One-third of women enrolled in Malawi's program to prevent human immunodeficiency virus mother-to-child-transmission (Option B+) adhered inadequately to antiretroviral therapy during pregnancy and breastfeeding. Long-term virological outcomes must be closely monitored, and effective interventions to improve adherence should be deployed.
Keywords: adherence, antiretroviral therapy, Option B+, mother-to-child-transmission, HIV
Abstract
Background. Adherence to antiretroviral therapy (ART) is crucial to preventing mother-to-child transmission of human immunodeficiency virus (HIV) and ensuring the long-term effectiveness of ART, yet data are sparse from African routine care programs on maternal adherence to triple ART.
Methods. We analyzed data from women who started ART at 13 large health facilities in Malawi between September 2011 and October 2013. We defined adherence as the percentage of days “covered” by pharmacy claims. Adherence of ≥90% was deemed adequate. We calculated inverse probability of censoring weights to adjust adherence estimates for informative censoring. We used descriptive statistics, survival analysis, and pooled logistic regression to compare adherence between pregnant and breastfeeding women eligible for ART under Option B+, and nonpregnant and nonbreastfeeding women who started ART with low CD4 cell counts or World Health Organization clinical stage 3/4 disease.
Results. Adherence was adequate for 73% of the women during pregnancy, for 66% in the first 3 months post partum, and for about 75% during months 4–21 post partum. About 70% of women who started ART during pregnancy and breastfeeding adhered adequately during the first 2 years of ART, but only about 30% of them had maintained adequate adherence at every visit. Risk factors for inadequate adherence included starting ART with an Option B+ indication, at a younger age, or at a district hospital or health center.
Conclusions. One-third of women retained in the Option B+ program adhered inadequately during pregnancy and breastfeeding, especially soon after delivery. Effective interventions to improve adherence among women in this program should be implemented.
In 2011, Malawi pioneered a simplified test and treat policy (Option B+) to effectively prevent mother-to-child transmission by providing lifelong antiretroviral therapy (ART) to pregnant and breastfeeding women infected with human immunodeficiency virus (HIV), regardless of immunological or clinical criteria [1, 2]. Option B+ was adopted by other low-resource countries and is now recommended by the World Health Organization (WHO) [3]. Under Option B+, HIV infection is diagnosed in many women during routine testing in antenatal care, when they are asymptomatic, and these women start treatment soon after that [4]. They receive a daily fixed-dose combination of efavirenz, tenofovir, and lamivudine. They collect new medication at each monthly visit for the first 6 months of treatment, and every 2–3 months thereafter [1, 2].
ART uptake greatly improved after Option B+ was implemented [5], but there is concern that women with asymptomatic HIV infections may not be retained or adhere poorly to treatment [6]. A recent study showed that about 30% of women who started ART under Option B+ were lost to follow-up (LTF) 3 years after starting ART, but no study has described adherence among women in the Option B+ program [7]. A systematic review of studies published before 2012 found that 73% of women adhered well during pregnancy, but only 53% adhered after delivery [8]. More recent studies suggest higher levels of adherence, during both pregnancy and the postpartum period [9–12]. Inadequate adherence to ART is associated with loss of viral suppression, increased risk of transmitting HIV, therapeutic failure, and viral resistance [13–17].
We examined adherence of women who began treatment between 1 September 2011 and 15 October 2013, at 13 health facilities in Malawi. We compared adherence between pregnant and breastfeeding women eligible for ART under Option B+, and women who were not pregnant and not breastfeeding and started ART with a CD4 cell count <350/µL or with WHO clinical stage 3/4 disease.
