Abstract
AIM
To describe racial/ethnic differences in treatment and survival among liver cancer patients in a population-based cancer registry.
METHODS
Invasive cases of primary hepatocellular carcinoma, n = 33270, diagnosed between January 1, 1988-December 31, 2012 and reported to the California Cancer Registry were analyzed by race/ethnicity, age, gender, geographical region, socio-economic status, time period of diagnosis, stage, surgical treatment, and survival. Patients were classified into 15 racial/ethnic groups: non-Hispanic White (White, n = 12710), Hispanic (n = 8500), Chinese (n = 2723), non-Hispanic Black (Black, n = 2609), Vietnamese (n = 2063), Filipino (n = 1479), Korean (n = 1099), Japanese (n = 658), American Indian/Alaskan Native (AIAN, n = 281), Laotian/Hmong (n = 244), Cambodian (n = 233), South Asian (n = 190), Hawai`ian/Pacific Islander (n = 172), Thai (n = 95), and Other Asian (n = 214). The main outcome measures were receipt of surgical treatment, and cause-specific and all-cause mortality.
RESULTS
After adjustment for socio-demographic characteristics, time period, and stage of disease, compared to Whites, Laotian/Hmong [odds ratio (OR) = 0.30, 95%CI: 0.17-0.53], Cambodian (OR = 0.65, 95%CI: 0.45-0.96), AIAN (OR = 0.66, 95%CI: 0.46-0.93), Black (OR = 0.76, 95%CI: 0.67-0.86), and Hispanic (OR = 0.78, 95%CI: 0.72-0.84) patients were less likely, whereas Chinese (OR = 1.58, 95%CI: 1.42-1.77), Koreans (OR = 1.45, 95%CI: 1.24-1.70), Japanese (OR = 1.41, 95%CI: 1.15-1.72), and Vietnamese (OR = 1.26, 95%CI: 1.12-1.42) were more likely to receive surgical treatment. After adjustment for the same covariates and treatment, cause-specific mortality was higher for Laotian/Hmong [(hazard ratio (HR) = 1.50, 95%CI: 1.29-1.73)], Cambodians (HR = 1.35, 95%CI: 1.16-1.58), and Blacks (HR = 1.07, 95%CI: 1.01-1.13), and lower for Chinese (HR = 0.82, 95%CI: 0.77-0.86), Filipinos (HR = 0.84, 95%CI: 0.78-0.90), Vietnamese (HR = 0.85, 95%CI: 0.80-0.90), Koreans (HR = 0.90, 95%CI: 0.83-0.97), and Hispanics (HR = 0.91, 95%CI: 0.88-0.94); results were similar for all-cause mortality.
CONCLUSION
Disaggregated data revealed substantial racial/ethnic differences in liver cancer treatment and survival, demonstrating the need for development of targeted interventions to mitigate disparities.
Keywords: Disparities, Treatment, Survival, Liver cancer, Hepatocellular carcinoma
Core tip: We found substantial racial/ethnic differences in treatment and survival in our analysis of 33270 cases of hepatocellular carcinoma from the world’s largest cancer registry in a single geo-political jurisdiction, diagnosed over a 25-year period and disaggregated into 15 racial/ethnic categories. Such granularity provides more precise identification of populations at risk by race/ethnicity, age, gender, socio-economic status, and stage of disease so that targeted interventions to mitigate disparities can be developed.
INTRODUCTION
Cancer of the liver and intrahepatic bile duct, of which approximately 80% is hepatocellular carcinoma (HCC)[1], led the 17 most common cancer sites with a 3.1% average annual increase in mortality rates between 2008 and 2012 among both men and women in the United States[2]. In contrast, mortality rates declined an average of 1.8% per year among men and 1.4% among women during the same time period for all cancer sites combined[2]. HCC’s prominence is further exemplified by the quadrupling of its incidence from 1.5 to 6.2 per 100000 between 1973 and 2011[3]. Worldwide, liver cancer has become the second leading cause of cancer deaths[4].
The principal risk factors for HCC are chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections[5]. After tobacco use, HBV infection, because of its etiological linkage to liver cancer, is the next most important cause of cancer worldwide[6,7]. HCV-related HCC has become the fastest increasing cause of cancer mortality in the United States[8]. In addition to alcohol consumption[8], other risk factors contributing to the increase in HCC in the United States are metabolic syndrome including diabetes[9,10] and obesity[8], which are risk factors for non-alcoholic steatohepatitis (NASH)[11]. There is evidence that the peak of the HCC epidemic may be near[3,12]. However, because HCC disproportionately affects populations of color[2]-African Americans, American Indians/Alaska Natives, Asian Americans, Hispanics, and Pacific Islanders-and because many of these racial/ethnic populations are increasing at faster rates than the population as a whole, the burden of liver cancer will continue to increase[2,13,14] unless detected earlier and properly treated or prevented[15].
Although the 5-year relative survival rate for liver cancer has risen in recent years, from 3.4% in 1975-77 to 18.1% in 2006-2012[16], it remains lower than that of most other common cancers[16]. Treatments have become more effective[11], and a larger proportion of patients are being diagnosed with early stage disease[17]. However not all HCC patients have benefited from these improvements[18]; consequently, racial/ethnic, socioeconomic, and geographic disparities in mortality persist[12,17].
Previous analyses of HCC survival and treatment characteristics have typically reported on HCC cases aggregated by ethnicity (Hispanic or non-Hispanic) accompanied by broad racial categories, e.g., American Indian/Alaska Native, Asian, Black, Native Hawaiian and Other Pacific Islander[19-21], or focused on Black-White comparisons[22]. Our previous study of more than 6000 HCC cases diagnosed in California in 1988-2007, which reported on specific Asian ethnicities, found substantial inter-ethnic variation in survival but did not include comparison with other racial/ethnic groups[23]. Rarely have HCC survival and treatment characteristics been characterized for 15 race/ethnic groups in a large geographically contiguous area over a period of 25 years.
The purpose of this study was to identify disparities in treatment and survival by race and ethnicity among more than 33000 California residents diagnosed with HCC from 1988-2012, and determine the extent to which variables such as age, gender, stage at diagnosis, and socioeconomic status explain these disparities.
MATERIALS AND METHODS
The data source for our study is the California Cancer Registry (CCR), the world’s largest population-based registry with ethnic-specific data in a single contiguous political jurisdiction. The CCR covers the entire state of California and includes three Surveillance, Epidemiology, and End Results (SEER) regions: the Greater Bay Area, Los Angeles County, and Greater California. The CCR has achieved the highest standards for cancer registry quality established by the North American Association of Central Cancer Registries (NAACCR) and the National Program of Cancer Registries (NPCR) for completeness and quality. Reporting of cancer cases to the CCR has been legislatively mandated in California since 1985. The CCR includes data from all cancer cases (except basal and squamous cell carcinoma of the skin and carcinoma in situ of the cervix), and its completeness is estimated to be 95% or greater.
The CCR follows standardized data collection and quality-control procedures in terms of racial/ethnic categorizations and cancer diagnoses[24]. Race/ethnicity information for the HCC cases is primarily based on information contained in the patient’s medical record. This information may be based on self-identification by the patient, on the assumptions by an admissions clerk or other medical personnel, or by inference using race/ethnicity of parents, birthplace, maiden name, or last name. To better identify Hispanics and Asian ethnic groups, cases were run through NAACCR Hispanic and Asian Identification Algorithm[25,26]. Cases are classified as non-Hispanic White (White), non-Hispanic Black (Black), Hispanic, American Indian/Alaskan Native, Asian American, and Native Hawaiian/Pacific Islander. Asian race is further divided into twelve groups, the nine largest in California in rank order according to their 2010 U.S. Census populations are as follows: Filipino, Chinese (including Taiwanese), Vietnamese, South Asian (Asian Indian, Pakistani, Bangladeshi, Sri Lankan), Korean, Japanese, Hmong and Laotian, Cambodian, and Thai.
In our study, Laotian and Hmong have been combined into one group because the majority of foreign-born Hmong were born in Laos[27], and older Hmong individuals may classify themselves as Laotian because they were formerly citizens of Laos[28]. South Asians, whose land of origin is the Indian subcontinent[29,30], are comprised of Asian Indian, Pakistani, other South Asian, Bangladeshi, Bhutanese, Nepalese, Sikh, and Sri Lankan. We combined cases from smaller or unknown Asian ethnic groups into an Other Asian category. Excluded from our analyses were 107 HCC cases with unknown race.
