Abstract
AIM
To reveal better diagnostic markers for differentiating neuroendocrine tumor (NET) from solid-pseudopapillary neoplasm (SPN), focusing primarily on immunohistochemical analysis.
METHODS
We reviewed 30 pancreatic surgical specimens of NET (24 cases) and SPN (6 cases). We carried out comprehensive immunohistochemical profiling using 9 markers: Synaptophysin, chromogranin A, pan-cytokeratin, E-cadherin, progesterone receptor, vimentin, α-1-antitrypsin, CD10, and β-catenin.
RESULTS
E-cadherin staining in NETs, and nuclear labeling of β-catenin in SPNs were the most sensitive and specific markers. Dot-like staining of chromogranin A might indicate the possibility of SPNs rather than NETs. The other six markers were not useful because their expression overlapped widely between NETs and SPNs. Moreover, two cases that had been initially diagnosed as NETs on the basis of their morphological features, demonstrated SPN-like immunohistochemical profiles. Careful diagnosis is crucial as we actually found two confusing cases showing disagreement between the tumor morphology and immunohistochemical profiles.
CONCLUSION
E-cadherin, chromogranin A, and β-catenin were the most useful markers which should be employed for differentiating between NET and SPN.
Keywords: Neuroendocrine tumor, Pancreas, Solid-pseudopapillary neoplasm, Immunohistochemistry, Diagnosis
Core tip: Neuroendocrine tumor (NET) and solid-pseudopapillary neoplasm (SPN) are two types of pancreatic tumor that were sometimes confused in differential diagnosis. We reviewed 30 pancreatic surgical specimens of NET (24 cases) and SPN (6 cases). We carried out comprehensive immunohistochemical profiling using 9 markers. E-cadherin staining in NETs, and nuclear labeling of β-catenin in SPNs were the most sensitive and specific markers. Dot-like staining of chromogranin A might indicate the possibility of SPNs rather than NETs. Moreover, two cases that had been initially diagnosed as NETs on the basis of their morphological features, demonstrated SPN-like immunohistochemical profiles.
INTRODUCTION
Neuroendocrine tumor (NET) and solid-pseudopapillary neoplasm (SPN) of the pancreas differ from each other significantly in terms of tumor aggressiveness, treatment, and prognosis. NETs are considered to have malignant potential, as 40%-90% of them (excluding insulinoma) show gross invasive growth or metastasis[1,2], whereas SPNs have low grade malignancy, exhibiting local invasion or metastasis in only 20% of cases[3]. Consequently, the five-year survival rate for patients with NETs is worse than that for patients with SPNs (65% of NETs vs 95% of SPNs)[3,4]. Surgical resection is the mainstay treatment for both types of tumor, irrespective of whether they are localized or metastatic. For patients with unresectable NET, chemotherapy using agents such as somatostatin analogues, α-interferon, sunitinib, or mTOR inhibitor can be applied[5,6]. On the other hand, clinical trials have yet to yield an effective form of chemotherapy for unresectable SPNs[7]. Due to these differences in clinical features and strategies, careful differentiation between NETs and SPNs is needed, as this can have a crucial bearing on outcome.
Morphological structures have often been the key guide for differentiating SPNs from NETs. SPNs have a distinctive appearance, exhibiting solid and pseudopapillary growth patterns. Grossly, they appear as a large encapsulated mass, containing yellowish solid areas, with cystic zones that are frequently necrotic or hemorrhagic[8]. Microscopically, this heterogeneous growth pattern demonstrates solid areas with alternating pseudopapillary structures, together with cystic spaces made up of poorly cohesive monomorphic cells with abundant degenerative changes[9,10]. However, SPNs can show considerable morphological overlap with NETs. That is, a proportion of NET cases may show cystic and necrotic areas composed of discohesive cells, and a proportion of SPN cases may show a predominantly solid growth pattern without pseudopapillary structures[9,11]. In a review of a case series, Liu et al[12] reported that three of 14 NETs had cystic components, whereas one of 10 SPNs did not exhibit cystic areas. Accordingly, it was not possible to distinguish these two types of tumor simply on the basis of their gross or microscopic features.
In addition to morphological evaluation, immunohistochemical analysis plays a crucial role in differentiating these two tumor types. Klimstra et al[13,14] have reported a simple algorithm for diagnostic evaluation of pancreatic neoplasms, and demonstrated that immunohistochemstry based on the direction of tumor differentiation can be useful for establishment of tumor entities. Basturk et al[15] reviewed earlier reports indicating that synaptophysin, chromogranin A, pan-cytokeratin, and E-cadherin were markers for NETs, whereas progesterone receptor, vimentin, α-1-antitrypsin, CD10, and nuclear labeling of β-catenin were markers for SPNs. However, the expressions of these markers show overlap between NETs and SPNs[12,16,17]. For example, synaptophysin, a neuroendocrine marker expressed in most NETs, is also expressed in a number of SPNs[18,19]. As the sensitivity and specificity of the markers used in earlier studies have varied, more practical procedures for correct diagnosis are anticipated.
