Abstract
Background. The regimen of linezolid and moxifloxacin was found to be efficacious in the hollow fiber system model of pediatric intracellular tuberculosis. However, its kill rate was slower than the standard 3-drug regimen of isoniazid, rifampin, and pyrazinamide. We wanted to examine the effect of adding a third oral agent, faropenem, to this dual combination.
Methods. We performed a series of studies in the hollow fiber system model of intracellular Mycobacterium tuberculosis, by mimicking pediatric pharmacokinetics of each antibiotic. First, we varied the percentage of time that faropenem persisted above minimum inhibitory concentration (TMIC) on the moxifloxacin-linezolid regimen. After choosing the best faropenem exposure, we performed experiments in which we varied the moxifloxacin and linezolid doses in the triple regimen. Finally, we performed longer-duration therapy validation experiments. Bacterial burden was quantified using both colony-forming units per milliliter (CFU/mL) and time to positivity (TTP). Kill slopes were modeled using exponential regression.
Results. TTP was a more sensitive measure of bacterial burden than CFU/mL. A faropenem TMIC > 62% was associated with steepest microbial kill slope. Regimens of standard linezolid and moxifloxacin plus faropenem TMIC > 60%, as well as higher-dose moxifloxacin, achieved slopes equivalent to those of the standard regimen based by both TTP and CFU/mL over 28 days of treatment.
Conclusions. We have developed an oral faropenem-linezolid-moxifloxacin (FLAME) regimen that is free of first-line drugs. The regimen could be effective against both multidrug-resistant and drug-susceptible tuberculosis in children.
Keywords: disseminated tuberculosis, regimen design, hollow fiber model, time-to-positivity
Treatment of tuberculosis in children, as in adults, involves using a combination of at least 3 drugs [1, 2]. We have demonstrated the efficacy of the combination of linezolid and moxifloxacin against intracellular Mycobacterium tuberculosis (Mtb) in the hollow fiber system model for pediatric intracellular tuberculosis (HFS-Peds) [3]. However, while effective, the kill rate was slower than that of the standard 3-drug regimen of isoniazid, rifampin, and pyrazinamide (herein termed “standard therapy”). Addition of a third oral agent to the backbone could improve the efficacy. One class of agents that could do this by virtue of a different mechanism of effect are β-lactams. Here, we performed a series of experiments using the HFS-Peds in which we added the β-lactam, faropenem.
Faropenem has an oral bioavailability of 70%–80% [4, 5]. The prodrug, faropenem medoxomil, results in several-fold higher exposures. Faropenem has a strong affinity for the high molecular penicillin binding proteins of the cell wall and inhibits transpeptidation, thereby interfering with cell wall synthesis [4, 5]. This differs from inhibition of bacterial protein synthesis by linezolid and gyrase inhibition by moxifloxacin, making the combination mechanistically attractive. Faropenem is more resistant to hydrolysis by β-lactamases than cephalosporins and carbapenems are, which enables it to work in β-lactamase–producing bacteria without a β-lactamase inhibitor [4–7]. This gives it an advantage over carbapenems, which have to be administered together with clavulanate (available only as Augmentin), and the associated diarrhea. Faropenem has been found to be clinically effective and safe in the treatment of upper respiratory gram-negative and gram-positive bacterial infections, bronchitis, pneumonia, otitis media, and urinary tract infections in children [8]. Recently, faropenem was also shown to possess activity against Mtb in susceptibility studies [9, 10]. Unlike the 3 first-line antituberculosis drugs of rifampin, isoniazid, and pyrazinamide, which are all associated with hepatotoxicity, faropenem is generally well tolerated, and its side-effect profile does not overlap with those of linezolid and moxifloxacin.
MATERIALS AND METHODS
Organism
Mtb H37Ra (ATCC 25177) was used throughout the experiments. Mtb culture stored at −80°C in Middlebrook 7H9 broth was thawed prior to the experiments, and grown into logarithmic phase growth in Middlebrook 7H9 broth supplemented with 10% oleic acid, dextrose, and catalase (OADC) at 37°C under 5% CO2 under slow shaking.
Materials and Drugs
Linezolid and moxifloxacin hydrochloride solution were purchased from the Baylor University Medical Center pharmacy. Faropenem sodium hydrate powder was purchased from BOC Sciences (Shirley, New York). Hollow fiber cartridges were purchased from FiberCell (Frederick, Maryland). BACTEC MGIT 960 Mycobacterial Growth Tube Indicator System (MGIT) was purchased from Becton Dickinson (Franklin Lakes, New Jersey), and MGIT tubes from BD.
Infection of THP-1 Cells With Mtb
Human-derived THP-1 monocytes (ATCC TIB-202) were cultured using prewarmed RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum at 37°C under 5% CO2. The cultures were checked for Mycoplasma contamination using a Mycoplasma PCR detection kit. The cells were maintained by subculturing every 72 hours, and then infected with log-phase growth Mtb at a bacteria-to-macrophage ratio of 1:1 by coincubation for 6 hours. Upon completion of the infection period, the monocytes were centrifuged at 200g for 5 minutes and washed with warm RPMI 1640 to remove carried-over extracellular bacteria. Cells were then counted using both a hemocytometer and Coulter counter.