The analysis included all invasive hepatocellular carcinoma (HCC) cases diagnosed between January 1, 1988 and December 31, 2012 and reported to the CCR as of December 2015. We used the International Classification of Diseases for Oncology, Third Edition site code (C22.0) and histology code (8170) to identify patients with HCC among all patients with primary liver cancer. Eligibility was restricted to HCC as the first primary cancer in order to eliminate survival differences due to the effects of other cancers. Only cases with diagnostic confirmation of HCC were included in our study (92.3%). Diagnostic confirmation of HCC was defined as having positive histology (56.7%), positive radiological test (27.6%), cytology (11.2%), laboratory test/marker study (4.2%), or direct visualization (0.3%). A total of 33270 invasive HCC cases that met the above requirements were analyzed for this study.
Patient vital status was updated using both passive and active follow-up methods. Passive follow-up methods included annual record linkages with the California State death file, National Death Index, Social Security Death Master File, Medicare and Medicaid, California Department of Motor Vehicles, Voter Registration, and National Change of Address. Active follow-up methods required contacting physician’s offices, hospitals, patient’s relatives, and patients. The follow-up period for this study began at HCC diagnosis and ended at the earlier of the date of death or last follow-up and December 31, 2013 (the end of the latest full year of case follow-up at the time these data were reported).
Statistical analysis
We used χ2 tests to examine bivariate relationships between race/ethnic groups and the variables displayed in Table 1. These variables included time period of diagnosis divided into five consecutive five-year intervals; age at diagnosis (< 50, 50-59, 60-69, 70-79, and 80 years or older); gender; geographical region (Los Angeles County, Greater San Francisco Bay Area, Central California, Northern California, and San Diego-Imperial-Orange Counties); stage of diagnosis (remote, regional, local, and unstaged); type of surgery (none, local, resection/transplant); and socioeconomic status (SES) on the basis of neighborhood income levels in quintiles. In categorizing type of surgery, resection and transplant were combined because SEER did not begin coding transplantation as a separate category until 1998.
Table 1.
Demographic and tumor characteristics by race/ethnic groups among patients with hepatocellular carcinoma in California, 1988-2012 (n = 33270)
| American Indian/Alaska Native | Black | Cambodian | Chinese | Filipino | Hawai`ian/Pacific Islander | Hispanic | Japanese | Korean | Laotian/Hmong | Other Asian | South Asian | Thai | Vietnamese | White | ||||||||||||||||
|
(n = 281) |
(n = 2609) |
(n = 233) |
(n = 2723) |
(n = 1479) |
(n = 172) |
(n = 8500) |
(n = 658) |
(n = 1099) |
(n = 244) |
(n = 214) |
(n = 190) |
(n = 95) |
(n = 2063) |
(n = 12710) |
||||||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Age at diagnosis | ||||||||||||||||||||||||||||||
| < 50 | 47 | 16.7 | 323 | 12.4 | 63 | 27.0 | 437 | 16.0 | 219 | 14.8 | 30 | 17.4 | 1170 | 13.8 | 30 | 4.6 | 172 | 15.7 | 65 | 26.6 | 44 | 20.6 | 13 | 6.8 | 29 | 30.5 | 326 | 15.8 | 1181 | 9.3 |
| 50-59 | 102 | 36.3 | 936 | 35.9 | 78 | 33.5 | 579 | 21.3 | 324 | 21.9 | 58 | 33.7 | 2604 | 30.6 | 116 | 17.6 | 284 | 25.8 | 62 | 25.4 | 58 | 27.1 | 44 | 23.2 | 27 | 28.4 | 533 | 25.8 | 3705 | 29.2 |
| 60-69 | 90 | 32.0 | 833 | 31.9 | 57 | 24.5 | 744 | 27.3 | 339 | 22.9 | 46 | 26.7 | 2424 | 28.5 | 219 | 33.3 | 346 | 31.5 | 72 | 29.5 | 59 | 27.6 | 65 | 34.2 | 22 | 23.2 | 596 | 28.9 | 3640 | 28.6 |
| 70-79 | 34 | 12.1 | 391 | 15.0 | 19 | 8.2 | 689 | 25.3 | 403 | 27.2 | 30 | 17.4 | 1657 | 19.5 | 206 | 31.3 | 233 | 21.2 | 31 | 12.7 | 44 | 20.6 | 45 | 23.7 | 13 | 13.7 | 471 | 22.8 | 2831 | 22.3 |
| ≥ 80 | 8 | 2.8 | 126 | 4.8 | 16 | 6.9 | 274 | 10.1 | 194 | 13.1 | 8 | 4.7 | 645 | 7.6 | 87 | 13.2 | 64 | 5.8 | 14 | 5.7 | 9 | 4.2 | 23 | 12.1 | -1 | -1 | 137 | 6.6 | 1353 | 10.6 |
| Gender | ||||||||||||||||||||||||||||||
| Male | 214 | 76.2 | 1971 | 75.6 | 182 | 78.1 | 2078 | 76.3 | 1111 | 75.1 | 138 | 80.2 | 6368 | 74.9 | 285 | 43.3 | 761 | 69.2 | 188 | 77.0 | 155 | 72.4 | 138 | 72.6 | 73 | 76.8 | 1622 | 78.6 | 9762 | 76.8 |
| Female | 67 | 23.8 | 638 | 24.4 | 51 | 21.9 | 645 | 23.7 | 368 | 24.9 | 34 | 19.8 | 2132 | 25.1 | 373 | 56.7 | 338 | 30.8 | 56 | 23.0 | 59 | 27.6 | 52 | 27.4 | 22 | 23.2 | 441 | 21.4 | 2948 | 23.2 |
| Socioeconomic status (SES) | ||||||||||||||||||||||||||||||
| 1 - Low SES | 75 | 26.7 | 867 | 33.2 | 113 | 48.5 | 377 | 13.8 | 204 | 13.8 | 30 | 17.4 | 2975 | 35.0 | 49 | 7.4 | 176 | 16.0 | 129 | 52.9 | 31 | 14.5 | 13 | 6.8 | 12 | 12.6 | 316 | 15.3 | 1599 | 12.6 |
| 2 | 82 | 29.2 | 586 | 24.8 | 42 | 18.0 | 428 | 15.7 | 286 | 19.3 | 42 | 24.4 | 2248 | 26.4 | 118 | 17.9 | 184 | 16.7 | 68 | 27.9 | 38 | 17.8 | 32 | 16.8 | 31 | 32.6 | 541 | 26.2 | 2512 | 19.8 |
| 3 | 72 | 25.6 | 483 | 18.5 | 33 | 14.2 | 488 | 17.9 | 367 | 24.8 | 40 | 23.3 | 1621 | 19.1 | 153 | 23.3 | 188 | 17.1 | 26 | 10.7 | 42 | 19.6 | 38 | 20.0 | 20 | 21.1 | 481 | 23.3 | 2977 | 23.4 |
| 4 | 36 | 12.8 | 409 | 15.7 | 26 | 11.2 | 660 | 24.2 | 402 | 27.2 | 34 | 19.8 | 1054 | 12.4 | 164 | 24.9 | 255 | 23.2 | 19 | 7.8 | 63 | 29.4 | 60 | 31.6 | 18 | 18.9 | 400 | 19.4 | 2942 | 23.1 |
| 5 - High SES | 16 | 5.7 | 203 | 7.8 | 19 | 8.2 | 770 | 28.3 | 220 | 14.9 | 26 | 15.1 | 602 | 7.1 | 174 | 26.4 | 296 | 26.9 | -1 | -1 | 40 | 18.7 | 47 | 24.7 | 14 | 14.7 | 325 | 15.8 | 2680 | 21.1 |
| Region | ||||||||||||||||||||||||||||||