In the present study, we reviewed the morphological and immunohistochemical profiles of 30 pancreatic tumors including 24 cases of NET and 6 cases of SPN. We comprehensively surveyed the usefulness of 9 markers in order to derive better diagnostic procedures for differentiating between the two tumor types. As a result, we discovered two confusing cases which showed both NET-like morphology and SPN-like immunohistochemical profiles, indicating the difficulties in differential diagnosis.
MATERIALS AND METHODS
Patients and tissue samples
Surgical specimens of primary NETs (24 cases) and SPNs (6 cases) of the pancreas were obtained at the Department of Surgery, University of Tsukuba Hospital, Tsukuba, Japan, between 2002 and 2011. All of the patients provided informed consent for analysis of their tissue samples in accordance with the ethics committee of University of Tsukuba Hospital. The specimens had been originally diagnosed by at least two pathologists in accordance with the World Health Organization (WHO) classification[10,20]. The grading of the NETs was re-evaluated on the basis of the WHO classification 2010, using the mitotic count and Ki-67 labeling index. Cases of Grade 1 and Grade 2 NET were included in the present study, but Grade 3 cases (neuroendocrine carcinoma) were excluded.
Immunohistochemical procedures
We performed immunohistochemical staining for synaptophysin, chromogranin A, pan-cytokeratin, E-cadherin, progesterone receptor, vimentin, α-1-antitrypsin, CD10, β-catenin, and Ki-67. Briefly, the resected tissues were fixed in 10% formalin and embedded in paraffin blocks, and the most representative block was chosen for each case. Each block was cut into serial sections 2 μm thick, and then deparaffinized with xylene and rehydrated with ethanol. After antigen retrieval and blocking of endogenous peroxidase activity with hydrogen peroxide, the sections were incubated with the primary antibodies (listed in Table 1, along with the antigen retrieval methods, dilutions, and incubation methods). All antigens except for progesterone receptor were detected using the EnVision+ System-HRP (Dako Japan, Tokyo, Japan); progesterone receptor was detected using an ultraView DAB universal kit (Roche Diagnostics, Tokyo, Japan). After visualization with diaminobenzidine chromogen, the sections were counterstained with hematoxylin, dehydrated with ethanol, made transparent with xylene, and mounted. All specimens were also stained with hematoxylin and eosin (H&E).
Table 1.
Immunohistochemical procedures
| Antigen (Clone) | Manufacturer | Antigen retrieval | Dilution | Incubation |
| solution/temperature/time | temperature/time | |||
| Synaptophysin (27G12) | Nichirei Biosciences, Tokyo, Japan | TB/105 °C/10 min | Prediluted | RT/30 min |
| Chromogranin A (polyclonal) | Dako Japan, Tokyo, Japan | CB/115 °C/10 min | 1:500 | RT/30 min |
| Pan-cytokeratin (AE1/AE3) | Dako Japan, Tokyo, Japan | TB/105 °C/10 min | 1:100 | RT/30 min |
| E-cadherin (36B5) | Thermo Scientific, Yokohama, Japan | TB/121 °C/10 min | 1:20 | RT/60 min |
| Progesterone receptor (IE2) | Roche Diagnostics, Tokyo, Japan | CB/115 °C/10 min | Prediluted | RT/30 min |
| Vimentin (V9) | Dako Japan, Tokyo, Japan | CB/100 °C/15 min | 1:100 | RT/30 min |
| α-1-antitrypsin (polyclonal) | Dako Japan, Tokyo, Japan | No retrieval | 1:100 | RT/30 min |
| CD10 (56C6) | Leica Microsystems, Tokyo, Japan | TB/105 °C/10 min | 1:40 | RT/30 min |
| β-catenin (β-Catenin-1) | Dako Japan, Tokyo, Japan | TB/105 °C/10 min | 1:200 | RT/30 min |
| Ki-67 (MIB-1) | Dako Japan, Tokyo, Japan | CB/100 °C/30 min | 1:25 | 4 °C/overnight |
TB: Tris-HCl buffer; CB: Citrate buffer; RT: Room temperature.
Interpretation of immunohistochemical staining and morphological features
Synaptophysin, chromogranin A, pan-cytokeratin, E-cadherin, vimentin, and α-1-antitrypsin were assessed for membranous and/or cytoplasmic staining. For progesterone receptor and β-catenin staining, nuclear labeling was evaluated as positive expression. All immunohistochemical markers except for Ki-67 were classified as strongly positive (++), weakly positive (+), or negative (-). Briefly, the distribution (no stain: 0-1%, focal: 2%-50%, or diffuse: 51%-100%) and the intensity (weak or strong) of cells in the stained sections were determined separately. Diffuse distribution with strong intensity was evaluated as strongly positive. Diffuse-weak, focal-strong or focal-weak combinations were all evaluated as weakly positive. No stain was evaluated as negative. For Ki-67 staining, nuclear labeling index of Ki-67 per 500-2000 cells was counted. Mitotic count was evaluated in at least 50 high-power fields using HE staining. Gross morphologic features were evaluated by analyzing pictures of the resected tumors and H&E-stained sections.