Determination of Minimum Inhibitory Concentration
Linezolid and moxifloxacin minimum inhibitory concentrations (MICs) were determined as described elsewhere in this supplement [3, 11]. For faropenem MIC, turbidity of Mtb culture in log-phase growth was adjusted to a bacterial density of 105 colony-forming units per milliliter (CFU/mL). Bacterial suspension was treated with faropenem concentrations of 0, 0.007, 0.015, 0.030, 0.06, 0.125, 0.25, 0.5, 1.0, 2.0, 4.0, and 8.0 mg/L in 96-well plates in triplicate, and incubated at 37°C with 5% CO2 for 7 days. Resazurin at 0.01% (v/v) was added to each well and incubated overnight under the same conditions and assessed for color development. The MIC was defined as the lowest drug concentration that prevented color change from blue to pink.
Detection of Growth by MGIT System
In a pilot experiment, serial 10-fold dilutions from 0 to −4 of turbidity-adjusted Mtb in log-phase growth were prepared. Then, 500 µL of bacterial suspension from each dilution was inoculated into MGIT tubes purchased from BD, following which 0.8 mL of OADC was added to supplement growth. The cultures were placed in the MGIT system for detection of Mtb growth. Bacterial suspension from each dilution tube was also simultaneously cultured on Middlebrook 7H10 solid agar with 10% OADC growth supplement. Cultures were incubated at 37°C at 5% CO2 and CFU counted after 21 days. Exponential regression analyses of time to positivity (TTP) in days in the MGIT system vs log10 CFU/mL on agar had an r2 = 0.995. This assay was used for monitoring bacterial growth in the hollow fiber.
Faropenem Effect Versus Intracellular Mtb
THP-1 monocytes were activated in 12-well plates with phorbol myristate acetate as described previously [3, 11]. After 72 hours, the adherent cells were infected with log-phase growth Mtb as described above, and cultures coincubated with faropenem concentrations of 0, 1.25, 2.5, 5, 10, 20, 40, or 80 mg/L, in triplicate. The relationship between Mtb log10 CFU/mL and faropenem concentration was analyzed using the inhibitory sigmoid maximal kill (Emax) model.
Linezolid, Moxifloxacin, and Faropenem Combination Study in the Hollow Fiber System
The details of the HFS-Peds have been recently described elsewhere [11, 12]. We performed 3 linezolid, moxifloxacin, and faropenem combination studies in this model. Faropenem exposures were expressed as the percentage of time concentration that persisted above the MIC in a 24-hour dosing interval (TMIC); as efficacy of this penem is linked to this parameter for other pathogens, we assumed the same for Mtb [13]. In the first study, we tested 3 different dosing schedules of faropenem, including once a day leading to a TMIC = 12.5%, twice a day associated with TMIC = 25%, and 3 times a day to achieve TMIC = 62.5% in combination with the dual regimen of linezolid and moxifloxacin chosen from prior studies, so as to identify the faropenem exposure associated with the best microbial response [3]. The study regimens were administered over 14 days and the study was geared toward an intensive look at the kill slopes early during therapy. The faropenem half-life was 1.0 hour [5]. Moxifloxacin was administered to achieve concentration-time profile in infants of 10 mg/kg/day and linezolid 20 mg/kg/day. We also wanted to compare the kill rates to the pediatric concentration-time profiles of drugs in standard therapy dosed as 10 mg/kg/day of isoniazid, 15 mg/kg/day rifampin, and 40 mg/kg/day pyrazinamide. Fresh media was infused into, and pumped out of, the HFS-Peds at predefined rates to mimic linezolid, moxifloxacin, isoniazid (slow acetylators), and rifampin half-lives (T1/2) of 3–4 hours encountered in children aged <3.5 years [14–17]. The pyrazinamide T1/2 in children was 5.5 hours a day [16, 18]. The concentration-time profiles of each antibiotic were validated by sampling the central compartment of each HFS-Peds at 7 time points over a 24-hour dosing interval following infusion of test doses. Peripheral compartments were sampled on days 0, 3, 5, 7, 9, 12, and 14, to enable quantification of Mtb and THP-1 cells so that the kill slopes could be examined for the different exposure regimens.
Based on the results of the first experiment, the best faropenem exposure for microbial kill was chosen for the second study. The intent of the second experiment was (i) to compare the slopes of the different experimental regimens in which the doses of moxifloxacin and linezolid combined with one faropenem exposure were varied, (ii) to add ethambutol as a fourth drug to accelerate microbial kill, and (iii) to examine the sensitivity and utility of TTP as a pharmacodynamic measure, particularly to determine limits of assay detection with log10 CFU/mL in a paucibacillary state. The MGIT liquid culture method has been found to be more sensitive than cultures on solid agar, and is now the standard used in the clinic and in clinical trials [19–21]. A low bacillary burden was used to mimic the paucibacillary state encountered in children. The treatment duration was for 10 days, and sampling of peripheral compartments was done on days 0, 3, 7, and 10. Samples were centrifuged at 21 100g for 5 minutes at room temperature and supernatant was removed. Then, the pellet was suspended in equal volume of phosphate-buffered saline with 0.05% Triton X and vortexed until disappearance of the pellet. Next, 0.5 mL of this suspension was introduced into the commercial MGIT tubes as described earlier. The MGIT tubes were then loaded into the MGIT machine and incubated per the manufacturer's instructions for detection of growth, and incubated for 56 days before being declared negative.
