Abstract
Children with tuberculosis are treated with drug regimens copied from adults despite significant differences in antibiotic pharmacokinetics, pathology, and the microbial burden between childhood and adult tuberculosis. We sought to develop a new and effective oral treatment regimen specific to children of different ages. We investigated and validated the concept that target drug concentrations associated with therapy failure and death in children are different from those of adults. On that basis, we proposed a 4-step program to rapidly develop treatment regimens for children. First, target drug concentrations for optimal efficacy are derived from preclinical models of disseminated tuberculosis that recapitulate pediatric pharmacokinetics, starting with monotherapy. Second, 2-drug combinations were examined for zones of synergy, antagonism, and additivity based on a whole exposure–response surface. Exposures associated with additivity or synergy were then combined and the regimen was compared to standard therapy. Third, several exposures of the third drug were added, and a 3-drug regimen was identified based on kill slopes in comparison to standard therapy. Fourth, computer-aided clinical trial simulations are used to identify clinical doses that achieve these kill rates in children in different age groups. The proposed program led to the development of a 3-drug combination regimen for children from scratch, independent of adult regimens, in <2 years. The regimens and doses can be tested in animal models and in clinical trials.
Keywords: disseminated tuberculosis, young children, drug regimen design, pharmacokinetics/pharmacodynamics, target setting
Young children (aged <5 years) exposed to Mycobacterium tuberculosis (Mtb) are at the highest risk of disseminated disease [1]. The number of tuberculosis cases in children varies between 3.5% and 11% of the total reported global tuberculosis cases, and an estimated 332 000 children are left undiagnosed or not reported in national surveys [2, 3]. Thus, childhood tuberculosis remains a hidden epidemic. In the case of multidrug-resistant (MDR) tuberculosis, the population is virtually “invisible,” and no treatment regimens of proven efficacy are available [4]. As drug concentrations associated with optimal outcome in children have hitherto been unknown, optimized drug doses for these “invisible children” have also not been available. Addressing these knowledge gaps should be a priority.
The therapeutic regimens currently used to treat pediatric tuberculosis are based on those developed for adults with cavitary pulmonary tuberculosis. The current practice is to dose children based on weight bands [5]. However, the relationship between weight and drug clearance or volume of distribution in children (and adults) is nonlinear, and often follows fractal geometry–based patterns. As a result, the peak concentration (“peak”) and 0- to 24-hour area under the curve (AUC0–24) of drugs achieved in children are often lower than those in adults treated with the same milligram per kilogram (mg/kg) dose [5]. We recently demonstrated that some important xenobiotic metabolism enzymes such as N-acetyltransferase 2 (NAT2) have ontogeny-driven variable function in children <5 years age, an effect that was more powerful than NAT2 genotype [6]. This further complicates imposition of doses derived in adults on infants and toddlers.
In an accompanying article in this supplement, we report that there are specific drug concentration thresholds associated with poor therapy outcome and death in chidren with tuberculosis; some were similar to findings in adults, but several were distinct [7]. Furthermore, the concentration thresholds and the ranking order of importance associated with therapeutic outcomes in infants and toddlers, the age group most prone to disseminated disease, were dramatically different from those in older children and adults. This means that the well-meaning approach that uses drug concentration targets identified in adults should now be supplanted by exposure targets specifically designed for children. Therefore, pharmacokinetic/pharmacodynamic (PK/PD) studies different from those performed for adults are needed, and specific intent should be paid to studies for toddlers and infants. The same approach will also be needed to study concentration-related toxicity in children. A recent analysis of factors contributing to US Food and Drug Administration registration failure of clinical trials of 44 pediatric products identified poor dosing and toxicity as important factors [8]. Here, we outline the principles behind a program whose intent was to design new dosing regimens for children with tuberculosis based on identifying optimal doses for efficacy that minimize toxicity.
STEP 1: DEFINING POPULATION PHARMACOKINETICS OF NEONATES, INFANTS, TODDLERS, AND PRESCHOOLERS
Children, especially the very young, have rapidly changing body size and physiology, and in some cases undergo a switch in isoenzymes important for xenobiotic metabolism [9]. The sad lesson of chloramphenicol toxicity in neonates illustrates the importance of considering enzyme maturation and changes in physiological processes such as renal excretion and dosing [10]. Neonates receiving intravenous loads of chloramphenicol developed cardiovascular collapse because the UDP-glucuronyltransferase enzyme system was immature and the renal function was not yet high enough to excrete the unconjugated antibiotic [11]. For linezolid, which is being considered for treatment of MDR tuberculosis in children, ontogeny clearly impacts systemic clearance of the drug [12]. The changes in systemic clearance and volume, hence AUC0–24 and peak, with age are shown elsewhere [13]. Thus, the first step in better target setting is identifying clinical pharmacokinetic parameters in the relevant age groups. Even more important is quantification of the between-child variability in the clearance and volumes of distribution because no 2 children are exactly alike, and there is age-dependent maturation. This means that when different children receive the same dose in mg/kg, they will achieve different concentration-time profiles.
