the combination of three-gene immunohistochemical panel and MRI-detected EMVI to assess prognosis in non-advanced rectal cancer patients.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was reviewed and approved by the institutional review board of Harbin Medical University.
Informed consent statement: The authors of this paper guarantee that all study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment.
Conflict-of-interest statement: The authors have no financial relationships to disclose.
Data sharing statement: No additional data are available.
Peer-review started: July 7, 2016
First decision: August 19, 2016
Article in press: September 14, 2016
P- Reviewer: Alric L, Chadokufa S, Rukavina M S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang FF
References
- 1.You YN, Baxter NN, Stewart A, Nelson H. Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: a nationwide cohort study from the National Cancer Database. Ann Surg. 2007;245:726–733. doi: 10.1097/01.sla.0000252590.95116.4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endreseth BH, Myrvold HE, Romundstad P, Hestvik UE, Bjerkeset T, Wibe A. Transanal excision vs. major surgery for T1 rectal cancer. Dis Colon Rectum. 2005;48:1380–1388. doi: 10.1007/s10350-005-0044-6. [DOI] [PubMed] [Google Scholar]
- 3.Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90:355–364. doi: 10.1002/bjs.4034. [DOI] [PubMed] [Google Scholar]
- 4.MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333:779. doi: 10.1136/bmj.38937.646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith NJ, Shihab O, Arnaout A, Swift RI, Brown G. MRI for detection of extramural vascular invasion in rectal cancer. AJR Am J Roentgenol. 2008;191:1517–1522. doi: 10.2214/AJR.08.1298. [DOI] [PubMed] [Google Scholar]
- 6.Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. The clinical significance of invasion of veins by rectal cancer. Br J Surg. 1980;67:439–442. doi: 10.1002/bjs.1800670619. [DOI] [PubMed] [Google Scholar]
- 7.Chand M, Siddiqui MR, Swift I, Brown G. Systematic review of prognostic importance of extramural venous invasion in rectal cancer. World J Gastroenterol. 2016;22:1721–1726. doi: 10.3748/wjg.v22.i4.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ong CA, Lao-Sirieix P, Fitzgerald RC. Biomarkers in Barrett’s esophagus and esophageal adenocarcinoma: predictors of progression and prognosis. World J Gastroenterol. 2010;16:5669–5681. doi: 10.3748/wjg.v16.i45.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagarde SM, ten Kate FJ, Richel DJ, Offerhaus GJ, van Lanschot JJ. Molecular prognostic factors in adenocarcinoma of the esophagus and gastroesophageal junction. Ann Surg Oncol. 2007;14:977–991. doi: 10.1245/s10434-006-9262-y. [DOI] [PubMed] [Google Scholar]
- 10.Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229–236. doi: 10.1002/bjs.5917. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Cai S, Wang X, Jiang Z. Identification and characterization of ANO9 in stage II and III colorectal carcinoma. Oncotarget. 2015;6:29324–29334. doi: 10.18632/oncotarget.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bugg WG, Andreou AK, Biswas D, Toms AP, Williams SM. The prognostic significance of MRI-detected extramural venous invasion in rectal carcinoma. Clin Radiol. 2014;69:619–623. doi: 10.1016/j.crad.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Ota DM, Jacobs L, Kuvshinoff B. Rectal cancer: the sphincter-sparing approach. Surg Clin North Am. 2002;82:983–993. doi: 10.1016/s0039-6109(02)00048-8. [DOI] [PubMed] [Google Scholar]
- 14.Bleday R. Local excision of rectal cancer. World J Surg. 1997;21:706–714. doi: 10.1007/s002689900295. [DOI] [PubMed] [Google Scholar]