METHODS
Study Design and Patient Eligibility
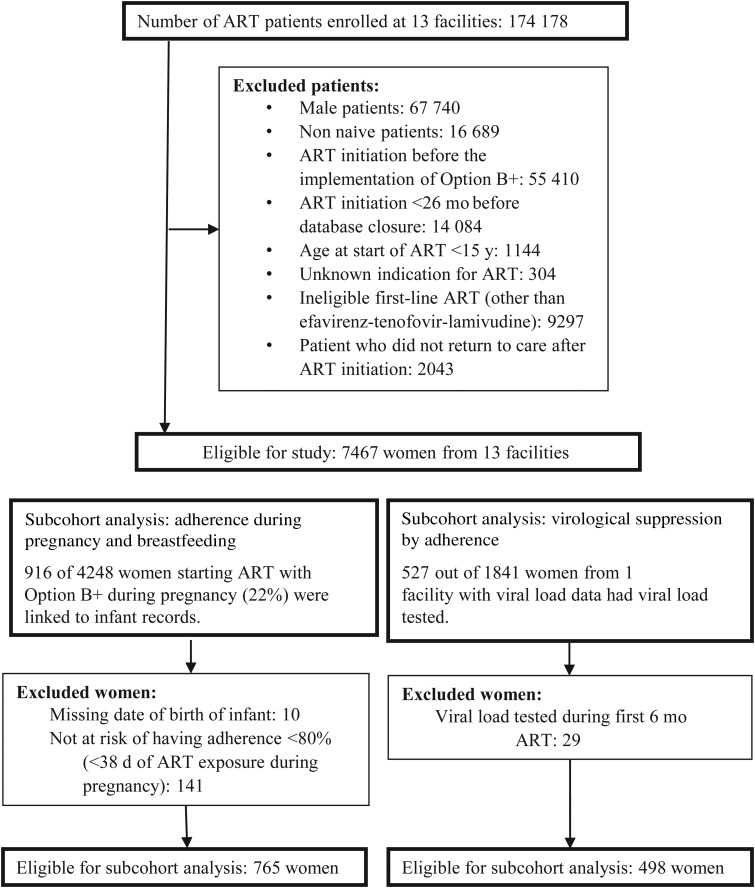
We included HIV-infected women (defined here as ≥15 years old), who started an ART regimen of efavirenz, tenofovir, and lamivudine between 1 September 2011 and 15 October 2013 and later returned to care after that (Figure 1). Our data came from 13 health facilities in Malawi that used electronic medical records systems (EMRS) during the study period and entered pharmacy data prospectively at the point of care. At most study facilities, women started ART in antenatal care and were referred to ART clinics 6 weeks post partum. Ethical approval was granted by the Malawi National Health Sciences Research Committee and the Cantonal Ethics Committee of Bern, Switzerland.
Figure 1.
Flow of eligibility of study participants. Abbreviation: ART, antiretroviral therapy.
Data Sources
Healthcare workers use an EMRS with barcode scanners and touchscreens to record data at the point of care [18]. Pharmacy claims are registered with barcode scanners. The EMRS records type of ART regimen, date of drug dispensation, number of returned pills, and number of pills dispensed. One facility had viral load data for 498 women (27%), and we compared adherence with viral suppression in this subgroup (Figure 1). Date of delivery is recorded in paper-based birth registers for HIV-exposed children and not in maternal ART records. We entered paper records into a database and used probabilistic record linkage to merge maternal and infant records. For the subgroup of women we linked, we compared antepartum and postpartum adherence (Figure 1).
Outcomes
We defined adherence as the proportion of days “covered” by pharmacy claims, calculated as the percentage of days drugs were available to women during a time interval [19]. Adherence was determined for each interval between clinic visits and for fixed time intervals (3 months and 2 years). In a subset of women (Figure 1), we compared virological suppression between women with different adherence levels and then defined a threshold for adequate adherence. Adherence of ≥90% was categorized “adequate” and adherence of <90% as “inadequate.” Virological suppression was defined as viral load value of <400 copies/mL, measured ≥6 months after start of ART. Timing of viral load testing was not standardized. We only considered results of viral load tests taken at least 6 months after ART initiation.
Procedures
We classified women by pregnancy and breastfeeding status at the start of ART: pregnant and eligible for ART under Option B+, breastfeeding and eligible for ART under Option B+, or not pregnant or breastfeeding and eligible for ART owing to WHO stage 3 or 4 disease or CD4 cell count <350/µL. Women were considered “retained in care” between the start of ART and their last drug refill. We classified women >60 days late for drug refills by the end of the study as LTF. Women who transferred to another facility were classified as “transferred out,” and those who informed healthcare workers that they had discontinued ART were classified as “stopped ART.”
Statistical Analysis
We calculate adjusted odds ratios (aORs) with 95% confidence intervals (CIs) for virological suppression by adherence strata (<80%, 80%–89%, 90%–99%, or 100%) to validate our adherence measure and determine the threshold for adequate adherence. We therefore used multivariable logistic regression adjusted for age, pregnancy and breastfeeding status at start of ART, and time receiving ART before viral load test.