The effect of pharmacokinetic parameters such as AUC0–24 and peak will be modified by the degree of susceptibility of the infecting Mtb isolate. The higher the concentration, the more bacteria are killed, whereas the higher the minimum inhibitory concentration (MIC), the fewer Mtb killed. Thus, we index pharmacokinetic parameters to the MIC, to generate pharmacodynamic parameters such as AUC0–24/MIC, peak/MIC and percentage of the dosing interval that concentrations persist above MIC (%TMIC). These quantities and ratios are termed drug exposures. Because of pharmacokinetic variability, and MIC variability in clinical isolates, children treated with a fixed dose will achieve different exposures from each other.
STEP 2: TARGET SETTING USING PK/PD PRINCIPLES WITH MONOTHERAPY
Preclinical models have been used for PK/PD target setting of antituberculosis agents in adults. The 2 most extensively used are hollow fiber systems and mouse models, as recently reviewed [14, 15]. For adults, these models have a fairly high degree of accuracy in identifying PK/PD exposures that are relevant to clinical treatment of tuberculosis [16]. The premise is that there is a predictable and reproducible relationship between drug exposure and effect, and this relationship holds across different disease models, including humans. This is important as it would be unethical in the case of fatal infections to perform a dose-response study in which some children are exposed to suboptimal doses. Thus, the dose response has to be explored in preclinical models.
The hollow fiber system model of tuberculosis recapitulates the concentration-time profile of the drugs encountered in humans [17]. In the case of children, these would be specific to the age group, based on population pharmacokinetic studies described in step 1 above. The important component is exposing these dynamic drug concentrations to Mtb that are in metabolic states of relevance to the disease. In adult tuberculosis, the hollow fiber system model has been used extensively to perform exposure–effect experiments for bactericidal and sterilizing effect, identification of drug exposures that may suppress drug resistance, and identification of susceptibility breakpoints [17, 18]. Recently, we developed an intracellular Mtb hollow fiber system model for exposure–effect studies that utilize pediatric pharmacokinetics [19].
In vivo models introduce additional complexities related to the host response and development of tuberculous lesions that may influence the relationship between plasma drug exposures and antituberculosis effect. Infection of BALB/c and other commonly used mouse strains with greater susceptibility to virulent Mtb produces disseminated infection characterized by nonnecrotic granuloma-like aggregates of inflammatory cells lesions in which Mtb is virtually entirely intracellular, whereas use of C3HeB/FeJ mice introduces the element of caseous necrosis and even cavitation, which may further improve the representation of tuberculosis among children >5 years of age [15]. Recent evidence indicating differential drug partitioning and drug concentration–response relationships in caseum vs cellular regions of lesions further emphasize the complementary role that these in vivo models may play [20–22]. The clearance of the drugs in mice is in some cases faster than in adults, and this faster clearance could be used as a surrogate for the pharmacokinetics in babies and toddlers [23]. This would be on a drug-by-drug basis, as not all drugs have more rapid clearance in young children; indeed, some may have poorer clearance as is the case of chloramphenicol. Nevertheless, mice could be used for exposure–effect studies, and in tandem with the hollow fiber system, to design antituberculosis doses for children that could optimize efficacy, suppress drug resistance, and minimize toxicity. The ultimate goal would be to develop new treatment regimens based on PK/PD principles and rank their efficacy.