RESULTS
Clinicopathological and morphological characteristics
The clinicopathological data for the examined cases are listed in Table 2. Postoperatively, 24 cases had been originally diagnosed as NET (NET-1 to NET-24), and 6 as SPN (SPN-1 to SPN-6). The mean age of the patients was 54 (range 31-77) years in the NET group, and 28.8 (range 20-43) years in the SPN group. In the NET group, 11 patients were male and 13 were female, whereas in the SPN group one was male and 5 were female. In the NET group, 4 cases (NET-1 to NET-4) were clinically diagnosed as multiple endocrine neoplasia type 1 (MEN1), 6 (NET-5 to NET-10) were diagnosed as insulinoma with clinical symptoms (e.g., hypoglycemia), and the other 14 (NET-11 to NET-24) did not have any genetic background, symptoms or blood examination data attributable to hormone hypersecretion. Preoperative enhanced computed tomography were performed in all cases, most of NET cases showed the enhancement of tumor in early phase, but SPN cases did not. Preoperative biopsy using ultrasonography was not performed in all cases. The mean tumor diameter of the surgical specimens was 3.0 (range 0.5-16) cm in the NET group, and 7.4 (range 3.8-13) cm in the SPN group. Assessment of tumor grading of NETs according to the mitotic count and Ki-67 labeling index showed that 19 cases were Grade 1, and 5 were Grade 2. In the SPN group, mitosis or Ki-67 labeling of cells was scarcely evident in the sections. At the end of 2012, metastasis and/or recurrence were found in 6 cases of NETs (NET-2, NET-3, NET-15, NET-20, NET-21, and NET-22), whereas they did not occur in SPN cases. Metastatic or recurrent NETs were treated by reoperation, chemotherapy and/or radiotherapy after initial operation.
Table 2.
Clinicopathological and morphological characteristics of neuroendocrine tumor (24 cases) and solid-pseudopapillary neoplasm (6 cases)
| Case | Age | Sex | Clinical findings | Tumor location | Operation | Diameter (cm) | Mitotic | Ki-67 | Grade | Solid or | PP |
| (yr) | count | (%) | for NETs | Cystic | pattern | ||||||
| NET-1 | 31 | F | MEN1 | H | PD | 4.5 | 0 | 0 | 1 | Solid and Cystic | Absent |
| NET-2 | 57 | F | MEN1 | H, T | EC, DP | 5.3 | 0 | 1 | 1 | Solid | Absent |
| NET-3 | 51 | F | MEN1 | H, B, T, L | EC, DP, LR | 6.0 | 0 | 0 | 1 | Solid | Absent |
| NET-4 | 45 | F | MEN1 | B, T | DP | 1.4 | 0 | 0 | 1 | Solid and Cystic | Absent |
| NET-5 | 42 | F | insulinoma | B | EC | 1.0 | 0 | 0 | 1 | Solid | Absent |
| NET-6 | 62 | F | insulinoma | H | EC | 1.2 | 0 | 0 | 1 | Solid | Absent |
| NET-7 | 73 | M | insulinoma | H | PD | 1.5 | 0 | 0 | 1 | Solid | Absent |
| NET-8 | 74 | F | insulinoma | T | DP | 1.0 | 0 | 0 | 1 | Solid | Absent |
| NET-9 | 67 | M | insulinoma | B | EC | 0.9 | 1 | 0 | 1 | Solid | Absent |
| NET-10 | 51 | F | insulinoma | B | MP | 1.1 | 7 | 2 | 2 | Solid | Absent |
| NET-11 | 45 | M | H | EC | 2.3 | 0 | 0 | 1 | Solid | Absent | |
| NET-12 | 68 | F | H | EC | 0.5 | 0 | 0 | 1 | Solid | Absent | |
| NET-13 | 77 | M | T | DP | 0.9 | 1 | 0 | 1 | Solid | Absent | |
| NET-14 | 57 | M | H | PD | 1.5 | 1 | 0 | 1 | Solid | Absent | |
| NET-15 | 45 | M | H | EC | 0.9 | 2 | 0 | 1 | Solid | Absent | |
| NET-16 | 51 | F | H | PD | 2.5 | 0 | 1 | 1 | Solid | Absent | |
| NET-17 | 59 | M | H | MP | 1.8 | 1 | 1 | 1 | Solid | Absent | |
| NET-18 | 59 | F | T | EC | 2.3 | 1 | 1 | 1 | Solid and Cystic | Absent | |
| NET-19 | 40 | F | T | DP | 3.3 | 0 | 4 | 2 | Solid and Cystic | Absent | |
| NET-20 | 53 | M | T | DP | 5.5 | 2 | 3 | 2 | Solid | Absent | |
| NET-21 | 39 | M | H, B, T | TP | 16 | 3 | 0 | 2 | Solid | Absent | |
| NET-22 | 51 | M | H | PD | 7.0 | 7 | 4 | 2 | Solid | Absent | |
| NET-23 | 58 | F | B | EC | 1.8 | 1 | 0 | 1 | Solid | Absent | |
| NET-24 | 32 | M | H | PD | 2.3 | 0 | 0 | 1 | Solid | Absent | |
| SPN-1 | 26 | F | H | EC | 8.5 | 0 | 0 | - | Solid and Cystic | Present | |
| SPN-2 | 34 | F | T | DP | 7.0 | 1 | 1 | - | Solid and Cystic | Present | |
| SPN-3 | 20 | F | T | DP | 7.5 | 0 | 0 | - | Solid and Cystic | Present | |
| SPN-4 | 23 | F | T | DP | 13 | 0 | 0 | - | Solid and Cystic | Present | |
| SPN-5 | 43 | F | B | MP | 3.8 | 0 | 0 | - | Solid and Cystic | Present | |
| SPN-6 | 27 | M | T | DP | 4.3 | 0 | 0 | - | Solid and Cystic | Present |
NET: Neuroendocrine tumor; SPN: Solid-pseudopapillary neoplasm; MEN: Multiple endocrine neoplasia; H: Pancreatic head; B: Pancreatic body; T: Pancreatic tail; L: Liver; PD: Pancreatoduodenectomy; MP: Middle pancreatectomy; DP: Distal pancreatectomy: TP: Total pancreatectomy; EC: Enucleation; LR: Liver resection; PP: Pattern pseudopapillary pattern; -: Not assessed.