| San Francisco- Oakland | 44 | 15.7 | 753 | 28.9 | 31 | 13.3 | 1482 | 54.4 | 517 | 35.0 | 62 | 36.0 | 1329 | 15.6 | 171 | 26.0 | 178 | 16.2 | 28 | 11.5 | 79 | 36.9 | 57 | 30.0 | 14 | 14.7 | 701 | 34.0 | 2637 | 20.7 |
| Central California | 78 | 27.8 | 335 | 12.8 | 24 | 10.3 | 78 | 2.9 | 109 | 7.4 | 21 | 12.2 | 2129 | 25.0 | 65 | 9.9 | 67 | 6.1 | 75 | 30.7 | 39 | 18.2 | 36 | 18.9 | 11 | 11.6 | 104 | 5.0 | 2705 | 21.3 |
| Northern California | 100 | 35.6 | 323 | 12.4 | 38 | 16.3 | 152 | 5.6 | 126 | 8.5 | 22 | 12.8 | 635 | 7.5 | 92 | 14.0 | 34 | 3.1 | 85 | 34.8 | 13 | 6.1 | 40 | 21.1 | 7 | 7.4 | 116 | 5.6 | 2578 | 20.3 |
| San Diego- Imperial-Orange | 33 | 11.7 | 206 | 7.9 | 22 | 9.4 | 173 | 6.4 | 263 | 17.8 | 28 | 16.3 | 1258 | 14.8 | 112 | 17.0 | 197 | 17.9 | 43 | 17.6 | 19 | 8.9 | 29 | 15.3 | 8 | 8.4 | 760 | 36.8 | 2208 | 17.4 |
| Los Angeles | 26 | 9.3 | 992 | 38.0 | 118 | 50.6 | 838 | 30.8 | 464 | 31.4 | 39 | 22.7 | 3149 | 37.0 | 218 | 33.1 | 623 | 56.7 | 13 | 5.3 | 64 | 29.9 | 28 | 14.7 | 55 | 57.9 | 382 | 18.5 | 2582 | 20.3 |
| Stage at diagnosis | ||||||||||||||||||||||||||||||
| Local | 109 | 38.8 | 987 | 37.8 | 96 | 41.2 | 1179 | 43.3 | 584 | 39.5 | 73 | 42.4 | 3825 | 45.0 | 288 | 43.8 | 450 | 40.9 | 71 | 29.1 | 102 | 47.7 | 84 | 44.2 | 38 | 40.0 | 947 | 45.9 | 5355 | 42.1 |
| Regional | 85 | 30.2 | 668 | 25.6 | 61 | 26.2 | 606 | 22.3 | 384 | 26.0 | 43 | 25.0 | 2010 | 23.6 | 142 | 21.6 | 267 | 24.3 | 52 | 21.3 | 48 | 22.4 | 53 | 27.9 | 22 | 23.2 | 498 | 24.1 | 2988 | 23.5 |
| Remote | 62 | 22.1 | 682 | 26.1 | 55 | 23.6 | 645 | 23.7 | 362 | 24.5 | 44 | 25.6 | 1812 | 21.3 | 149 | 22.6 | 242 | 22.0 | 88 | 36.1 | 49 | 22.9 | 40 | 21.1 | 24 | 25.3 | 437 | 21.2 | 2905 | 22.9 |
| Unstaged | 25 | 8.9 | 272 | 10.4 | 21 | 9.0 | 293 | 10.8 | 149 | 10.1 | 12 | 7.0 | 853 | 10.0 | 79 | 12.0 | 140 | 12.7 | 33 | 13.5 | 15 | 7.0 | 13 | 6.8 | 11 | 11.6 | 181 | 8.8 | 1462 | 11.5 |
| Time period of diagnosis | ||||||||||||||||||||||||||||||
| 1988-1992 | 9 | 3.2 | 217 | 8.3 | 22 | 9.4 | 334 | 12.3 | 161 | 10.9 | 14 | 8.1 | 557 | 6.6 | 84 | 12.8 | 114 | 10.4 | 29 | 11.9 | 9 | 4.2 | 12 | 6.3 | 7 | 7.4 | 128 | 6.2 | 1257 | 9.9 |
| 1993-1997 | 14 | 5.0 | 319 | 12.2 | 30 | 12.9 | 435 | 16.0 | 195 | 13.2 | 25 | 14.5 | 809 | 9.5 | 108 | 16.4 | 178 | 16.2 | 41 | 16.8 | 11 | 5.1 | 15 | 7.9 | 12 | 12.6 | 271 | 13.1 | 1681 | 13.2 |
| 1998-2002 | 49 | 17.4 | 472 | 18.1 | 45 | 19.3 | 543 | 19.9 | 304 | 20.6 | 32 | 18.6 | 1461 | 17.2 | 139 | 21.1 | 267 | 24.3 | 56 | 23.0 | 51 | 23.8 | 29 | 15.3 | 21 | 22.1 | 429 | 20.8 | 2252 | 17.7 |
| 2003-2007 | 88 | 31.3 | 683 | 26.2 | 61 | 26.2 | 695 | 25.5 | 408 | 27.6 | 41 | 23.8 | 2340 | 27.5 | 166 | 25.2 | 270 | 24.6 | 49 | 20.1 | 50 | 23.4 | 61 | 32.1 | 16 | 16.8 | 575 | 27.9 | 3208 | 25.2 |
| 2008-2012 | 121 | 43.1 | 918 | 35.2 | 75 | 32.2 | 716 | 26.3 | 411 | 27.8 | 60 | 34.9 | 3333 | 39.2 | 161 | 24.5 | 270 | 24.6 | 69 | 28.3 | 93 | 43.5 | 73 | 38.4 | 39 | 41.1 | 660 | 32.0 | 4312 | 33.9 |
| Type of surgery | ||||||||||||||||||||||||||||||
| None | 238 | 84.7 | 2214 | 84.9 | 198 | 85.0 | 2007 | 73.7 | 1220 | 82.5 | 138 | 80.2 | 7062 | 83.1 | 491 | 74.6 | 805 | 73.2 | 230 | 94.3 | 158 | 73.8 | 145 | 76.3 | 75 | 78.9 | 1536 | 74.4 | 10117 | 79.6 |
| Local | 20 | 7.1 | 150 | 5.7 | 13 | 5.6 | 184 | 6.8 | 60 | 4.1 | 10 | 5.8 | 550 | 6.5 | 58 | 8.8 | 83 | 7.6 | 7 | 2.9 | 16 | 7.5 | 18 | 9.5 | -1 | -1 | 194 | 9.4 | 925 | 7.3 |
| Resection/transplant | 23 | 8.2 | 245 | 9.4 | 22 | 9.4 | 532 | 19.5 | 199 | 13.5 | 24 | 14.0 | 888 | 10.4 | 109 | 16.6 | 211 | 19.2 | 7 | 2.9 | 40 | 18.7 | 27 | 14.2 | 16 | 16.8 | 333 | 16.1 | 1668 | 13.1 |
Less than 5 cases. χ2, P < 0.0001 for racial/ethnic differences in all variables tabulated.
Individual patient-level SES data are not collected by the CCR, and neighborhood SES was calculated using two methods. For cases diagnosed from 1988 through 2005, the index of SES was a composite variable created by principal components analysis using a number of variables from 1990 and 2000 Census data at the block group level. The Census variables used in creating the aggregate SES measure included: education index, median household income, proportion below 200% of the poverty level, median rent, median house value, proportion with a blue collar job, and proportion older than 16 in the workforce without a job. Block group quintiles based on statewide measurement of the SES variable were used in the analysis[31]. For cases diagnosed from 2006 through 2012, a composite variable was also created by principal components analysis using variables from the American Community Survey (ACS) at the block group level. The index used the following variables: education index, percent persons with a ratio of household income to poverty line 2 or higher (percent persons above 200% poverty line), percent persons with a blue collar job, percent persons employed, median rental, median value of owner-occupied housing unit, and median household income. The SES index could not be calculated if any of the seven components were missing. Missing values were imputed using multiple imputation, and the SES index was based on the imputed data[32].
The main difference between the two SES indexes is that the index based on the ACS used the inverse complement of two variables used from the 2000 Census: percent unemployed (2000 Census) and percent less than 200% of poverty line (ACS). Cases missing Census block group due to incomplete address at time of diagnosis (4.9% of patients) were randomly allocated to census block groups within county of residence because excluding these cases has been shown to bias results. Each case was assigned a neighborhood SES quintile, based on the distribution of SES across census block groups in California.