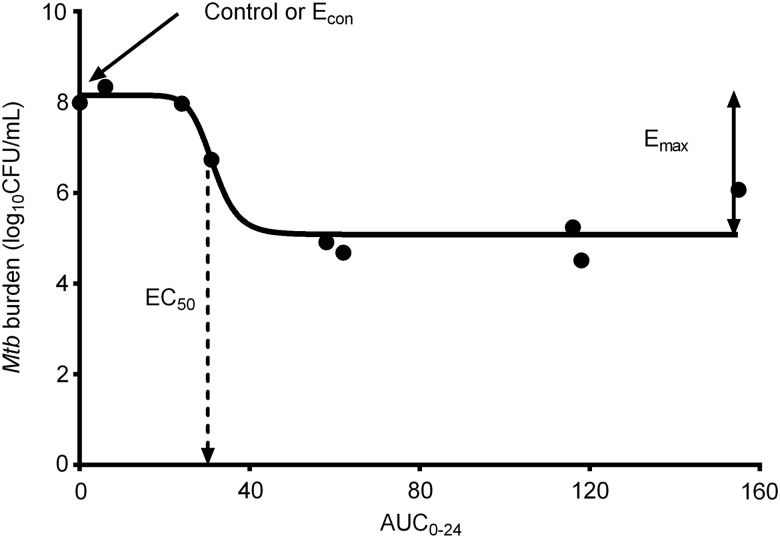
The relationship between drug exposure and total microbial burden in these preclinical models is analyzed using the inhibitory sigmoid maximal microbial kill (Emax) model, shown in Figure 1. The slope of the steep portion of this sigmoid curve is described by the Hill factor (H). The second parameter to consider is the maximal amount of Mtb kill by the drug (Emax), which is the efficacy of the drug. The drug exposure associated with 50% of Emax is the EC50, which denotes the potency of the drug. On the sigmoid curve, the bacterial burden in nontreated systems or animals is denoted as Econ. The exposure–response relationship is used to calculate the drug exposure associated with either 80% or 90% of Emax (EC80 and EC90). These are called “optimal exposure” because 100% Emax is on an asymptote. That these EC50, EC80, and EC90 exposure values are invariant and, as such, can be transported from preclinical models to humans is a basic tenet of the science of antibiotic pharmacodynamics.
Figure 1.
Exposure–effect relationship. As shown in the figure, at low doses the bacterial burden does not differ much from nontreated controls (Econ). As the drug exposure increases, there is an inflection point after which slight increases in drug exposure lead to large changes, the steep portion of the curve. The slope on this steep portion is the Hill factor. Close to maximal microbial kill (Emax) there is a second inflection at which large increases in drug exposure lead to very little change in bacterial burden. Abbreviations: AUC0–24, 0- to 24-hour area under the curve; CFU, colony-forming units; EC50, drug exposure associated with 50% of Emax; Econ, bacterial burden in nontreated controls.
Experiments can also be performed in preclinical models to identify the effect of the dose schedule. This is because when exposures such as AUC0–24/MIC ratio, peak/MIC, and %TMIC are utilized in inhibitory sigmoid Emax models, often one of these has a closer relationship to microbial effect compared to the others, as indicated by a highest coefficient of determination (r2). The r2 tells us what percentage of effect is explained by the particular exposure ratio of either AUC0–24/MIC, peak/MIC, or %TMIC. A dosing schedule that maximizes this r2 is associated with the best microbial kill for that drug, and is said to be linked to effect. This pattern is also invariant.
STEP 3: IDENTIFYING OPTIMAL EXPOSURES OF DRUG COMBINATIONS
Tuberculosis is treated with combination therapy because monotherapy frequently leads to emergence of resistance. After the exposure–effect studies, the next step is thus to combine 2 drugs. However, this is immediately preceded by the question of which exposures to use in the combination. How do we know which exposures to combine? This is a step fraught with danger, given 2 linked concepts, toxicity and efficacy. Some drugs may be more toxic in combination than as single agents. This problem of “the toxicity of poisons applied jointly” is what motivated Chester Bliss in 1939 to introduce the concept for drugs that inhibit different sites or receptors [24]. A similar concept applies to combining drugs for greater efficacy, which is tantamount to poisoning Mtb more effectively with the combination of antibiotics. If one examined all possible combinations of drug concentrations of 2 drugs A and B and depicted their effect on a giant surface, there are 3 possibilities at each point of the surface. The inhibitory sigmoid Emax in Figure 1 depicts the effect of the exposure or concentration of each drug A or B alone; in other words, the exposure for the companion drug is zero. At each exposure, one can add the effect of the exposure of drug A and drug B alone to give an expected amount of microbial kill if the effect of the 2 drugs was independent and additive. However, if one then measured the actual effect (Mtb burden) of the combination and found it less than the expected additive effect, then drugs A and B are antagonistic at those concentrations. Simply stated, if the observed effect is less than the additive effect, or observed effect minus expected additive effect is less than zero, then there is antagonism. On our giant surface with effect measured as up (above the surface) or down (below the surface), then values less than zero (antagonism) produce valleys below the surface. If, on the other hand, the observed effect is similar or equal to the expected additive effect, then the difference is zero and the effect lies along the plane of the surface. If, however, the observed effect is greater than the expected sum of the effects of A and B at those concentrations, then effect is above the surface and this synergy is depicted by a hill or mountain.