We performed a third hollow fiber study with drug regimen exposures identical to those of the second. This time we started with a higher bacillary burden, and extended the treatment duration for 28 days. Sampling of central compartment to validate that intended concentration-time profiles of antibiotic had been achieved was performed as described above. The peripheral compartments were sampled on days 0, 3, 7, 14, 21, and 28 for determination of both log10 CFU/mL and TTP. This study design allowed us to examine the longer-term bacterial decline, determine if there was total sterilization of the systems, compare kill rates to standard therapy, and also determine if there was rebound/failure of the regimen beyond the first 2 weeks.
Regression Analysis Methods for Bacterial Burden
We examined for both linear regression, as well as an exponential decline model. Exponential decline is based on the simple exponential decay relationship:
where N is Mtb bacterial burden, t is time in days, and the bacterial burden in each hollow fiber system at t = 0 is N0. The standard solution for this is given by:
For the best model, we compared the r2 for standard therapy, given that both exponential decline models and linear models have been applied to standard therapy in patients. We report both results; however, the model with the higher r2 was chosen for the final ranking of kill rates.
Drug Concentration Assays
Concentrations of antibiotics in the samples collected from the central compartment of the HFS were analyzed using liquid chromatography–tandem mass spectrometry. The assays to measure rifampin, isoniazid, pyrazinamide, linezolid, and moxifloxacin have been described in the past [3, 11, 12]. Faropenem-d4 and sulfamethoxazole-d4 (internal standards) were purchased from Toronto Research Chemicals (Toronto, Canada). Calibrator, controls, and internal standard were included in each analytical run for quantitation. Stock solutions of faropenem and internal standard were prepared in 80:20 methanol:water at a concentration of 1 mg/mL and stored at −20°C. A 7-point calibration curve was prepared by diluting faropenem stock solution in drug-free media (0.1, 0.2, 1, 2, 5, 10, and 20 µg/mL). Quality control samples were prepared by spiking media with stock standards for 2 levels of controls of 0.8 µg/mL and 16 µg/mL. Samples were prepared in 96-well plates by the addition of 10 µL of calibrator, quality controls, or sample to 190 µL 0.1% formic acid in water containing 1 µg/mL internal standard followed by vortex. Chromatographic separation was achieved on an Acquity UPLC HSS T3 1.8-µm 50 × 2.1-mm analytical column (Waters) maintained at 30°C at a flow of 0.2 mL/minute with a binary gradient with a total run time of 6 minutes. The observed ion (m/z) values of the fragment ions were 308.0 → 177.9. Sample injection and separation was performed by an Acquity UPLC interfaced with a Xevo TQ mass spectrometer (Waters). All data were collected using MassLynx software version 4.1 SCN810. The limit of quantitation for this assay was 0.1 µg/mL. The interday % coefficient of variation (%CV) for the assay was 2%–5%, which was identical to the interday %CV.
RESULTS
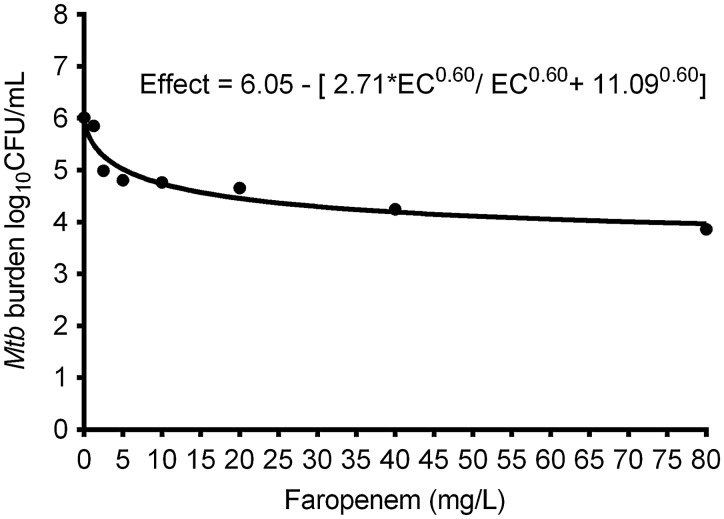
The faropenem MIC of Mtb H37Ra strain was 1.0 mg/L. The inhibitory sigmoid Emax relationship between faropenem exposure and intracellular Mtb is shown in Figure 1. The figure shows that faropenem effectively kills intracellular Mtb as monotherapy.
Figure 1.
Exposure-effect relationship of faropenem and Mycobacterium tuberculosis (Mtb) on day 7 using inhibitory sigmoid maximal kill (Emax) model. Treatment with static concentrations of faropenem for 7 days resulted in a Emax of 2.71 log10 colony-forming units (CFU)/mL with an exposure mediating 50% of Emax of 11.09 mg/L (r2 = 0.91), as shown in the equation, which is a kill rate similar to, and better than, most currently used antituberculosis agents.