In the NET group, 20 cases showed a predominantly solid growth pattern, and 4 cases had cystic areas. In the SPN group, all 6 cases showed a solid and cystic growth pattern. The cystic lesions of SPNs were typically larger than those of NETs. NETs were composed of relatively uniform cells forming various organoid histological patterns, characterized by nesting, trabecular, glandular, gyriform, tubuloacinar, or pseudorosette arrangements. All of the cases in the SPN group showed pseudopapillary structures with poorly cohesive cells, whereas no such features were evident in the NET group.
Immunohistochemical findings
The immunohistochemical profiles are summarized in Table 3. Synaptophysin was positive in all NETs (100%), whereas in 4 SPNs (67%). Chromogranin A was positive in all NETs (100%); however, two cases (NET-23 and NET-24) showed a dot-like pattern, whereas 22 cases showed diffuse staining. On the other hand, all SPNs (100%) showed a dot-like pattern of chromogranin A immunostaining. Pan-cytokeratin was positive in 20 NETs (83%), but in only one (17%) of the SPNs. E-cadherin was positive in 22 NETs (92%), with the exception of NET-23 and NET-24, whereas all the SPNs were negative for E-cadherin. Progesterone receptor, vimentin, α-1-antitrypsin, CD10, and β-catenin (nuclear/cytoplasmic expression) were positive in all SPNs (100%), whereas among the NETs, progesterone receptor was positive in 20 (83%), vimentin was positive in 10 (42%), α-1-antitrypsin was positive in 14 (58%), CD10 was positive in 8 (33%), and β-catenin was positive in 2 (8%).
Table 3.
Immunohistochemical profiles of neuroendocrine tumor and solid-pseudopapillary neoplasm
| Case | Syn | CgA | CK | E-cad | PgR | Vim | αATP | CD10 | βcat (N/C) |
| NET-1 | ++ | ++ | ++ | ++ | - | - | + | ++ | - |
| NET-2 | ++ | ++ | ++ | ++ | ++ | - | + | ++ | - |
| NET-3 | ++ | ++ | ++ | ++ | ++ | - | ++ | ++ | - |
| NET-4 | ++ | ++ | ++ | ++ | ++ | ++ | - | - | - |
| NET-5 | ++ | ++ | + | ++ | ++ | - | - | - | - |
| NET-6 | ++ | ++ | + | ++ | ++ | - | - | - | - |
| NET-7 | ++ | ++ | ++ | ++ | ++ | - | ++ | - | - |
| NET-8 | ++ | ++ | + | ++ | ++ | - | ++ | - | - |
| NET-9 | ++ | ++ | - | ++ | - | - | - | - | - |
| NET-10 | ++ | ++ | + | ++ | ++ | ++ | ++ | - | - |
| NET-11 | ++ | ++ | - | ++ | ++ | - | + | - | - |
| NET-12 | ++ | ++ | + | + | ++ | ++ | - | - | - |
| NET-13 | ++ | ++ | ++ | ++ | ++ | ++ | - | - | - |
| NET-14 | ++ | ++ | ++ | ++ | - | ++ | ++ | - | - |
| NET-15 | ++ | ++ | ++ | ++ | ++ | - | - | - | - |
| NET-16 | ++ | ++ | + | + | ++ | - | ++ | ++ | - |
| NET-17 | ++ | ++ | ++ | ++ | ++ | - | - | ++ | - |
| NET-18 | ++ | ++ | ++ | ++ | ++ | ++ | - | ++ | - |
| NET-19 | ++ | ++ | ++ | ++ | ++ | ++ | - | - | - |
| NET-20 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | - | - |
| NET-21 | ++ | ++ | ++ | ++ | ++ | - | ++ | - | - |
| NET-22 | ++ | ++ | ++ | ++ | - | - | ++ | - | - |
| NET-23 | ++ | + (dot) | - | - | ++ | ++ | ++ | ++ | ++ |
| NET-24 | ++ | + (dot) | - | - | ++ | ++ | ++ | ++ | ++ |
| SPN-1 | - | + (dot) | - | - | ++ | ++ | ++ | ++ | ++ |
| SPN-2 | - | + (dot) | - | - | ++ | ++ | ++ | ++ | ++ |
| SPN-3 | ++ | + (dot) | + | - | ++ | ++ | ++ | ++ | ++ |
| SPN-4 | ++ | + (dot) | - | - | ++ | ++ | ++ | ++ | ++ |
| SPN-5 | ++ | + (dot) | - | - | ++ | ++ | ++ | ++ | ++ |
| SPN-6 | ++ | + (dot) | - | - | ++ | ++ | ++ | ++ | ++ |
NET: Neuroendocrine tumor; SPN: Solid-pseudopapillary neoplasm; Syn: Synaptophysin; CgA: Chromogranin A; CK: Pan-cytokeratin; E-cad: E-cadherin; PgR: Progesterone receptor; Vim: Vimentin; αATP: α-1-antitrypsin; βcat (N/C): β-catenin (nuclear/cytoplasmic staining); ++: Strongly positive; +: Weakly positive; -: Negative; dot: Dot-like pattern.