It so happens that for most combinations of drugs, there are some concentrations at which there is antagonism, whereas at some concentrations there is synergy, while yet at other concentration combinations there is additivity. Thus, when one studies a grid of such possible concentrations, there is a mixture of peaks and valleys and flat surfaces [25]. Because of the number of mice or hollow fibers one would need to employ to test all such combinations, we resort to testing this out with static drug concentrations in 24- and 48-well plates. In the case of intracellular infection, we first infect the macrophages with Mtb, and then coincubate at many of these possible concentration combinations, and after a week (or longer) examine for extent of microbial kill. We then find exposure combination regions dominated by hills and flat planes, while avoiding those in valleys. We then design regimens based on these exposure combinations to test for efficacy in the hollow fiber system, which makes the number of units more manageable. Such experiments incorporate the necessary positive and negative controls from monotherapy studies.
Next, several exposures of a third drug are added to the chosen dual regimen in the hollow fiber system model. The doses of the third drug are chosen across a range of exposures. As an example, if drug C's effect is linked to %TMIC, then one could examine adding exposures of 20%, 40%, 60%, or 80% %TMIC to the dual regimen and then comparing the slopes of kill of the regimens. The standard first-line regimen is always the control that allows us to determine how much faster (or slower) the rate of microbial sterilization will be compared to standard therapy. After that choice, the hollow fiber study is repeated with the optimal exposures to prospectively validate the exposures and confirm the slope relationships.
STEP 4: MONTE CARLO SIMULATIONS TO TRANSLATE RESULTS TO CHILDREN IN THE CLINIC
Once the exposures associated with the steepest microbial kill slope in the hollow fiber system are known, the next step is to find the doses that would achieve these exposures in the highest proportions of children of different ages who have tuberculosis [26]. Monte Carlo simulations, originally invented by Ulam and Metropolis for work on fissile material during the construction of the atomic bomb, and later used in the anti-infective PK/PD, are most widely used for this process [27, 28]. The steps taken to achieve this and safeguards to ensure validity of the results have been recently summarized [29, 30]. The first step takes into account the pharmacokinetic variability as identified in step 1. For each dose, the pharmacokinetic parameter estimates and their covariance matrix are used to generate pharmacokinetic parameters of 10 000 children (eg, full-term infants) and the distribution of values such as peak and AUC0–24. Then, at each MIC in the entire MIC distribution, the drug exposure is calculated. The MIC distribution is that of Mtb isolates encountered in the clinic, and may vary from region to region [31–33]. Then, the exposures are compared to the target exposure derived from experiments in steps 2 and 3 above, to determine if the target exposure is achieved or exceeded by the dose—that is, target attainment probability. After that, the target attainment is calculated or summated for the entire MIC distribution, yielding the cumulative fraction of response. These steps keep getting repeated as the dose is increased, allowing for an in silico dose response. The dose associated with a cumulative fraction of response of >90% of children is then chosen as the optimal dose. If a drug's toxicity is concentration dependent, the drug concentrations identified for each dose can also be examined to identify the proportion of children who would achieve the concentration known to be associated with increased toxicity. In that way, a dose associated with optimal efficacy but minimal toxicity can then be chosen. These simulations are repeated for each drug in the regimen. In adult tuberculosis, and in other infectious diseases, this approach has been found to be highly accurate in identifying optimal clinical doses [29, 34]. The variety of disease in children is also taken into account at this stage; if pulmonary disease is being treated, for example, then penetration indices of the drugs into lung are taken into consideration. This step is crucial in, for example, the treatment of tuberculous meningitis in children, given that many drugs have poor penetration into subarachnoid space, are transported out by components of the blood–brain barrier, or both.
CONCLUSIONS
Drug exposures and concentrations are the most important determinants of response to antibiotics [35, 36]. Here, we outline steps in a program to identify the target exposures associated with optimal microbial kill in children for monotherapy, dual therapy, and triple therapy. The outputs of these results are then used in Monte Carlo simulations to identify clinical doses for different age groups of children. We implemented this program, which led to the identification of an entirely new regimen for treatment of children with disseminated tuberculosis in a time period <2 years [25, 37–39].