The drug exposures achieved in the first hollow fiber study were a linezolid 0- to 24-hour area under the curve (AUC0–24)/MIC of 61.93, a moxifloxacin AUC0–24/MIC of 247.56, isoniazid AUC0–24/MIC of 524.33, rifampin AUC0–24/MIC of 113.21, and pyrazinamide AUC0–24/MIC of 45.18. The faropenem TMICs observed after drug assays of the replicate HFS-TB in this experiment were exactly 12.5%, 25%, and 62.5%. Figure 2A shows the THP-1 counts over the 14-day experiment. The figure shows no effect by the different faropenem dosing exposures on THP-1 cell viability; indeed, the counts were similar to nontreated controls. Figure 2B shows the microbial kill slopes for each regimen over the 14 days, based on log10 CFU/mL. Figure 2B shows that the regimen with faropenem with TMIC of 62.5% had the best kill rates among the faropenem regimens, based on the exponential decline model. The time required to sterilize each system by the faropenem TMIC > 62% triple regimen was similar to that for standard therapy.
Figure 2.
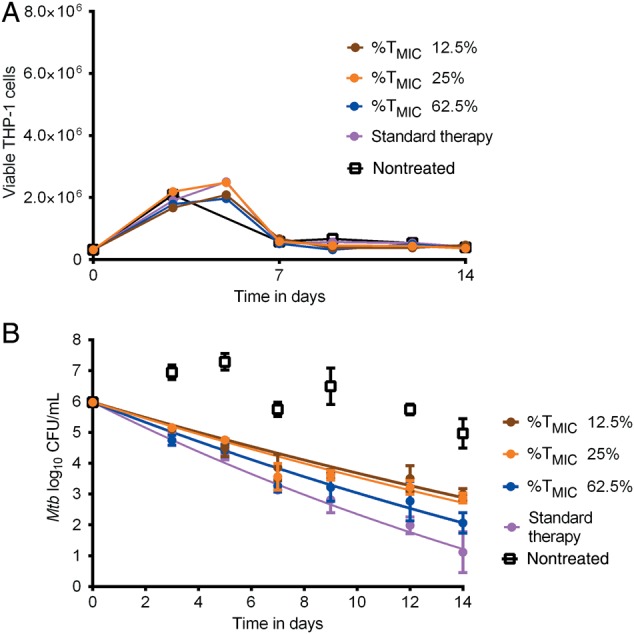
Faropenem exposure-response in combination regimen. A, Effect of different faropenem % of time concentration persists above minimum inhibitory concentration (%TMIC) and linezolid-moxifloxacin regimen on THP-1 cells. There was no effect of faropenem exposures on THP-1 cells. B, Effect of different faropenem exposures in combination with the linezolid-moxifloxacin regimen based on an exponential decline model for Mycobacterium tuberculosis (Mtb) log10 colony-forming units (CFU)/mL. The faropenem TMIC of 62.5% had a steeper slope than 12.5% and 25%. There was no convergence for the exponential decline model for nontreated controls, as expected, given they did not decline.
In the next hollow fiber experiments, a faropenem TMIC of 62.5% was used in combination with linezolid and moxifloxacin; drug concentrations were not measured in this study. There was no change in macrophage count over the course of 10 days. Figure 3A shows the change in log10 CFU/mL, and shows that based on colony counts, the experimental regimen of faropenem and standard doses of linezolid and moxifloxacin (FLM) had a kill rate similar to the standard therapy regimen. Since it looked as if the high-dose moxifloxacin regimen (FLMHi) plus ethambutol could have a steeper decline, we performed 2-way analysis of variance (ANOVA) to examine the effect of regimen and day of therapy, which revealed that the day of therapy accounted for 93.29% of the total variation (P < .0001) whereas the regimen accounted for only 0.783% (P = .1473). Next, we examined TTP as a pharmacodynamic measure, given that in Figure 3A the regimens seemed to have sterilized the HFS-Peds by the last day of therapy. Figure 3B shows that based on TTP, some regimens such as standard therapy had not yet sterilized the systems; thus, TTP was a more sensitive pharmacodynamic parameter for identifying live bacteria, especially at CFU/mL assay limits. Second, Figure 3B shows that the experimental regimen FLM killed slightly better than the standard therapy regimen based on TTP, whereas the FLMHi regimens with and without ethambutol were even better. Indeed, a 2-way ANOVA revealed that while day of sampling explained 55.16% of TTP variation, the regimen explained 17.41% of variation (P < .0001).
Figure 3.