Logistic regression was used to evaluate the association between race/ethnicity and receipt of surgical treatment (any vs none) before and after adjustment for time period of diagnosis, age, gender, geographic region, SES quintile, and stage at diagnosis; because prioritization for transplantation for HCC changed in 2002[33], we included an interaction between time period and stage in the multivariable model, allowing estimation of stage effects for each time period and time period effects at the referent level of stage. Odds ratios (OR) and 95%CI are shown in Table 2.
Table 2.
Factors associated with receipt of surgical treatment for hepatocellular carcinoma in California, 1988-2012 (n = 33270)
|
Receipt of surgical treatment |
||||
| Unadjusted OR | 95%CI | Adjusted1 OR | 95%CI | |
| American Indian/Alaska Native | 0.71a | 0.51-0.98a | 0.66a | 0.46-0.93a |
| Black | 0.70a | 0.62-0.78a | 0.76a | 0.67-0.86a |
| Cambodian | 0.69a | 0.48-0.99a | 0.65a | 0.45-0.96a |
| Chinese | 1.39a | 1.27-1.53a | 1.58a | 1.42-1.77a |
| Filipino | 0.83a | 0.72-0.95a | 0.92 | 0.79-1.07 |
| Hawai`ian/Pacific Islander | 0.96 | 0.66-1.40 | 0.94 | 0.62-1.40 |
| Hispanic | 0.79a | 0.74-0.85a | 0.78a | 0.72-0.84a |
| Japanese | 1.33a | 1.11-1.59a | 1.41a | 1.15-1.72a |
| Korean | 1.43a | 1.24-1.64a | 1.45a | 1.24-1.70a |
| Laotian/Hmong | 0.24a | 0.14-0.41a | 0.30a | 0.17-0.53a |
| Other Asian | 1.38a | 1.02-1.88a | 1.27 | 0.91-1.78 |
| South Asian | 1.21 | 0.86-1.70 | 1.13 | 0.78-1.63 |
| Thai | 1.04 | 0.63-1.71 | 1.04 | 0.61-1.78 |
| Vietnamese | 1.34a | 1.20-1.49a | 1.26a | 1.12-1.42a |
| White | 1.00 | 1.00 | ||
| Age at diagnosis | ||||
| < 50 | 1.00 | |||
| 50-59 | 0.83a | 0.76-0.92a | ||
| 60-69 | 0.76a | 0.69-0.84a | ||
| 70-79 | 0.47a | 0.42-0.52a | ||
| ≥ 80 | 0.20a | 0.17-0.24a | ||
| Gender | ||||
| Female | 1.00 | |||
| Male | 0.82a | 0.77-0.88a | ||
| Socioeconomic Status | ||||
| 1 - Low SES | 1.00 | |||
| 2 | 1.25a | 1.13-1.37a | ||
| 3 | 1.29a | 1.17-1.42a | ||
| 4 | 1.65a | 1.49-1.82a | ||
| 5 - High SES | 1.84a | 1.66-2.05a | ||
| Region | ||||
| Los Angeles | 1.00 | |||
| San Francisco-Oakland | 0.81a | 0.74-0.88a | ||
| Central California | 0.96 | 0.87-1.05 | ||
| Northern California | 1.09 | 0.98-1.20 | ||
| San Diego-Imperial-Orange | 1.34a | 1.22-1.47a | ||
| Stage at diagnosis: 1988-1992 | ||||
| Remote | 1.00 | |||
| Regional | 3.30a | 2.29-4.75a | ||
| Local | 8.28a | 6.09-11.26a | ||
| Unstaged | 0.27a | 0.15-0.48a | ||
| Stage at diagnosis: 1993-1997 | ||||
| Remote | 1.00 | |||
| Regional | 2.88a | 2.02-4.11a | ||
| Local | 8.81a | 6.79-11.43a | ||
| Unstaged | 0.47a | 0.27-0.79a | ||
| Stage at diagnosis: 1998-2002 | ||||
| Remote | 1.00 | |||
| Regional | 2.61a | 2.01-3.40a | ||
| Local | 8.07a | 6.49-10.04a | ||
| Unstaged | 0.78 | 0.52-1.17 | ||
| Stage at diagnosis: 2003-2007 | ||||
| Remote | 1.00 | |||
| Regional | 4.44a | 3.44-5.73a | ||
| Local | 12.97a | 10.16-16.56a | ||
| Unstaged | 0.93 | 0.58-1.49 | ||
| Stage at diagnosis: 2008-2012 | ||||
| Remote | 1.00 | |||
| Regional | 4.50a | 3.43-5.92a | ||
| Local | 13.6a | 10.48-17.66a | ||
| Unstaged | 1.12 | 0.68-1.87 | ||
| Time period of diagnosis: remote stage tumors | ||||
| 1988-1992 | 1.00 | |||
| 1993-1997 | 0.75 | 0.53-1.06 | ||
| 1998-2002 | 0.89 | 0.64-1.23 | ||
| 2003-2007 | 0.83 | 0.58-1.17 | ||
| 2008-2012 | 0.59a | 0.41-0.85a | ||
Adjusted for all other factors presented in the table using multivariable logistic regression.
P < 0.05. Time period X stage interaction P = 0.0014.
Kaplan-Meier methods were used to estimate cause-specific and overall survival curves for each of the race/ethnic groups, and the log-rank test was used to assess racial/ethnic differences in survival. Median survival times with 95%CI are presented in Figure 1. Cox proportional hazards models were used to evaluate the association between race/ethnicity and survival, before and after adjustment for the effects of time period of diagnosis, age, gender, geographic region, SES quintile, stage at diagnosis, and type of surgery. Both cause-specific and all cause hazard ratios were calculated. Using non-Hispanic White as the referent group, hazard ratios (HR) and 95%CI were calculated for death from HCC. Survival time was measured in weeks from the date of diagnosis to death or censoring. People who were still alive on December 31, 2013 were censored on that date; in cause-specific survival analyses, because the outcome of interest was death due to HCC, people who died of other causes before that date were censored at date of death. All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC); statistical significance was assessed at the 0.05 level (2-sided).
Figure 1.
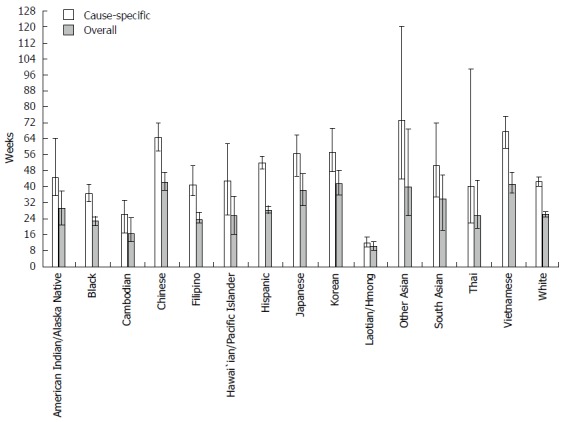
Median survival for patients with hepatocellular carcinoma by race/ethnicity in California, 1988-2012. Note: Error bars are limits of 95%CI.
RESULTS
Patient characteristics
A total of 33270 patients in the designated race/ethnic groups were diagnosed with HCC in California in 1988-2012 and reported to the CCR as of December 31, 2015. The largest number were identified as non-Hispanic White (n = 12710), followed by Hispanic (n = 8500), Chinese (n = 2723), non-Hispanic Black (n = 2609), Vietnamese (n = 2063), Filipino (n = 1479), and Korean (n = 1099). As shown in Table 1, the distributions of all patient characteristics differed significantly by race ethnicity (P < 0.0001). There was a male predominance of cases in all groups (69%-80%), except Japanese (43%). Overall, 12% were under age 50 at diagnosis, with highest proportions under 50 among Thai (31%), Cambodians (27%), and Laotian/Hmong (27%). There were substantial disparities in SES: those most likely to live in lowest quintile neighborhoods were Laotian/Hmong (53%), Cambodians (48%), Hispanics (35%), and Blacks (33%). There were also disparities in stage at diagnosis and receipt of treatment. Those least likely to be diagnosed with local stage tumors (43% overall) were Laotian/Hmong (29%), Blacks (38%), AIANs (39%), and Filipinos (39%). Those least likely to receive a resection or transplant (13% overall) were Laotian/Hmong (3%), AIANs (8%), Blacks (9%), and Cambodians (9%).