Notes
Author contributions. T. G., E. N., and S. S. designed the program; S. S. wrote the first draft of the manuscript; S. S., D. D., J. G. P., T. T., S. S., E. N., and T. G. wrote the manuscript, with specific special input into one of the steps that are within the area of expertise of each.
Financial support. Funding for this study was provided by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant number R56 AI111985).
Supplement sponsorship. This article appears as part of the supplement “A Development Paradigm for Novel Combination Regimens for Multidrug-Resistant and Drug-Susceptible Tuberculosis in Children: FLAME for Work and Play,” sponsored by the Center for Infectious Diseases Research and Experimental Therapeutics (CIDRET), Baylor Institute for Immunology Research, Baylor Research Institute.
Potential conflicts of interest. T. G. is a consultant for Astellas Pharma USA and LuminaCare solutions, and founded Jacaranda Biomed, Inc. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cruz AT, Martinez BJ. Childhood tuberculosis in the United States: shifting the focus to prevention. Int J Tuberc Lung Dis 2015; 19(suppl 1):50–3. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med 2012; 367:348–61. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins HE, Tolman AW, Yuen CM et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet 2014; 383:1572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becerra MC, Swaminathan S. Commentary: a targets framework: dismantling the invisibility trap for children with drug-resistant tuberculosis. J Public Health Policy 2014; 35:425–54. [DOI] [PubMed] [Google Scholar]
- 5.International Union Against Tuberculosis and Lung Disease. Desk-guide for diagnosis and management of TB in children. Paris: The Union, 2014. [Google Scholar]
- 6.Rogers Z, Hiruy H, Pasipanodya J et al. The non-linear child: ontogeny, isoniazid concentration, and NAT2 gene interactions modulate enzyme reaction kinetics and metabolism. EBioMedicine 2016. Available at: http://dx.doi.org/10.1016/j.ebiom.2016.07.031. Accessed 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swaminathan S, Pasipanodya J, Ramachandran G et al. Drug concentration thresholds predictive of therapy failure and death in children with tuberculosis: bread crumb trails in random forests. Clin Infect Dis 2016; 63(suppl 3):S63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Momper JD, Mulugeta Y, Burckart GJ. Failed pediatric drug development trials. Clin Pharmacol Ther 2015; 98:245–51. [DOI] [PubMed] [Google Scholar]
- 9.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med 2003; 349:1157–67. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland JM. Fatal cardiovascular collapse of infants receiving large amounts of chloramphenicol. AMA J Dis Child 1959; 97:761–7. [DOI] [PubMed] [Google Scholar]
- 11.Weiss CF, Glazko AJ, Weston JK. Chloramphenicol in the newborn infant. A physiologic explanation of its toxicity when given in excessive doses. N Engl J Med 1960; 262:787–94. [DOI] [PubMed] [Google Scholar]
- 12.Kearns GL, Jungbluth GL, Abdel-Rahman SM et al. Impact of ontogeny on linezolid disposition in neonates and infants. Clin Pharmacol Ther 2003; 74:413–22. [DOI] [PubMed] [Google Scholar]
- 13.Kearns GL, Abdel-Rahman SM, Blumer JL et al. Single dose pharmacokinetics of linezolid in infants and children. Pediatr Infect Dis J 2000; 19:1178–84. [DOI] [PubMed] [Google Scholar]
- 14.Pasipanodya JG, Nuermberger E, Romero K, Hanna D, Gumbo T. Systematic analysis of hollow fiber model of tuberculosis experiments. Clin Infect Dis 2015; 61:S10–7. [DOI] [PubMed] [Google Scholar]
- 15.Gumbo T, Lenaerts AJ, Hanna D, Romero K, Nuermberger E. Nonclinical models for antituberculosis drug development: a landscape analysis. J Infect Dis 2015; 211(suppl 3):S83–95. [DOI] [PubMed] [Google Scholar]
- 16.Gumbo T, Pasipanodya JG, Romero K, Hanna D, Nuermberger E. Forecasting accuracy of the hollow fiber model of tuberculosis for clinical therapeutic outcomes. Clin Infect Dis 2015; 61:S25–31. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava S, Gumbo T. In vitro and in vivo modeling of tuberculosis drugs and its impact on optimization of doses and regimens. Curr Pharm Des 2011; 17:2881–8. [DOI] [PubMed] [Google Scholar]