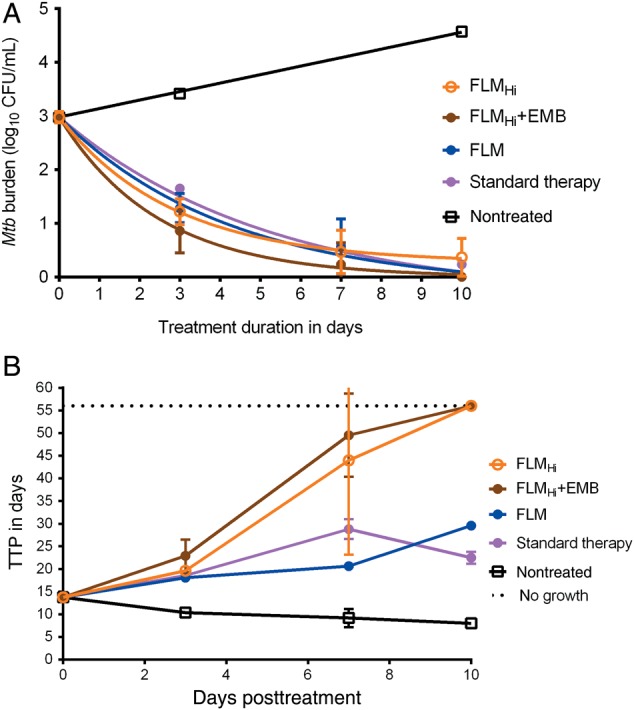
Microbial responses to optimized faropenem-based regimens in a paucibacillary model. A, The experimental faropenem, linezolid, and moxifloxacin (FLM) regimen and high-dose moxifloxacin (FLMHi) plus ethambutol (EMB), as well as the standard therapy regimen, had killed Mycobacterium tuberculosis (Mtb) in the pediatric hollow fiber system model for intracellular tuberculosis systems below colony-forming unit (CFU)/mL assay limits, suggesting complete sterilization. B, The time to positivity (TTP) assay revealed that there was still bacterial growth with the FLM regimen and standard regimen; the former has statistically higher TTP compared with standard regimen at the end of experiment, suggesting better kill rates.
The third hollow fiber experiment drug concentration measurements revealed linezolid, isoniazid, rifampin, and pyrazinamide exposures as in the first experiment. The moxifloxacin AUC0–24/MIC was 121.6 in the FLM regimen and 243.2 in the FLMHi regimen; faropenem TMIC was exactly 62.5%. These exposures achieved log10 CFU/mL kill slopes shown in Figure 4A, based on a linear regression model. The figure would seem to suggest equivalence of the FLMHi plus ethambutol regimen and standard therapy based on overlapping 95% confidence intervals (CIs), and superiority to FLM. However, an analysis of the same data using the exponential decline model revealed superior r2 values and lower corrected Akaike information criteria scores, which means the exponential decline model was better. The exponential decline model slopes are shown in Figure 4B. The kill rate constants based on log10 CFU/mL assay were 0.098 (95% CI, .082–.114; r2 = 0.92) per day for FLMHi plus ethambutol, 0.096 (95% CI, .085–.106; r2 = 0.95) per day for FLMHi, and 0.048 (95% CI, .037–.059; r2 = 0.81) per day for FLM. On the other hand, the kill rate constant for the standard therapy regimen was 0.127 (95% CI, .104–.149; r2 = 0.92) per day, while the nontreated controls showed no decline and thus the model did not converge. Thus, based on this, the kill rate constants and 95% confidence intervals overlapped between 2 faropenem regimens and standard therapy, with the FLM regimen slightly less steep than the rest. Figure 4B shows that for some regimens, CFU/mL values were below the limit of detection from day 14 onward. However Figure 4C shows that by using TTP as a response measure, there was still bacterial growth in all systems up to day 28. Figure 4C shows that based on TTP, all drug treatment regimens, including FLM, were actually equivalent at the end of experiment, but had significantly lower bacterial burden than nontreated controls. Thus, all the regimens killed at similar rates based on TTP over the 1-month duration of the experiment.
Figure 4.
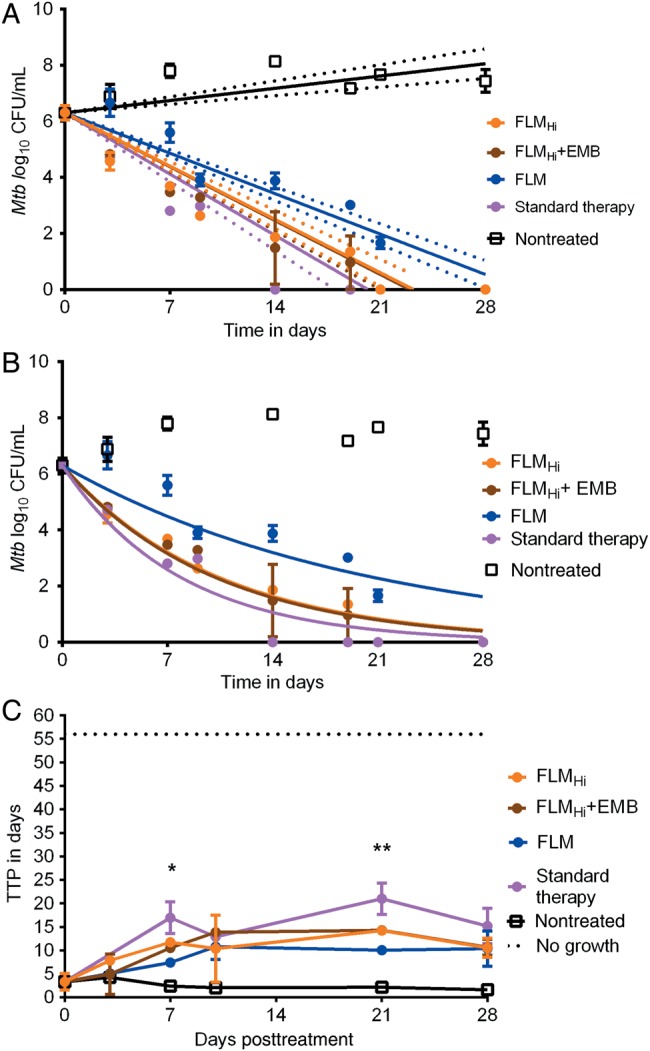
Effect of a new faropenem, linezolid, and moxifloxacin (FLM) regimen over 1 month. A, Kill slopes of the different regimens based on a linear regression model and 95% confidence bounds. The r2 for the FLM regimen was 0.92, for high-dose moxifloxacin (FLMHi) was 0.84, for FLMHi with ethambutol (EMB) was 0.84, and for standard therapy was 0.73. B, Exponential decline model. The decline rates of all the regimens were similar to standard therapy. C, Time to positivity (TTP) of contents from each hollow fiber system shows that there is no sterilization up to day 28 with TTP when compared to Mycobacterium tuberculosis (Mtb) log colony-forming units (CFU)/mL, which revealed sterilization. In this experiment we started with a higher bacterial burden, an acid test. Results of multiple t test comparison shown as *P value of .045 on day 7 and **P = .011 for day 21. However, by day 28 the TTPs were similar in all drug treatment regimens.
DISCUSSION