We followed up women for a maximum of 2 years with ART. Women who stopped ART, died, were LTF, or transferred out were censored on the day they submitted their last pharmacy claim. In an earlier analysis of data from the same cohort of women, we showed that censoring did not occur at random: women with poor adherence had higher dropout rates than those with good adherence [7]. This mechanism of “informative censoring” biases analysis of patterns of adherence over time [20]. We used stabilized inverse probability of censoring weighting (IPCW) to adjust for informative censoring. Weights were calculated as the inverse of the probability of being under follow-up, given observed adherence (<80%, 80%–89%, 90%–99%, or 100%), pregnancy and breastfeeding status at start of ART, age (15–19, 20–24, 25–29, or ≥30 years), WHO clinical stage at start of ART, and facility type (health center, district hospital, faith-based hospital, or central hospital).
We used descriptive statistics to examine the characteristics of women at start of ART. We used Fine and Gray's method to estimate cumulative incidences of LTF, transferring out, death, and stopping ART [21]. Mortality, transferring out, stopping ART, and LTF were competing events. We used the Kaplan–Meier method to estimate retention in care and the probability that women would maintain adequate adherence. We split follow-up time into 3-month intervals and determined the proportions of women with adherence ≥90% or <90% in each interval. For the subset of women who could be linked to infants (Figure 1), we determined proportions of women with adherence ≥90% or <90% during pregnancy and in 3-month intervals post partum. We used pooled logistic regression with cluster-based robust standard errors to calculate adjusted hazard ratios (aHRs) for adequate adherence. Analysis was adjusted for age, pregnancy and breastfeeding status at the start of ART, facility type, and WHO clinical stage. We used Stata software, version 14, for all statistical analyses.
RESULTS
Characteristics of Study Participants
A total of 7467 women met our eligibility criteria: 4248 (57%) were pregnant, 1614 (22%) were breastfeeding, and 1605 (22%) were neither pregnant nor breastfeeding at the start of ART (Figure 1; Table 1). Pregnant and breastfeeding women started ART younger and had less advanced HIV disease than women who were not pregnant or breastfeeding.
Table 1.
Women's Characteristics at Start of Antiretroviral Therapy
| Characteristic | Women by Pregnancy and Breastfeeding Status at Start of ART, No. (%)a |
|||
|---|---|---|---|---|
| Pregnant (Option B+) (n = 4248; 56.9%) | Breastfeeding (Option B+) (n = 1614; 21.6%) | Not Pregnant or Breastfeeding (n = 1605; 21.5%) | Total (n = 7467; 100.0%) | |
| Age group | ||||
| 15–19 y | 279 (6.6) | 79 (4.9) | 48 (3.0) | 406 (5.4) |
| 20–24 y | 958 (22.6) | 360 (22.3) | 169 (10.5) | 1487 (19.9) |
| 25–29 y | 1731 (40.7) | 646 (40.0) | 424 (26.4) | 2801 (37.5) |
| ≥30 y | 1280 (30.1) | 529 (32.8) | 964 (60.1) | 2773 (37.1) |
| Age, median (IQR), y | 28 (24–32) | 28 (24–32) | 33 (27–39) | 29 (24–33) |
| Year of ART initiation | ||||
| 2011 | 516 (12.1) | 495 (30.7) | 248 (15.5) | 1259 (16.9) |
| 2012 | 2236 (52.6) | 803 (49.8) | 475 (29.6) | 3514 (47.1) |
| 2013 | 1496 (35.2) | 316 (19.6) | 882 (55.0) | 2694 (36.1) |
| WHO clinical stage | ||||
| 1 | 4187 (98.6) | 1435 (88.9) | 369 (23.0) | 5991 (80.2) |
| 2 | 38 (0.9) | 87 (5.4) | 169 (10.5) | 294 (3.9) |
| 3 | 15 (0.4) | 1 (0.1) | 749 (46.7) | 765 (10.2) |
| 4 | 8 (0.2) | 0 (0.0) | 206 (12.8) | 214 (2.9) |
| Unknown | 0 (0.0) | 91 (5.6) | 112 (7.0) | 203 (2.7) |
| Facility type | ||||
| Central hospital | 462 (10.9) | 180 (11.2) | 464 (28.9) | 1106 (14.8) |
| District hospital | 2546 (59.9) | 858 (53.2) | 723 (45.0) | 4127 (55.3) |
| Mission hospital | 349 (8.2) | 267 (16.5) | 180 (11.2) | 796 (10.7) |
| Health center | 891 (21.0) | 309 (19.1) | 238 (14.8) | 1438 (19.3) |
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range; WHO, World Health Organization.
a Data represent No. (%) of women unless otherwise indicated.