- 18.Gumbo T. New susceptibility breakpoints for first-line antituberculosis drugs based on antimicrobial pharmacokinetic/pharmacodynamic science and population pharmacokinetic variability. Antimicrob Agents Chemother 2010; 54:1484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srivastava S, Pasipanodya JG, Ramachandran G et al. A long-term co-perfused disseminated tuberculosis-3D liver hollow fiber model for both drug efficacy and hepatotoxicity in babies. EBioMedicine 2016; 6:126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irwin SM, Gruppo V, Brooks E et al. Limited activity of clofazimine as a single drug in a mouse model of tuberculosis exhibiting caseous necrotic granulomas. Antimicrob Agents Chemother 2014; 58:4026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanoix JP, Ioerger T, Ormond A et al. Selective inactivity of pyrazinamide against tuberculosis in C3HeB/FeJ mice is best explained by neutral pH of caseum. Antimicrob Agents Chemother 2015; 60:735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prideaux B, Via LE, Zimmerman MD et al. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat Med 2015; 21:1223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakai C, Iwaano S, Yamazaki Y et al. Species differences in the pharmacokinetic parameters of cytochrome P450 probe substrates between experimental animals, such as mice, rats, dogs, monkeys, and microminipigs, and humans. J Drug Metab Toxicol 2014; 5:173. [Google Scholar]
- 24.Bliss CI. The toxicity of poisons applied jointly. Ann Appl Biol 1939; 26:585–615. [Google Scholar]
- 25.Deshpande D, Srivastava S, Nuermberger E, Pasipanodya J, Swaminathan S, Gumbo T. Concentration-dependent synergy and antagonism of linezolid and moxifloxacin in the treatment of childhood tuberculosis: the dynamic duo. Clin Infect Dis 2016; 63(suppl 3):S88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeena PM, Bishai WR, Pasipanodya JG, Gumbo T. In silico children and the glass mouse model: clinical trial simulations to identify and individualize optimal isoniazid doses in children with tuberculosis. Antimicrob Agents Chemother 2011; 55:539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metropolis N, Ulam S. The Monte Carlo method. J Am Stat Assoc 1949; 44:335–41. [DOI] [PubMed] [Google Scholar]
- 28.Drusano GL, Preston SL, Hardalo C et al. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob Agents Chemother 2001; 45:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gumbo T, Angulo-Barturen I, Ferrer-Bazaga S. Pharmacokinetic-pharmacodynamic and dose-response relationships of antituberculosis drugs: recommendations and standards for industry and academia. J Infect Dis 2015; 211(suppl 3):S96–106.. [DOI] [PubMed] [Google Scholar]
- 30.Pasipanodya J, Gumbo T. An oracle: antituberculosis pharmacokinetics-pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future. Antimicrob Agents Chemother 2011; 55:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ford CB, Shah RR, Maeda MK et al. Mycobacterium tuberculosis mutation rate estimates from different lineages predict substantial differences in the emergence of drug-resistant tuberculosis. Nat Genet 2013; 45:784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gumbo T. Biological variability and the emergence of multidrug-resistant tuberculosis. Nat Genet 2013; 45:720–1. [DOI] [PubMed] [Google Scholar]
- 33.Pasipanodya J, Srivastava S, Gumbo T. New susceptibility breakpoints and the regional variability of MIC distribution in Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother 2012; 56:5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gumbo T, Pasipanodya JG, Nuermberger E, Romero K, Hanna D. Correlations between the hollow fiber model of tuberculosis and therapeutic events in tuberculosis patients: learn and confirm. Clin Infect Dis 2015; 61:S18–24. [DOI] [PubMed] [Google Scholar]
- 35.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 2013; 208:1464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chigutsa E, Pasipanodya JG, Visser ME et al. Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob Agents Chemother 2015; 59:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deshpande D, Srivastava S, Pasipanodya JG et al. Linezolid for infants and toddlers with disseminated tuberculosis: first steps. Clin Infect Dis 2016; 63(suppl 3):S80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deshpande D, Srivastava S, Nuermberger E, Pasipanodya J, Swaminathan S, Gumbo T. A faropenem, linezolid, and moxifloxacin regimen for both drug-susceptible and multidrug-resistant tuberculosis in children: FLAME path on the Milky Way. Clin Infect Dis 2016; 63(suppl 3):S95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srivastava S, Deshpande D, Pasipanodya J et al. Optimal clinical doses of faropenem, linezolid, and moxifloxacin in children with disseminated tuberculosis: Goldilocks. Clin Infect Dis 2016; 63(suppl 3):S109–9. [DOI] [PMC free article] [PubMed] [Google Scholar]