First, here and in the accompanying articles in this supplement, we went through a series of steps for multidrug regimen design specific to intracellular tuberculosis in children [3, 11, 22]. This approach is, to our knowledge, the first of its kind not copied from adults. The approach takes into consideration pharmacokinetic/pharmacodynamic-derived target exposures associated with optimal microbial kill as monotherapy as well as the exposures associated with synergy and additivity when using drugs in combination. Here, we effected the third step, which is the sequential addition of a third (and potentially a fourth) agent. Use of the standard regimen ensures comparison of a pharmacodynamic target whose success rate is already known in the clinic to judge the speed of kill (ie, CFU/mL and TTP slopes). This comparison could potentially indicate if a new regimen could kill all Mtb in children in 6 months or less. If slopes are similar to the standard regimen, then likely 6 months will be enough with the experimental regimen, whereas if the slope for the experimental regimen is faster, then potentially shorter therapy duration could work. This approach requires further validation based on comparison of cure rates in children. As regards to the regimen, elsewhere in sterilizing effect models, when the same regimen was compared with standard therapy over 2 months of intensive phase, we found that it actually outperformed the rifampin-pyrazinamide–based standard therapy in terms of slope (manuscripts in preparation). This suggests the regimen could work even in adult-type tuberculosis in teenagers. The time from the first linezolid monotherapy experiment in this series to counting the last CFUs in the current study, and completion of simulations was 1 year and 4 months (with change in our laboratory location in the process). In other words, this deliberate approach is dramatically faster, cheaper, and safer than trying to perform this sequentially in clinical studies of children that would try to switch out 1 drug at a time.
Second, using this approach we have developed a faropenem-linezolid-moxifloxacin (with and without ethambutol) or “FLAME” regimen that is independent of rifampin, isoniazid, and pyrazinamide, and thus can be tested in clinical trials to treat both multidrug-resistant (MDR) tuberculosis and drug-susceptible tuberculosis, given that obtaining cultures for microbiologic diagnosis is difficult, particularly in very young children [23]. Moreover, the current treatment for drug-susceptible tuberculous meningitis is associated with death in 20% of children and probability of survival without neurologic sequelae of only 40%. In this context, the good cerebrospinal fluid (CSF) penetration by linezolid could be an advantage. Furthermore, the faropenem-linezolid portion of the regimen will also work for extensively drug-resistant (XDR) tuberculosis in children. As the microbial kill slopes were similar to those of standard short-course chemotherapy for drug-susceptible tuberculosis, this new regimen could be examined for cure of children with MDR tuberculosis in 6 months. This is more so given that we had optimized the exposures in the standard regimen (reflected by half-lives of isoniazid and rifampin), and hence AUC/MIC, on the high end of the distribution. Thus, the equivalence of effect we identified is unlikely to be due to suboptimal dosing in the standard regimen. In the future, hollow fiber studies adding another antibiotic to regimen, or switching out congeners (either oxazolidinones or quinolones or penems) to achieve faster Mtb kill rates than standard therapy could be examined. The addition of a fourth drug (especially one that also works against XDR tuberculosis) that improves the slope by a factor of 2 or 3 could create a regimen administered for shorter duration of therapy for all forms of tuberculosis, including drug-resistant ones.