The majority of NETs were positive for synaptophysin, chromogranin A, pan-cytokeratin, E-cadherin, and progesterone receptor. Some cases of NET were positive for vimentin, α-1-antitrypsin, CD10, and β-catenin. On the other hand, all SPNs showed the same immunohistochemical profiles, being positive for progesterone receptor, vimentin, α-1-antitrypsin, CD10, and β-catenin. Moreover, they were also negative for E-cadherin, and showed a dot-like pattern of chromogranin A immunostaining. In the NET group we examined two cases (NET-23 and NET-24) that showed distinctive expression in comparison with the others; although these cases had been originally diagnosed as NETs, they showed the same profiles as SPNs (positive for progesterone receptor, vimentin, α-1-antitrypsin, CD10 and β-catenin, a dot-like chromogranin A pattern, and negative for E-cadherin). Microscopically, these cases did not show a pseudopapillary pattern. Representative staining patterns in a typical case of NET (NET-21), confusing case (NET-24), and a typical case of SPN (SPN-5) are shown in Figure 1.
Figure 1.
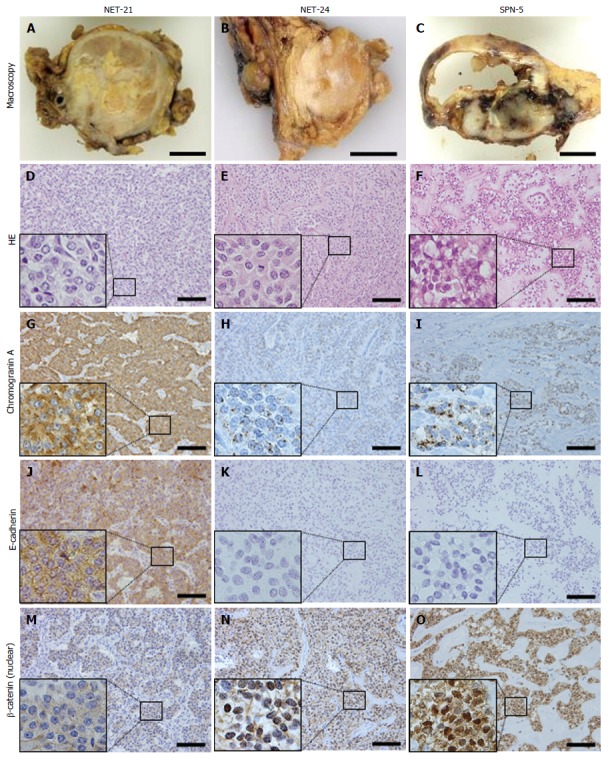
Macroscopic features and representative staining of a typical case of neuroendocrine tumor (case NET-21: A, D, G, J, M), a confusing case (case NET-24: B, E, H, K, N), and a typical case of solid-pseudopapillary neoplasm (case SPN-5: C, F, I, L, O). The typical NET case showed a solid growth pattern (A) with homogeneous cells, being strongly positive for chromogranin A and E-cadherin, and did not show nuclear labeling of β-catenin. In contrast, the typical SPN case showed a solid and cystic growth pattern (C) containing pseudopapillary structures formed by poorly cohesive cells, with a dot-like pattern of chromogranin A, negativity for E-cadherin, and nuclear labeling for β-catenin. NET-24, originally diagnosed as NET, mimicked the macroscopic features of NET, but exhibited the same immunohistochemical profile as SPN. HE, hematoxylin and eosin stain. Asterisk, cystic lesion of SPN case. Scale bars, 1 cm (macroscopic image) and 100 μm (microscopic image). Small boxes indicate representative magnified fields.