Receipt of surgical treatment
Compared to Whites, Laotian/Hmong (OR = 0.24, 95%CI: 0.14-0.41), Cambodians (OR = 0.69, 95%CI: 0.48-0.99), Blacks (OR = 0.70, 95%CI: 0.62-0.78), AIAN (OR = 0.71, 95%CI: 0.51-0.98), Hispanics (OR = 0.79, 95%CI: 0.74-0.85), and Filipinos (OR = 0.83, 95%CI: 0.72-0.95) were less likely, and Koreans (OR = 1.43, 95%CI: 1.24-1.64), Chinese (OR = 1.39, 95%CI: 1.27-1.53), Other Asians (OR = 1.38, 95%CI: 1.02-1.88), Vietnamese (OR = 1.34, 95%CI:1.20-1.49), and Japanese (OR = 1.33, 95%CI: 1.11-1.59) were more likely to receive surgical treatment (Table 2). After adjustment for demographic characteristics, time period, and stage of disease, Laotian/Hmong (OR = 0.30, 95%CI: 0.17-0.53), Cambodian (OR = 0.65, 95%CI: 0.45-0.96), AIAN (OR = 0.66, 95%CI: 0.46-0.93), Black (OR = 0.76, 95%CI: 0.67-0.86), and Hispanic (OR=0.78, 95%CI 0.72-0.84) patients were less likely, whereas Chinese (OR = 1.58, 95%CI:1.42-1.77), Koreans (OR = 1.45, 95%CI: 1.24-1.70), Japanese (OR = 1.41, 95%CI: 1.15-1.72), and Vietnamese (OR = 1.26, 95%CI: 1.12-1.42) were more likely to receive surgical treatment. The odds of treatment decreased with age (over 80 vs under 50: OR = 0.20, 95%CI: 0.17-0.24) and increased with SES (highest vs. lowest quintile: OR = 1.84, 95%CI: 1.66-2.05); males were less likely than females to be treated (OR = 0.82, 95%CI: 0.77-0.88). The time X stage interaction was statistically significant (P = 0.0014): patients with local stage disease had a greater advantage over those with remote stage disease in 2003-2012 (ORs = 13.0 and 13.6) than in 1988-2002 (ORs = 8.28, 8.81, and 8.07).
Survival
Kaplan-Meier analysis: Both cause-specific and overall survival differed significantly by race/ethnicity (log-rank P < 0.0001). Cause-specific median survival in weeks (Figure 1) was lowest for Laotian/Hmong (11.6, 95%CI: 9.6-14.4), followed by Cambodians (26.1, 95%CI: 16.7-33.1), Blacks (36.3, 95%CI: 32.1-41.0), Thai (39.9, 95%CI: 21.7-98.7), Filipinos (41.0, 95%CI: 35.4-50.0), Whites (42.4, 95%CI: 40.1-44.6), Hawai`ians/Pacific Islanders (42.7, 95%CI: 25.7-61.4), AIANs [44.6, 95%CI: (35.6-64.1)], South Asians (50.6, 95%CI: 34.4-72.0), Hispanics (51.9, 95%CI: 48.4-55.3), Japanese (56.3, 95%CI: 44.9-65.9), Koreans (57.4, 95%CI: 47.6-69.4), Chinese (64.4, 95%CI: 57.7-71.7), Vietnamese (67.3, 95%CI: 59.1-75.0), and Other Asians (73.3, 95%CI: 43.7-120.3). Results were similar for all cause survival.
Cox proportional hazards models: Compared to Whites, higher cause-specific mortality was experienced by Laotian/Hmong [hazard ratio (HR) = 1.91, 95%CI: 1.65-2.21], Cambodians (HR = 1.38, 95%CI: 1.18-1.60), and Blacks (HR = 1.12, 95%CI: 1.06-1.18), and lower mortality by Other Asians (HR = 0.80, 95%CI 0.67-0.96), Chinese (HR = 0.81, 95%CI: 0.77-0.86), Vietnamese (HR = 0.83, 95%CI: 0.79-0.89), Koreans (HR = 0.87, 95%CI: 0.80-0.93), and Hispanics (HR = 0.91, 95%CI: 0.88-0.95) (Table 3). After adjustment for demographics, time period, stage of disease, and treatment, mortality remained higher for Laotian/Hmong (HR = 1.50, 95%CI: 1.29-1.73), Cambodians (HR = 1.35, 95%CI: 1.16-1.58), and Blacks (HR = 1.07, 95%CI: 1.01-1.13), and was lower for Chinese (HR = 0.82, 95%CI: 0.77-0.86), Filipinos (HR = 0.84, 95%CI: 0.78-0.90), Vietnamese (HR = 0.85, 95%CI: 0.80-0.90), Koreans (HR = 0.90, 95%CI: 0.83-0.97), and Hispanics (HR = 0.91, 9 95%CI: 0.88-0.94). Lower mortality was associated with younger age, female gender, earlier stage disease, receipt of surgical treatment, higher SES, and later time period of diagnosis. Results were similar for all-cause mortality.
Table 3.
Factors associated with survival from hepatocellular carcinoma in California, 1988-2012 (n = 33270)
|
Cause-specific survival |
All cause survival |
|||||||
| Unadjusted HR | 95%CI | Adjusted1 HR | 95%CI | UnadjustedHR | 95%CI | Adjusted1 HR | 95%CI | |
| American Indian/ Alaska Native | 0.92 | 0.79-1.07 | 0.90 | 0.77-1.04 | 0.94 | 0.82-1.07 | 0.91 | 0.80-1.04 |
| Black | 1.12a | 1.06-1.18a | 1.07a | 1.01-1.13a | 1.12a | 1.07-1.17a | 1.06a | 1.01-1.11a |
| Cambodian | 1.38a | 1.18-1.60a | 1.35a | 1.16-1.58a | 1.31a | 1.15-1.51a | 1.27a | 1.11-1.46a |
| Chinese | 0.81a | 0.77-0.86a | 0.82a | 0.77-0.86a | 0.75a | 0.72-0.79a | 0.76a | 0.72-0.79a |
| Filipino | 0.95 | 0.88-1.01 | 0.84a | 0.78-0.90a | 0.98 | 0.92-1.04 | 0.88a | 0.83-0.93a |
| Hawai`ian/Pacific Islander | 0.95 | 0.78-1.15 | 0.98 | 0.81-1.19 | 0.97 | 0.82-1.14 | 1.00 | 0.85-1.18 |
| Hispanic | 0.91a | 0.88-0.95a | 0.91a | 0.88-0.94a | 0.98 | 0.95-1.01 | 0.95a | 0.92-0.99a |
| Japanese | 0.95 | 0.86-1.04 | 0.93 | 0.84-1.02 | 0.88a | 0.81-0.96a | 0.86a | 0.79-0.94a |
| Korean | 0.87a | 0.80-0.93a | 0.90a | 0.83-0.97a | 0.80a | 0.74-0.85a | 0.82a | 0.76-0.88a |
| Laotian/Hmong | 1.91a | 1.65-2.21a | 1.50a | 1.29-1.73a | 1.74a | 1.52-1.98a | 1.37a | 1.20-1.57a |
| Other Asian | 0.80a | 0.67-0.96a | 0.98 | 0.82-1.17 | 0.80a | 0.68-0.93a | 0.96 | 0.82-1.12 |
| South Asian | 0.91 | 0.75-1.09 | 0.88 | 0.74-1.06 | 0.93 | 0.79-1.09 | 0.91 | 0.78-1.06 |
| Thai | 1.00 | 0.77-1.29 | 1.08 | 0.84-1.40 | 0.96 | 0.77-1.21 | 1.04 | 0.82-1.30 |
| Vietnamese | 0.83a | 0.79-0.89a | 0.85a | 0.80-0.90a | 0.79a | 0.75-0.84a | 0.80a | 0.76-0.84a |
| White | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Age at diagnosis | ||||||||
| < 50 | 1.00 | 1.00 | ||||||
| 50-59 | 1.01 | 0.96-1.06 | 1.04 | 1.00-1.09 | ||||
| 60-69 | 1.06a | 1.01-1.11a | 1.08a | 1.04-1.13a | ||||
| 70-79 | 1.29a | 1.23-1.35a | 1.29a | 1.24-1.35a | ||||
| ≥ 80 | 1.54a | 1.45-1.64a | 1.57a | 1.49-1.65a | ||||
| Gender | ||||||||
| Female | 1.00 | 1.00 | ||||||
| Male | 1.10a | 1.07-1.14a | 1.09a | 1.06-1.12a | ||||
| Socioeconomic status | ||||||||
| 1 - Low SES | 1.00 | 1.00 | ||||||
| 2 | 0.95a | 0.92-0.99a | 0.94a | 0.91-0.98a | ||||
| 3 | 0.91a | 0.88-0.95a | 0.90a | 0.87-0.94a | ||||
| 4 | 0.88a | 0.84-0.92a | 0.86a | 0.83-0.89a | ||||
| 5 - High SES | 0.81a | 0.77-0.85a | 0.79a | 0.75-0.82a | ||||
| Region | ||||||||
| Los Angeles | 1.00 | 1.00 | ||||||
| San Francisco- Oakland | 1.03 | 0.99-1.07 | 1.00 | 0.97-1.03 | ||||
| Central California | 1.06a | 1.02-1.11a | 1.07a | 1.03-1.11a | ||||
| Northern California | 1.14a | 1.08-1.19a | 1.08a | 1.04-1.12a | ||||
| San Diego- Imperial-Orange | 1.09a | 1.05-1.14a | 1.08a | 1.04-1.13a | ||||
| Stage at diagnosis | ||||||||
| Remote | 1.00 | 1.00 | ||||||
| Regional | 0.70a | 0.67-0.73a | 0.71a | 0.69-0.74a | ||||
| Local | 0.39a | 0.38-0.41a | 0.44a | 0.43-0.46a | ||||
| Unstaged | 0.76a | 0.72-0.79a | 0.77a | 0.74-0.80a | ||||
| Time period of diagnosis | ||||||||
| 1988-1992 | 1.00 | 1.00 | ||||||
| 1993-1997 | 0.86a | 0.82-0.91a | 0.90a | 0.86-0.95a | ||||
| 1998-2002 | 0.75a | 0.71-0.79a | 0.77a | 0.74-0.81a | ||||
| 2003-2007 | 0.69a | 0.66-0.73a | 0.72a | 0.68-0.75a | ||||
| 2008-2012 | 0.54a | 0.51-0.57a | 0.57a | 0.54-0.60a | ||||
| Type of surgery | ||||||||
| None | 1.00 | 1.00 | ||||||
| Local | 0.40a | 0.38-0.43a | 0.43a | 0.41-0.46a | ||||
| Resection or transplant | 0.26a | 0.25-0.28a | 0.29a | 0.28-0.31a | ||||