Finally, in the current study we validated TTP as a pharmacodynamic measure for hollow fiber tuberculosis studies. Comparing TTP will allow easier translation to the clinic. In the clinic, liquid media have been shown to be more sensitive than solid media for culture of mycobacteria from sputum; TTP at commencement of therapy correlates with treatment outcome and mortality for pulmonary and extrapulmonary tuberculosis [24–32]. This method also gave a more sensitive index for measuring total sterilization compared with solid agar colony counts in the hollow fiber, given that positive cultures were encountered up to 28 days of therapy despite negative cultures on agar. In addition, in earlier studies we also introduced host–pathogen RNA sequencing to these HFS-Peds studies, which would provide yet another measure of total sterilization. These measures will improve sensitivity of prediction of such clinical microbial measures as relapse.
There are limitations to our study. We used an avirulent strain of Mtb in all our studies. This is because virulent strains kill macrophages within days, making it difficult to maintain a long-term co-culture system, as we demonstrated in a prior study [12]. However, the effect of drugs in this system that uses this avirulent strain and the ranking of drugs in combination regimens for the standard therapy seem to be well reflected in clinical outcomes in children with intrapulmonary, extrapulmonary, and disseminated tuberculosis [12]. Moreover, comparison of kill rates of the intracellular isogenic virulent strain in studies done by others to our current studies on adherent THP-1 cells reveals virtually the same kill curves, at least in 12-well plates, suggesting similar responses between virulent and avirulent strains [9]. We also intend to perform further validation in a mouse model of disseminated tuberculosis, still being developed and validated.
In summary, we have developed a combination FLAME regimen, for treatment of intracellular tuberculosis in children: the first steps along the mythical path created by the young Khoisan girl who gave us the Milky Way [33]. The regimen displays similar kill rates to the current standard 3-drug regimen of isoniazid, rifampin, and pyrazinamide for drug-susceptible tuberculosis. In doing so, we have created a regimen that is likely to work against both MDR tuberculosis and drug-susceptible tuberculosis, and has good CSF penetration rates for possible treatment of tuberculous meningitis.
Notes
Author contributions. T. G., and E. N. designed the study; D. D., S. S., and T. G. performed the hollow fiber studies; D. D. wrote the first draft of the manuscript; D. D., S. S., E. N., J. G. P., S. S., and T. G. wrote the manuscript.
Financial support. Funding for this study was provided by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant number R56 AI111985).
Supplement sponsorship. This article appears as part of the supplement “A Development Paradigm for Novel Combination Regimens for Multidrug-Resistant and Drug-Susceptible Tuberculosis in Children: FLAME for Work and Play,” sponsored by the Center for Infectious Diseases Research and Experimental Therapeutics (CIDRET), Baylor Institute for Immunology Research, Baylor Research Institute.
Potential conflicts of interest. T. G. is a consultant for Astellas Pharma USA and LuminaCare solutions, and founded Jacaranda Biomed, Inc. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Blumberg HM, Burman WJ, Chaisson RE et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 2003; 167:603–62. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. 2nd ed. Geneva, Switzerland: WHO, 2014. [PubMed] [Google Scholar]
- 3.Deshpande D, Srivastava S, Nuermberger E, Pasipanodya JG, Swaminathan S, Gumbo T. Concentration-dependent synergy and antagonism of linezolid and moxifloxacin in the treatment of childhood tuberculosis: the dynamic duo. Clin Infect Dis 2016; 63(suppl 3):S88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalhoff A, Nasu T, Okamoto K. Beta-lactamase stability of faropenem. Chemotherapy 2003; 49:229–36. [DOI] [PubMed] [Google Scholar]
- 5.Gettig JP, Crank CW, Philbrick AH. Faropenem medoxomil. Ann Pharmacother 2008; 42:80–90. [DOI] [PubMed] [Google Scholar]
- 6.Mushtaq S, Hope R, Warner M, Livermore DM. Activity of faropenem against cephalosporin-resistant Enterobacteriaceae. J Antimicrob Chemother 2007; 59:1025–30. [DOI] [PubMed] [Google Scholar]
- 7.Schurek KN, Wiebe R, Karlowsky JA, Rubinstein E, Hoban DJ, Zhanel GG. Faropenem: review of a new oral penem. Expert Rev Anti Infect Ther 2007; 5:185–98. [DOI] [PubMed] [Google Scholar]
- 8.Yokota T, Azagami S, Abe T et al. Efficacy and safety of faropenem in pediatric patients with bacterial infectious diseases. Jpn J Antibiot 2008; 61:366–78. [PubMed] [Google Scholar]