DISCUSSION
Differentiating between pancreatic NETs and SPNs is crucial but still challenging even with precise morphological and histological analysis. Several immunohistochemical markers are reported to be useful for the differential diagnosis; however, not all of these markers are conclusive. Our immunohistochemical review of surgical specimens of NETs and SPNs demonstrated that E-cadherin, and nuclear labeling of β-catenin were the most sensitive and specific among the examined markers for differentiating the two type of tumors.
As earlier studies suggested, NETs in our case series also showed more malignant potential and needed various postoperative treatments compared with SPNs; therefore, differential diagnosis of the two tumors should be performed carefully. The key gross and microscopic features for differential diagnosis between NETs and SPNs are that SPNs typically show a mixture of solid and cystic growth areas composed of pseudopapillary structures, as was the case in all the SPNs we examined, whereas NETs generally show a solid growth pattern without pseudopapillae (Table 2). However, it should be noted that NETs sometimes show areas of cystic degeneration (NET-1, NET-4, NET-18 and NET-19). Clinical characteristics other than histopathology may sometimes be helpful for differential diagnosis between NETs and SPNs (Table 2). Clinical symptoms may be key feature of NETs, as some are associated with hypersecretion of hormones including insulin, glucagon, somatostatin, or gastrin[21]. Genetic background, such as MEN1, or von Hippel-Lindau disease, may also suggest a high likelihood of NET[22]. Although these features play a substantial role in differential diagnosis, their usefulness may sometimes be limited, and in fact in the present series of 24 NET cases diagnosis on this basis was made in only 10, including 4 cases of MEN1 (NET-1 to NET-4) and 6 cases of functional insulinoma (NET-5 to NET-10). The other 14 NET cases (NET-11 to NET-24) required further detailed differential diagnosis on the basis of pathology. Although SPNs occur predominantly in young female patients, this is not always pathognomonic, since males can also be sometimes affected[23], as was the case for SPN-6. Thus, more objective diagnostic modalities need to be developed.
Here we addressed the usefulness of immunohistochemical expression of various markers, either alone or in combination, for differential diagnosis between NETs and SPNs. Synaptophysin and chromogranin A, which are representative well-known neuroendocrine markers, were usually strongly positive in the majority of NETs. However, these are not specific markers for NETs, as 4 of the 6 SPNs we examined were also positive for synaptophysin, as reported earlier[18]. In contrast, chromogranin A has been regarded as typically negative in SPNs[16,18]. Here we found that all SPNs were weakly positive for chromogranin A, and that its distribution was quite unique, exhibiting a dot-like pattern formed by a relatively small number of chromogranin granules. Jirásek et al[24] have suggested that this expression of chromogranin A might reflect weak differentiation from a neuroendocrine lineage. In fact several studies have reported positivity for chromogranin A in SPNs[12,17,25]; these cases might be resulted in the criteria that dot-like pattern was evaluated as positive expression of chromogranin A. E-cadherin, a major epithelial adhesion molecule, was generally expressed in the majority of NETs, whereas it was not expressed in SPNs[26]. Loss of E-cadherin may explain the histological characteristics of SPNs, which show a pseudopapillary pattern perhaps resulting from loss of cell cohesiveness. Abnormal accumulation of β-catenin in the nucleus, caused by prolonged degradation of mutated β-catenin protein correlated with loss of E-cadherin, was observed in 95% of SPNs[27], whereas NET was generally negative (except for cases NET-23 and NET-24, as discussed in the next paragraph). Progesterone receptor, vimentin, α-1-antitrypsin, and CD10, also known to be markers for SPNs, were expressed in all of the SPNs we examined, but were less specific. Therefore, the main message of our present study is that membranous/cytoplasmic expression of E-cadherin in NETs, and nuclear staining for β-catenin in SPNs were useful immunohistochemical markers, which should be routinely applied to the differential diagnosis between NETs and SPNs. In addition, chromogranin A immunostaining should be interpreted carefully since a dot like pattern of chromogranin A might indicate the possibility of SPNs rather than NETs.
The two confusing cases in this series (NET-23 and NET-24) had been initially diagnosed as NETs because they did not show the typical morphological structure of SPN, i.e., solid and cystic growth and a pseudopapillary pattern. However, they expressed SPN-like immunohistochemical profiles. The issue here is whether these two cases were truly NETs or truly SPNs. The WHO classification already states that a few SPNs display a solid growth pattern and lack pseudopapillary structures[9,10], and recommends that immunohistochemistry including nuclear β-catenin staining is potentially helpful for diagnosis of these SPNs. Moreover, hyaline globules, which are an architectural feature known to occur predominantly in SPNs rather than in NETs[28], were actually observed in all 6 of the present cases of SPN. Cases NET-23 and NET-24 also exhibited hyaline globules (data not shown), in addition to nuclear β-catenin staining. Therefore, we consider that these two cases might be SPNs lacking pseudopapillary structures which had been initially diagnosed as NETs.
The limitation of our study is that the number of cases we evaluated was small because SPN is rare pancreatic tumor. However, our present data should be useful for improving our routine diagnostic approach for NETs. In our institution, NETs have been initially diagnosed on the basis of gross and microscopic features, followed by supplementary immunohistochemistry for neuroendocrine markers including synaptophysin and chromogranin A. However, application of only neuroendocrine markers to “morphologically” NET-like cases is insufficient, since a substantial proportion of SPN cases mimicking the morphology of NETs will be present among these NET-like cases. Therefore, we propose that the immunohistochemical analysis should be extended to SPN-specific markers such as β-catenin, even if the tumors appear to have a NET-like morphology.