Adjusted for all other factors presented in the table using Cox proportional hazards regression models.
P < 0.05.
DISCUSSION
We found substantial racial/ethnic variation in receipt of curative treatment, even after accounting for stage of disease and SES, which also varied considerably. These results are consistent with those of others[34], who also found that Blacks and Hispanics were less likely and Asians as a whole were more likely to receive treatment (although less likely to receive a transplant) than Whites. Patients with local stage disease were more likely to receive curative treatment than those with distant stage disease, and this advantage increased after changes to transplant guidelines in 2002 allowing for and increasing prioritization of transplantation in cases of local stage disease. Others have found that the change in guidelines did not lead to decreasing disparities in treatment[35].
We found that even after accounting for treatment differences, disparities in survival remained, with Blacks, Laotian/Hmong, and Cambodians experiencing significantly higher mortality than Whites, Hispanics, and most other Asian ethnicities. Those other Asian ethnic groups, such as Chinese and Vietnamese, have been the focus of longer histories of HBV screening than Blacks, Laotian/Hmong, and Cambodians and perhaps are the beneficiaries of earlier detection. Other studies have found Black-White differences to persist after adjustment for receipt of surgical treatment[19,22,36]. One study noted that even among transplant patients, Blacks had shorter and Asian/Pacific Islander patients had longer survival compared to Whites, with causes of death that suggested variation in the amount of immunosuppression accounted for differences in survival[19]. Consistent with others[37], we found that females had a survival advantage.
Differences in survival may be in part due to differences in comorbidities, stage of underlying liver disease, and etiology of HCC. A study using National Health and Nutrition Examination (NHANES) data found that risk factors for liver disease varied by race/ethnicity and gender, with Mexican Americans more likely than Blacks and Whites to have elevated aminotransferase activity, and Blacks more likely than Mexican Americans and Whites to have hepatitis B or C infection. Among men, Mexican Americans were more likely than Whites to be heavy/binge drinkers; among women, Mexican Americans and Blacks were more likely to be obese or diabetic but less likely to be heavy/binge drinkers than Whites[38]. Among East and Southeast Asians, HBV is the most common cause of HCC[1,20], except for Japanese, among whom HCV is more common[1]. HBV-associated HCC can occur without cirrhosis, which may confer a survival advantage as the typical complications of portal hypertension are not present[3].
HCC in the setting of a non-cirrhotic liver (NCL) is rare and has different etiologic, genetic, and pathologic characteristics from cirrhotic HCC, including a lower prevalence of HBV, HCV, and alcohol abuse, a lower rate of p53 mutation, and more advanced tumor stage at diagnosis[39]. Risk factors for the development of HCC in NCL include metabolic syndrome and non-alcoholic fatty liver disease, which may co-exist with viral hepatitis or alcohol abuse[40]. Hepatic resection is generally the best treatment choice for HCC patients with NCL, leading to better overall and disease-free survival than those of cirrhotic patients; in fact, survival after resection among NCL patients with non-advanced tumors is comparable to that of cirrhotic patients with early tumors who receive liver transplantation[39].
Compared to other etiologies, HCV-related HCC has been associated with poorer overall and recurrence-free survival after surgery[41]. Among patients with cirrhosis, those with chronic HCV experienced lower survival at 1, 3, and 5 years after liver transplantation compared to those without HCV. An accelerated progression to cirrhosis in HCV patients post-transplant may be responsible for this phenomenon seen in an era when treatment with interferon-based therapies was minimally effective in this population[42]. These outcomes will need to be revisited in the era of highly effective direct acting antiviral medications[41]. There is also evidence of racial differences in protein expression in HCV-associated HCC, indicating a possible biological mechanism for some disparities[43].
Other disparities in survival are likely due to differences in access to care and quality of treatment[18,36], as well as knowledge and attitudes regarding liver disease[44-46]. It is clear that gaps in both patient and provider knowledge lead to decreased screening and vaccination rates among those at risk for chronic hepatitis B[47-51]. Trends in earlier stage at diagnosis and leveling off of liver cancer incidence rates among Asians as a whole have been attributed to HBV testing and surveillance of those chronically infected[3,21]. Now that all-oral, curative treatment for HCV is available, HCV testing of people born in 1945-1965 is recommended[52]. Nevertheless, barriers to HBV and HCV testing and treatment remain[21,53-55]. However, attempts to intentionally link HBV screening results with linkage to care, while not optimized yet, are promising[56].
In summary, this paper reports on and analyzes 33270 HCC cases among Californians who were diagnosed over a 25-year period from 1988 to 2012. To our knowledge, these data represent the largest and most racially/ethnic diverse study of HCC cases collected through a registry with a Gold Certification (highest award) from the North American Association of Central Cancer Registries. Previously published reports of HCC cases utilizing the California Cancer Registry were focused on specific population groups, e.g., Asian Americans[23] or combined analyses of two population groups, e.g., Asian Americans and Hispanics[57] or were otherwise limited in numbers of cases, geographic scope, time period. Papers utilizing the SEER cancer registries, including those in California, were limited to a focus on a single racial category, e.g., American Indians and Alaska Natives[58] or did not analyze as many disaggregated ethnic groups. Our findings underscore the need for disaggregation-those least likely to be treated and those with the highest mortality were Asian, as were those most likely to be treated and those with the lowest mortality. Our analyses provided greater granularity by including as separate categories: Cambodian, Chinese, Filipino, Hawai`ian/Pacific Islander, Japanese, Korean, Laotian/Hmong, Other Asian, South Asian, Thai, and Vietnamese, almost all of whom are at higher risk for HCC compared to the population-at-large. The greater granularity also enabled us to specifically identify populations-at-risk who share common socio-ethnic characteristics. Thus, the findings from this paper offer the potential for more precise targeting of interventions by ethnic group, and hence language preference, and geographical area.