- 15.Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, Miedema B, Ota D, Sargent D. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–596. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 16.Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18:3004–3016. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 17.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KS, Kwak Y, Nam KH, Kim DW, Kang SB, Choe G, Kim WH, Lee HS. c-MYC Copy-Number Gain Is an Independent Prognostic Factor in Patients with Colorectal Cancer. PLoS One. 2015;10:e0139727. doi: 10.1371/journal.pone.0139727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toon CW, Chou A, Clarkson A, DeSilva K, Houang M, Chan JC, Sioson LL, Jankova L, Gill AJ. Immunohistochemistry for myc predicts survival in colorectal cancer. PLoS One. 2014;9:e87456. doi: 10.1371/journal.pone.0087456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith DR, Goh HS. Overexpression of the c-myc proto-oncogene in colorectal carcinoma is associated with a reduced mortality that is abrogated by point mutation of the p53 tumor suppressor gene. Clin Cancer Res. 1996;2:1049–1053. [PubMed] [Google Scholar]
- 21.Erisman MD, Litwin S, Keidan RD, Comis RL, Astrin SM. Noncorrelation of the expression of the c-myc oncogene in colorectal carcinoma with recurrence of disease or patient survival. Cancer Res. 1988;48:1350–1355. [PubMed] [Google Scholar]
- 22.Lin CY, Lovén J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavezzi AM, Ottaviani G, De Ruberto F, Fichera G, Matturri L. Prognostic significance of different biomarkers (DNA content, PCNA, karyotype) in colorectal adenomas. Anticancer Res. 2002;22:2077–2081. [PubMed] [Google Scholar]
- 24.Kovac D, Rubinic M, Krasevic M, Krizanac S, Petrovecki M, Stimac D, Melato M. Proliferating cell nuclear antigen (PCNA) as a prognostic factor for colorectal cancer. Anticancer Res. 1995;15:2301–2302. [PubMed] [Google Scholar]
- 25.Guzińska-Ustymowicz K, Pryczynicz A, Kemona A, Czyzewska J. Correlation between proliferation markers: PCNA, Ki-67, MCM-2 and antiapoptotic protein Bcl-2 in colorectal cancer. Anticancer Res. 2009;29:3049–3052. [PubMed] [Google Scholar]
- 26.Ponton A, Coulombe B, Skup D. Decreased expression of tissue inhibitor of metalloproteinases in metastatic tumor cells leading to increased levels of collagenase activity. Cancer Res. 1991;51:2138–2143. [PubMed] [Google Scholar]
- 27.Khokha R, Waterhouse P, Yagel S, Lala PK, Overall CM, Norton G, Denhardt DT. Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science. 1989;243:947–950. doi: 10.1126/science.2465572. [DOI] [PubMed] [Google Scholar]
- 28.Pellegrini P, Contasta I, Berghella AM, Gargano E, Mammarella C, Adorno D. Simultaneous measurement of soluble carcinoembryonic antigen and the tissue inhibitor of metalloproteinase TIMP1 serum levels for use as markers of pre-invasive to invasive colorectal cancer. Cancer Immunol Immunother. 2000;49:388–394. doi: 10.1007/s002620000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. Spread of rectal cancer within veins. Histologic features and clinical significance. Am J Surg. 1981;141:15–17. doi: 10.1016/0002-9610(81)90004-0. [DOI] [PubMed] [Google Scholar]
- 30.Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, Williams GT. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227:371–377. doi: 10.1148/radiol.2272011747. [DOI] [PubMed] [Google Scholar]
- 31.Sohn B, Lim JS, Kim H, Myoung S, Choi J, Kim NK, Kim MJ. MRI-detected extramural vascular invasion is an independent prognostic factor for synchronous metastasis in patients with rectal cancer. Eur Radiol. 2015;25:1347–1355. doi: 10.1007/s00330-014-3527-9. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Wang L, Bayaxi N, Li J, Verhaegh W, Janevski A, Varadan V, Ren Y, Merkle D, Meng X, et al. A microRNA panel to discriminate carcinomas from high-grade intraepithelial neoplasms in colonoscopy biopsy tissue. Gut. 2013;62:280–289. doi: 10.1136/gutjnl-2011-301554. [DOI] [PubMed] [Google Scholar]