In conclusion, we have carried out comprehensive immunohistochemical profiling of 24 cases of NET and 6 cases of SPN of the pancreas. E-cadherin and β-catenin are the most useful immunostaining markers for differentiating between NETs and SPNs. On the other hand, we also found two cases which showed disagreement between the tumor morphology and immunohistochemical profiles. These cases strongly indicate the careful and precise assessments are crucial for differential diagnosis between NETs and SPNs.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Tomoyo Takeuchi and Dr. Dongping Li (Tsukuba Human Tissue Diagnostic Center, University of Tsukuba Hospital) for their skillful technical assistance with immunohistochemical staining.
COMMENTS
Background
Neuroendocrine tumor (NET) and solid-pseudopapillary neoplasm (SPN) are two types of pancreatic tumor that were sometimes confused in differential diagnosis. Morphological structures and immunohistochemical profiles have often been the key guide for differentiating SPNs from NETs. However, morphological or immunohistochemical features show overlap between NETs and SPNs.
Research frontiers
NET and SPN sometimes show malignant character such as liver metastasis; however additional therapy after resection is different between the two tumors.
Innovations and breakthroughs
The immunohistochemical review of surgical specimens of NETs and SPNs demonstrated that E-cadherin, and nuclear labeling of β-catenin were the most sensitive and specific among the examined markers for differentiating the two type of tumors.
Applications
The authors propose that the immunohistochemical analysis should be extended to SPN-specific markers such as β-catenin, even if the tumors appear to have a NET-like morphology.
Terminology
SPN of the pancreas is an uncommon low grade malignant neoplasm. Exact histogenesis of SPN remains uncertain. It is well known for its predilection in young women.
Peer-review
In the present study, the authors reviewed the morphological and immunohistochemical profiles of 30 pancreatic tumors including 24 cases of NET and 6 cases of SPN. The authors comprehensively surveyed the usefulness of 9 markers in order to derive better diagnostic procedures for differentiating between the two tumor types.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was performed and reviewed in accordance with the ethics committee of University of Tsukuba Hospital.
Informed consent statement: All of the patients provided informed consent for analysis of their tissue samples in accordance with the ethics committee of University of Tsukuba Hospital.
Conflict-of-interest statement: The authors have no potential conflicts of interest to disclose.
Data sharing statement: No additional data are available.
Peer-review started: May 6, 2016
First decision: June 20, 2016
Article in press: August 1, 2016
P- Reviewer: Junginger T, Malak M S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
References
- 1.Jensen RT. Pancreatic endocrine tumors: recent advances. Ann Oncol. 1999;10 Suppl 4:170–176. [PubMed] [Google Scholar]
- 2.Mansour JC, Chen H. Pancreatic endocrine tumors. J Surg Res. 2004;120:139–161. doi: 10.1016/j.jss.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965–972. doi: 10.1016/j.jamcollsurg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Hochwald SN, Zee S, Conlon KC, Colleoni R, Louie O, Brennan MF, Klimstra DS. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20:2633–2642. doi: 10.1200/JCO.2002.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Oberg K, Akerström G, Rindi G, Jelic S. Neuroendocrine gastroenteropancreatic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v223–v227. doi: 10.1093/annonc/mdq192. [DOI] [PubMed] [Google Scholar]
- 6.Reidy-Lagunes D, Thornton R. Pancreatic neuroendocrine and carcinoid tumors: what’s new, what’s old, and what’s different? Curr Oncol Rep. 2012;14:249–256. doi: 10.1007/s11912-012-0232-1. [DOI] [PubMed] [Google Scholar]
- 7.Romics L, Oláh A, Belágyi T, Hajdú N, Gyurus P, Ruszinkó V. Solid pseudopapillary neoplasm of the pancreas--proposed algorithms for diagnosis and surgical treatment. Langenbecks Arch Surg. 2010;395:747–755. doi: 10.1007/s00423-010-0599-0. [DOI] [PubMed] [Google Scholar]
- 8.Santini D, Poli F, Lega S. Solid-papillary tumors of the pancreas: histopathology. JOP. 2006;7:131–136. [PubMed] [Google Scholar]
- 9.Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol. 2007;20 Suppl 1:S94–112. doi: 10.1038/modpathol.3800686. [DOI] [PubMed] [Google Scholar]