Despite the advantages of being able to access and analyze the largest cancer registry in a geographically contiguous political jurisdiction, we recognize several limitations. We were not able to assess racial/ethnic differences in receipt of transplant over this time period because the CCR did not distinguish between transplant and resection until 1998. The CCR does not include data on risk factors, such as exposure to viral infections, cirrhosis, alcohol consumption, or documentation of an individual’s metabolic syndrome/diabetes or body mass index as a measure of obesity. These latter risk factors are increasingly influential in HCC etiology[11]. No data are captured regarding the patients’ English fluency or other potential measures of acculturation and access to care. The aggregate socioeconomic status variables are not measures of the individual patients but rather that of their Census block group[31,32]. Although the CCR employs extensive follow-up procedures it is possible that some patients returned to their home countries to die[59]. Finally, there was limited power to detect treatment and survival differences for racial/ethnic groups with small numbers of cases.
In conclusion, nonetheless these findings demonstrate substantial racial/ethnic disparities in HCC treatment and survival that were not explained by disease stage, time period of diagnosis, or socio-demographic factors. Continued effort is required to improve access and attitudes towards HBV and HCV testing and follow-up, address other etiological risk factors such as alcoholism and obesity, develop targeted therapies, and provide high quality treatment to all patients.
COMMENTS
Background
Cancer of the liver and intrahepatic bile duct, of which approximately 80% is hepatocellular carcinoma (HCC), led the 17 most common cancer sites with a 3.1% average annual increase in mortality rates between 2008 and 2012 among both men and women in the United States.
Research frontiers
The authors’ previous study of more than 6000 HCC cases diagnosed in California in 1988-2007, which reported on specific Asian ethnicities, found substantial inter-ethnic variation in survival but did not include comparison with other racial/ethnic groups. Rarely have HCC survival and treatment characteristics been characterized for 15 race/ethnic groups in a large geographically contiguous area over a period of 25 years.
Innovations and breakthroughs
This paper reports on and analyzes 33270 HCC cases among Californians who were diagnosed over a 25-year period from 1988 to 2012. To the best knowledge of the authors, these data represent the largest and most racially/ethnic diverse study of HCC cases collected through a Gold Certification (highest award) North American Association of Central Cancer Registries.
Applications
Nonetheless these findings demonstrate substantial racial/ethnic disparities in HCC treatment and survival that were not explained by disease stage, time period of diagnosis, or socio-demographic factors.
Peer-review
Non-cirrhotic liver with HCC has a different prognosis from a liver cirrhosis and HCV-related cirrhosis has a different survival rates in comparison to other etiologies.
ACKNOWLEDGMENTS
The production of this paper was supported in part by a cooperative agreement from the National Cancer Institute, U54CA153499, but the views of this paper are those of the authors and not necessarily those of the National Cancer Institute.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest relevant to this article were reported.
Data sharing statement: No additional data are available.
Peer-review started: June 28, 2016
First decision: July 29, 2016
Article in press: September 12, 2016
P- Reviewer: Santambrogio R S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver. 2016;10:332–339. doi: 10.5009/gnl15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61:191–199. doi: 10.1002/hep.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248:171–183. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen MS Jr, Dang J. Hepatitis B among Asian Americans: Prevalence, progress, and prospects for control. World J Gastroenterol. 2015;21:11924–11930. doi: 10.3748/wjg.v21.i42.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 9.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 10.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 11.Waller LP, Deshpande V, Pyrsopoulos N. Hepatocellular carcinoma: A comprehensive review. World J Hepatol. 2015;7:2648–2663. doi: 10.4254/wjh.v7.i26.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109:542–553. doi: 10.1038/ajg.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 14.Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol. 2016;34:1787–1794. doi: 10.1200/JCO.2015.64.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen MS Jr. Preventing Hepatitis B-induced Liver Cancer: Implications for Eliminating Health Disparities. J Health Dispar Res Pract. 2010;4:88–99. [PMC free article] [PubMed] [Google Scholar]
- 16.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) SEER Cancer Statistics Review, 1975-2013. National Cancer Institute: Surveillance, Epidemiology, and End Results Program; 2016. Available from: http://seer.cancer.gov/csr/1975_2013/ [Google Scholar]
- 17.Rodriguez DN, Torruellas C, Cress RD. Trends in early-stage hepatocellular carcinoma, California 1988-2010. Cancer Causes Control. 2016;27:325–331. doi: 10.1007/s10552-015-0705-2. [DOI] [PubMed] [Google Scholar]
- 18.Harlan LC, Parsons HM, Wiggins CL, Stevens JL, Patt YZ. Treatment of hepatocellular carcinoma in the community: disparities in standard therapy. Liver Cancer. 2015;4:70–83. doi: 10.1159/000367729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Njei B, Ditah I, Lim JK. Persistent racial disparities in survival among u.s. Adults with hepatocellular carcinoma after liver transplantation: the paradox of all-cause and cause-specific mortality. Gastrointest Cancer Res. 2013;6:73–74. [PMC free article] [PubMed] [Google Scholar]
- 20.Wong RJ, Corley DA. Survival differences by race/ethnicity and treatment for localized hepatocellular carcinoma within the United States. Dig Dis Sci. 2009;54:2031–2039. doi: 10.1007/s10620-008-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ha J, Yan M, Aguilar M, Bhuket T, Tana MM, Liu B, Gish RG, Wong RJ. Race/ethnicity-specific disparities in cancer incidence, burden of disease, and overall survival among patients with hepatocellular carcinoma in the United States. Cancer. 2016;122:2512–2523. doi: 10.1002/cncr.30103. [DOI] [PubMed] [Google Scholar]
- 22.Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98:1934–1939. [PMC free article] [PubMed] [Google Scholar]
- 23.Kwong SL, Stewart SL, Aoki CA, Chen MS Jr. Disparities in hepatocellular carcinoma survival among Californians of Asian ancestry, 1988 to 2007. Cancer Epidemiol Biomarkers Prev. 2010;19:2747–2757. doi: 10.1158/1055-9965.EPI-10-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwong SL, Chen MS Jr, Snipes KP, Bal DG, Wright WE. Asian subgroups and cancer incidence and mortality rates in California. Cancer. 2005;104:2975–2981. doi: 10.1002/cncr.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NAACCR Race and Ethnicity Work Group. NAACCR Asian Pacific Islander identification algorithm (NAPIIA v1.2.1). Springfield, IL: North American Association of Central Cancer Registries, 2011 [Google Scholar]
- 26.NAACCR Race and Ethnicity Work Group. NAACCR guideline for enhancing Hispanic/Latino identification: revised NAACR Hispanic/Latino identification algorithm (NHIA v2.2.1). Springfield, IL: North American Association of Central Cancer Registries, 2011 [Google Scholar]
- 27.Fang DM, Lee S, Stewart S, Ly MY, Chen MS Jr. Factors associated with pap testing among Hmong women. J Health Care Poor Underserved. 2010;21:839–850. doi: 10.1353/hpu.0.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang RC, Mills PK, Riordan DG. Cervical cancer among Hmong women in California, 1988 to 2000. Am J Prev Med. 2004;27:132–138. doi: 10.1016/j.amepre.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Shankar LD, Srikanth R. A Part, Yet Apart. South Asians in Asian America: Temple University Press, 1998 [Google Scholar]