- 9.Dhar N, Dubee V, Ballell L et al. Rapid cytolysis of Mycobacterium tuberculosis by faropenem, an orally bioavailable beta-lactam antibiotic. Antimicrob Agents Chemother 2015; 59:1308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solapure S, Dinesh N, Shandil R et al. In vitro and in vivo efficacy of beta-lactams against replicating and slowly growing/nonreplicating Mycobacterium tuberculosis. Antimicrob Agents Chemother 2013; 57:2506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deshpande D, Srivastava S, Pasipanodya JG et al. Linezolid for infants and toddlers with disseminated tuberculosis. Clin Infect Dis 2016; 63(suppl 3):S80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava S, Pasipanodya JG, Ramachandran G et al. A long-term co-perfused disseminated tuberculosis-3D liver hollow fiber model for both drug efficacy and hepatotoxicity in babies. EBioMedicine 2016; 6:126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill SC, Rubino CM, Bassett J et al. Pharmacokinetic-pharmacodynamic assessment of faropenem in a lethal murine Bacillus anthracis inhalation postexposure prophylaxis model. Antimicrob Agents Chemother 2010; 54:1678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jungbluth GL, Welshman IR, Hopkins NK. Linezolid pharmacokinetics in pediatric patients: an overview. Pediatr Infect Dis J 2003; 22:S153–7. [DOI] [PubMed] [Google Scholar]
- 15.Thee S, Garcia-Prats AJ, Draper HR et al. Pharmacokinetics and safety of moxifloxacin in children with multidrug-resistant tuberculosis. Clin Infect Dis 2015; 60:549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiruy H, Rogers Z, Mbowane C et al. Subtherapeutic concentrations of first-line anti-TB drugs in South African children treated according to current guidelines: the PHATISA study. J Antimicrob Chemother 2015; 70:1115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safety, tolerability and pharmacokinetics of single dose intravenous moxifloxacin in pediatric patients, 2011. Available at: http://trialfinder.bayerscheringpharma.de/html/pdf/11826_Study_Synopsis_CTP.pdf. Accessed 19 July 2016. [DOI] [PMC free article] [PubMed]
- 18.Swaminathan S, Pasipanodya J, Ramachandran G, Srivastava S, Deshpande D, Gumbo T. Drug concentration thresholds predictive of therapy failure and death in children with tuberculosis: bread crumb trails in random forests. Clin Infect Dis 2016; 63(suppl 3):S63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joloba ML, Johnson JL, Namale A et al. Quantitative sputum bacillary load during rifampin-containing short course chemotherapy in human immunodeficiency virus-infected and non-infected adults with pulmonary tuberculosis. Int J Tuberc Lung Dis 2000; 4:528–36. [PubMed] [Google Scholar]
- 20.MacKenzie WR, Heilig CM, Bozeman L et al. Geographic differences in time to culture conversion in liquid media: Tuberculosis Trials Consortium study 28. Culture conversion is delayed in Africa. PLoS One 2011; 6:e18358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rustomjee R, Lienhardt C, Kanyok T et al. A phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 2008; 12:128–38. [PubMed] [Google Scholar]
- 22.Srivastava S, Deshpande D, Thomas T, Swaminathan S, Nuermberger E, Gumbo T. A combination regimen design program based on pharmacodynamic target setting for childhood tuberculosis: design rules for the playground. Clin Infect Dis 2016; 63(suppl 3):S75–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marais BJ, Pai M. New approaches and emerging technologies in the diagnosis of childhood tuberculosis. Paediatr Respir Rev 2007; 8:124–33. [DOI] [PubMed] [Google Scholar]
- 24.Abe C, Hosojima S, Fukasawa Y et al. Comparison of MB-Check, BACTEC, and egg-based media for recovery of mycobacteria. J Clin Microbiol 1992; 30:878–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfyffer GE, Welscher HM, Kissling P et al. Comparison of the mycobacteria growth indicator tube (MGIT) with radiometric and solid culture for recovery of acid-fast bacilli. J Clin Microbiol 1997; 35:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chew WK, Lasaitis RM, Schio FA, Gilbert GL. Clinical evaluation of the mycobacteria growth indicator tube (MGIT) compared with radiometric (Bactec) and solid media for isolation of mycobacterium species. J Med Microbiol 1998; 47:821–7. [DOI] [PubMed] [Google Scholar]
- 27.Epstein MD, Schluger NW, Davidow AL, Bonk S, Rom WN, Hanna B. Time to detection of Mycobacterium tuberculosis in sputum culture correlates with outcome in patients receiving treatment for pulmonary tuberculosis. Chest 1998; 113:379–86. [DOI] [PubMed] [Google Scholar]
- 28.Somoskovi A, Magyar P. Comparison of the mycobacteria growth indicator tube with MB redox, Lowenstein-Jensen, and Middlebrook 7H11 media for recovery of mycobacteria in clinical specimens. J Clin Microbiol 1999; 37:1366–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pheiffer C, Carroll NM, Beyers N et al. Time to detection of Mycobacterium tuberculosis in BACTEC systems as a viable alternative to colony counting. Int J Tuberc Lung Dis 2008; 12:792–8. [PubMed] [Google Scholar]
- 30.Chihota VN, Grant AD, Fielding K et al. Liquid vs. solid culture for tuberculosis: performance and cost in a resource-constrained setting. Int J Tuberc Lung Dis 2010; 14:1024–31. [PubMed] [Google Scholar]
- 31.Hesseling AC, Walzl G, Enarson DA et al. Baseline sputum time to detection predicts month two culture conversion and relapse in non- HIV-infected patients. Int J Tuberc Lung Dis 2010; 14:560–70. [PubMed] [Google Scholar]
- 32.Pasipanodya JG, Mubanga M, Ntsekhe M et al. Tuberculous pericarditis is multibacillary and bacterial burden drives high mortality. EBioMedicine 2015; 2:1634–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lesau C, Lesau W. African folk tales. Mount Vernon, NY: Peter Pauper Press, 1963. [Google Scholar]