- 10.Kloppel G, Hruban RH, Klimstra DS, Maitra A, Morohoshi T, Notohara K, Shimizu M, Terris B. Solid-pseudopapillary neoplasm of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise N, eds. WHO Classification of Tumours of the Digestive System. Lyon: WHO Press, 2010: 327-330 In: Bosman FT, Carneiro F, Hruban RH, Theise N, eds, et al., editors. [Google Scholar]
- 11.Notohara K, Wani Y, Fujisawa M. Solid pseudopapillary neoplasm: pathological diagnosis and distinction from other solid cellular tumours of the pancreas. Diagn Histopat. 2008;14:266–274. [Google Scholar]
- 12.Liu BA, Li ZM, Su ZS, She XL. Pathological differential diagnosis of solid-pseudopapillary neoplasm and endocrine tumors of the pancreas. World J Gastroenterol. 2010;16:1025–1030. doi: 10.3748/wjg.v16.i8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klimstra DS, Pitman MB, Hruban RH. An algorithmic approach to the diagnosis of pancreatic neoplasms. Arch Pathol Lab Med. 2009;133:454–464. doi: 10.5858/133.3.454. [DOI] [PubMed] [Google Scholar]
- 14.Hruban RH, Pitman MB, Klimstra DS. Tumors of the pancreas. Washington DC: American registry of pathology, 2007: 231-304 [Google Scholar]
- 15.Basturk O, Farris III AB, Adsay NV. Immunohistology of the pancreas, biliary tract, and liver. In: Dabbs DJ, ed. Diagnostic immunohistocchemistry: theranostic and genomic applications. Philadelphia: Saunders Elsevier, 2010: 541-559 In: Dabbs DJ, ed, et al., editors. [Google Scholar]
- 16.Notohara K, Hamazaki S, Tsukayama C, Nakamoto S, Kawabata K, Mizobuchi K, Sakamoto K, Okada S. Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol. 2000;24:1361–1371. doi: 10.1097/00000478-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Choi YL, Oh YL, Kim SH, Park CK, Ahn G. Comparative study of non-functional islet cell tumors and pancreatic solid and papillary neoplasms: biological behavior and immunohistochemistry. Pathol Int. 2002;52:358–366. doi: 10.1046/j.1440-1827.2002.01361.x. [DOI] [PubMed] [Google Scholar]
- 18.Stömmer P, Kraus J, Stolte M, Giedl J. Solid and cystic pancreatic tumors. Clinical, histochemical, and electron microscopic features in ten cases. Cancer. 1991;67:1635–1641. doi: 10.1002/1097-0142(19910315)67:6<1635::aid-cncr2820670627>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 19.Kosmahl M, Seada LS, Jänig U, Harms D, Klöppel G. Solid-pseudopapillary tumor of the pancreas: its origin revisited. Virchows Arch. 2000;436:473–480. doi: 10.1007/s004280050475. [DOI] [PubMed] [Google Scholar]
- 20.Klimstra DS, Arnold R, Capella C, Hruban RH, Kloppel G, Komminoth P, Solcia E, Rindi G. Neuroendocrine neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise N, eds. WHO Classification of Tumours of the Digestive System. Lyon: WHO Press, 2010: 322-326 In: Bosman FT, Carneiro F, Hruban RH, Theise N, eds, et al., editors. [Google Scholar]
- 21.Oberg K. Pancreatic endocrine tumors. Semin Oncol. 2010;37:594–618. doi: 10.1053/j.seminoncol.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Asa SL. Pancreatic endocrine tumors. Mod Pathol. 2011;24 Suppl 2:S66–S77. doi: 10.1038/modpathol.2010.127. [DOI] [PubMed] [Google Scholar]
- 23.Tien YW, Ser KH, Hu RH, Lee CY, Jeng YM, Lee PH. Solid pseudopapillary neoplasms of the pancreas: is there a pathologic basis for the observed gender differences in incidence? Surgery. 2005;137:591–596. doi: 10.1016/j.surg.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Jirásek T, Hozák P, Mandys V. Different patterns of chromogranin A and Leu-7 (CD57) expression in gastrointestinal carcinoids: immunohistochemical and confocal laser scanning microscopy study. Neoplasma. 2003;50:1–7. [PubMed] [Google Scholar]
- 25.Li L, Li J, Hao C, Zhang C, Mu K, Wang Y, Zhang T. Immunohistochemical evaluation of solid pseudopapillary tumors of the pancreas: the expression pattern of CD99 is highly unique. Cancer Lett. 2011;310:9–14. doi: 10.1016/j.canlet.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Kim MJ, Jang SJ, Yu E. Loss of E-cadherin and cytoplasmic-nuclear expression of beta-catenin are the most useful immunoprofiles in the diagnosis of solid-pseudopapillary neoplasm of the pancreas. Hum Pathol. 2008;39:251–258. doi: 10.1016/j.humpath.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Abraham SC, Klimstra DS, Wilentz RE, Yeo CJ, Conlon K, Brennan M, Cameron JL, Wu TT, Hruban RH. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002;160:1361–1369. doi: 10.1016/s0002-9440(10)62563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meriden Z, Shi C, Edil BH, Ellison T, Wolfgang CL, Cornish TC, Schulick RD, Hruban RH. Hyaline globules in neuroendocrine and solid-pseudopapillary neoplasms of the pancreas: a clue to the diagnosis. Am J Surg Pathol. 2011;35:981–988. doi: 10.1097/PAS.0b013e31821a9a14. [DOI] [PMC free article] [PubMed] [Google Scholar]