- 30.Parikh-Patel A, Mills PK, Jain RV. Breast cancer survival among South Asian women in California (United States) Cancer Causes Control. 2006;17:267–272. doi: 10.1007/s10552-005-0520-2. [DOI] [PubMed] [Google Scholar]
- 31.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–711. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Schupp CW, Harrati A Clark C, Keegan THM, Gomez SL. Developing an area-based socioeconomic measure from American Community Survey data. Fremont, California: Cancer Prevention Institute of California, 2014 [Google Scholar]
- 33.Robbins AS, Daily MF, Aoki CA, Chen MS Jr, Troppmann C, Perez RV. Decreasing disparity in liver transplantation among white and Asian patients with hepatocellular carcinoma: California, 1998-2005. Cancer. 2008;113:2173–2179. doi: 10.1002/cncr.23766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ha J, Yan M, Aguilar M, Tana M, Liu B, Frenette CT, Bhuket T, Wong RJ. Race/Ethnicity-specific Disparities in Hepatocellular Carcinoma Stage at Diagnosis and its Impact on Receipt of Curative Therapies. J Clin Gastroenterol. 2016;50:423–430. doi: 10.1097/MCG.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 35.Robbins AS, Cox DD, Johnson LB, Ward EM. Persistent disparities in liver transplantation for patients with hepatocellular carcinoma in the United States, 1998 through 2007. Cancer. 2011;117:4531–4539. doi: 10.1002/cncr.26063. [DOI] [PubMed] [Google Scholar]
- 36.Mathur AK, Osborne NH, Lynch RJ, Ghaferi AA, Dimick JB, Sonnenday CJ. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg. 2010;145:1158–1163. doi: 10.1001/archsurg.2010.272. [DOI] [PubMed] [Google Scholar]
- 37.Dohmen K, Shigematsu H, Irie K, Ishibashi H. Longer survival in female than male with hepatocellular carcinoma. J Gastroenterol Hepatol. 2003;18:267–272. doi: 10.1046/j.1440-1746.2003.02936.x. [DOI] [PubMed] [Google Scholar]
- 38.Flores YN, Yee HF, Leng M, Escarce JJ, Bastani R, Salmerón J, Morales LS. Risk factors for chronic liver disease in Blacks, Mexican Americans, and Whites in the United States: results from NHANES IV, 1999-2004. Am J Gastroenterol. 2008;103:2231–2238. doi: 10.1111/j.1572-0241.2008.02022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trevisani F, Frigerio M, Santi V, Grignaschi A, Bernardi M. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis. 2010;42:341–347. doi: 10.1016/j.dld.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Schütte K, Schulz C, Poranzke J, Antweiler K, Bornschein J, Bretschneider T, Arend J, Ricke J, Malfertheiner P. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117. doi: 10.1186/1471-230X-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shindoh J, Hashimoto M, Watanabe G. Surgical approach for hepatitis C virus-related hepatocellular carcinoma. World J Hepatol. 2015;7:70–77. doi: 10.4254/wjh.v7.i1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bozorgzadeh A, Orloff M, Abt P, Tsoulfas G, Younan D, Kashyap R, Jain A, Mantry P, Maliakkal B, Khorana A, et al. Survival outcomes in liver transplantation for hepatocellular carcinoma, comparing impact of hepatitis C versus other etiology of cirrhosis. Liver Transpl. 2007;13:807–813. doi: 10.1002/lt.21054. [DOI] [PubMed] [Google Scholar]
- 43.Dillon ST, Bhasin MK, Feng X, Koh DW, Daoud SS. Quantitative proteomic analysis in HCV-induced HCC reveals sets of proteins with potential significance for racial disparity. J Transl Med. 2013;11:239. doi: 10.1186/1479-5876-11-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maxwell AE, Stewart SL, Glenn BA, Wong WK, Yasui Y, Chang LC, Taylor VM, Nguyen TT, Chen MS Jr, Bastani R. Theoretically informed correlates of hepatitis B knowledge among four Asian groups: the health behavior framework. Asian Pac J Cancer Prev. 2012;13:1687–1692. doi: 10.7314/apjcp.2012.13.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burnham B, Wallington S, Jillson IA, Trandafili H, Shetty K, Wang J, Loffredo CA. Knowledge, attitudes, and beliefs of patients with chronic liver disease. Am J Health Behav. 2014;38:737–744. doi: 10.5993/AJHB.38.5.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Safo SA, Batchelder A, Peyser D, Litwin AH. The common sense model applied to hepatitis C: a qualitative analysis of the impact of disease comparison and witnessed death on hepatitis C illness perception. Harm Reduct J. 2015;12:20. doi: 10.1186/s12954-015-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H, Tu SP, Teh CZ, Yip MP, Choe JH, Hislop TG, Taylor VM, Thompson B. Lay beliefs about hepatitis among North American Chinese: implications for hepatitis prevention. J Community Health. 2006;31:94–112. doi: 10.1007/s10900-005-9000-6. [DOI] [PubMed] [Google Scholar]
- 48.Wu CA, Lin SY, So SK, Chang ET. Hepatitis B and liver cancer knowledge and preventive practices among Asian Americans in the San Francisco Bay Area, California. Asian Pac J Cancer Prev. 2007;8:127–134. [PubMed] [Google Scholar]
- 49.Chao SD, Wang BM, Chang ET, Ma L, So SK. Medical training fails to prepare providers to care for patients with chronic hepatitis B infection. World J Gastroenterol. 2015;21:6914–6923. doi: 10.3748/wjg.v21.i22.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robotin M, Patton Y, George J. Getting it right: the impact of a continuing medical education program on hepatitis B knowledge of Australian primary care providers. Int J Gen Med. 2013;6:115–122. doi: 10.2147/IJGM.S41299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Upadhyaya N, Chang R, Davis C, Conti MC, Salinas-Garcia D, Tang H. Chronic hepatitis B: perceptions in Asian American communities and diagnosis and management practices among primary care physicians. Postgrad Med. 2010;122:165–175. doi: 10.3810/pgm.2010.09.2213. [DOI] [PubMed] [Google Scholar]
- 52.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, Jewett A, Baack B, Rein DB, Patel N, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1–32. [PubMed] [Google Scholar]
- 53.Zeremski M, Zibbell JE, Martinez AD, Kritz S, Smith BD, Talal AH. Hepatitis C virus control among persons who inject drugs requires overcoming barriers to care. World J Gastroenterol. 2013;19:7846–7851. doi: 10.3748/wjg.v19.i44.7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ha NB, Trinh HN, Nguyen TT, Leduc TS, Bui C, Ha NB, Wong CR, Tran AT, Nguyen MH. Prevalence, risk factors, and disease knowledge of chronic hepatitis B infection in Vietnamese Americans in California. J Cancer Educ. 2013;28:319–324. doi: 10.1007/s13187-013-0466-0. [DOI] [PubMed] [Google Scholar]
- 55.Ditah I, Al Bawardy B, Gonzalez HC, Saberi B, Ditah C, Kamath PS, Charlton M. Lack of health insurance limits the benefits of hepatitis C virus screening: insights from the National Health and Nutrition Examination Hepatitis C follow-up study. Am J Gastroenterol. 2015;110:1126–1133. doi: 10.1038/ajg.2015.31. [DOI] [PubMed] [Google Scholar]
- 56.Dang JH, Chen MS Jr. Increasing Hepatitis B Testing and Linkage to Care of Foreign-Born Asians, Sacramento, California, 2012-2013. Public Health Rep. 2016;131 Suppl 2:119–124. doi: 10.1177/00333549161310S218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang ET, Yang J, Alfaro-Velcamp T, So SK, Glaser SL, Gomez SL. Disparities in liver cancer incidence by nativity, acculturation, and socioeconomic status in California Hispanics and Asians. Cancer Epidemiol Biomarkers Prev. 2010;19:3106–3118. doi: 10.1158/1055-9965.EPI-10-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jim MA, Perdue DG, Richardson LC, Espey DK, Redd JT, Martin HJ, Kwong SL, Kelly JJ, Henderson JA, Ahmed F. Primary liver cancer incidence among American Indians and Alaska Natives, US, 1999-2004. Cancer. 2008;113:1244–1255. doi: 10.1002/cncr.23728. [DOI] [PubMed] [Google Scholar]
- 59.Gomez SL, Clarke CA, Shema SJ, Chang ET, Keegan TH, Glaser SL. Disparities in breast cancer survival among Asian women by ethnicity and immigrant status: a population-based study. Am J Public Health. 2010;100:861–869. doi: 10.2105/AJPH.2009.176651. [DOI] [PMC free article] [PubMed] [Google Scholar]