Adherence and Virological Suppression
One facility had viral load data, and we compared adherence with virological suppression in this subgroup. Out of 1841 women included in the study facility, 498 (279 Option B+ women) were eligible for this analysis (Figure 1). The median time between ART initiation and the viral load test was 504 days (interquartile range, 259–743). Of tested patients, 448 (90.0%) were virologically suppressed. Women whose adherence between start of ART and the viral load test was <90% were much less likely to be virologically suppressed than those whose adherence was 100% (aOR for adherence <80%, 0.15 [95% CI, .06–.38]; aOR for adherence 80%–89%, 0.37 [.14–.95]). Women whose adherence was 90%–99% had a similar chance of virological suppression (aOR, 0.96; 95% CI, .43–2.17) compared with those with 100% adherence. Based on these results, we considered <90% adherence inadequate and ≥90% adequate.
Adherence Among Women Retained in Care
Kaplan–Meier estimates for retention after 2 years by pregnancy and breastfeeding status at ART initiation were 59.4% (95% CI, 57.9%–60.1%) for pregnant women, 65.2% (62.9%–67.5%) for breastfeeding women, and 68.0% (65.7%–70.3%) for women who were not pregnant or breastfeeding. The cumulative incidence of LTF by year 2 was 24.5% (95% CI, 23.2%–25.8%) among women who started ART during pregnancy, 20.2% (95% CI, 18.3%–22.2%) among those who started ART while breastfeeding, and 17.0% (95% CI, 15.2%–18.8%) among those who were neither pregnant nor breastfeeding when they started treatment. About 15% of the women from all groups transferred out, <1% stopped ART, and <1% died.
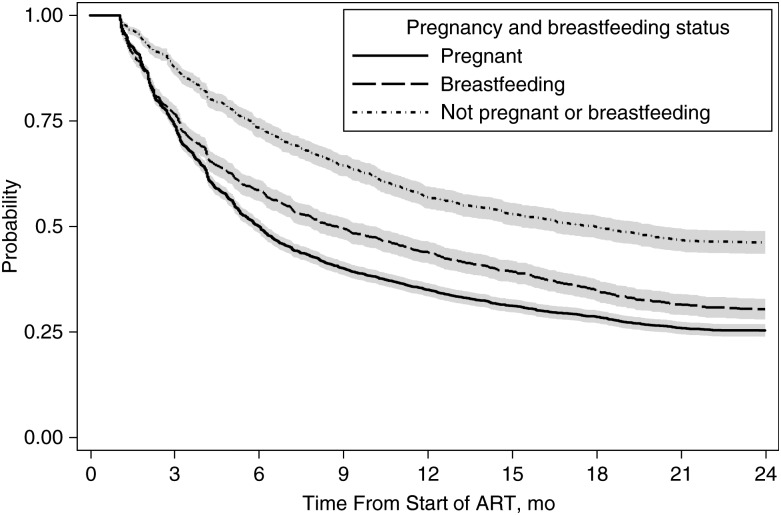
Among women retained after 2 years, adequate adherence (≥90%) during the first 2 years of ART was 67.0% (95% CI, 65.1%–68.8%) for women who started ART during pregnancy, 72.9% (70.1%–75.6%) for those who started ART while breastfeeding, and 78.1% (75.6%–80.7%) for women who were neither pregnant nor breastfeeding. The probability of maintaining adequate adherence at every follow-up visit during the first 2 years on ART was 0.25 (95% CI, .24–.27) in women who started ART during pregnancy, 0.30 (.28–.33) in those who started ART while breastfeeding, and 0.46 (.44–.49) in women those were neither pregnant nor breastfeeding (Figure 2).
Figure 2.
Probability of maintaining adequate adherence (≥90%) at every clinic visit (lines) and 95% confidence intervals (shaded areas) compared according to pregnancy and breastfeeding status at the start of antiretroviral therapy (ART).
Adherence Pattern Over Time on ART
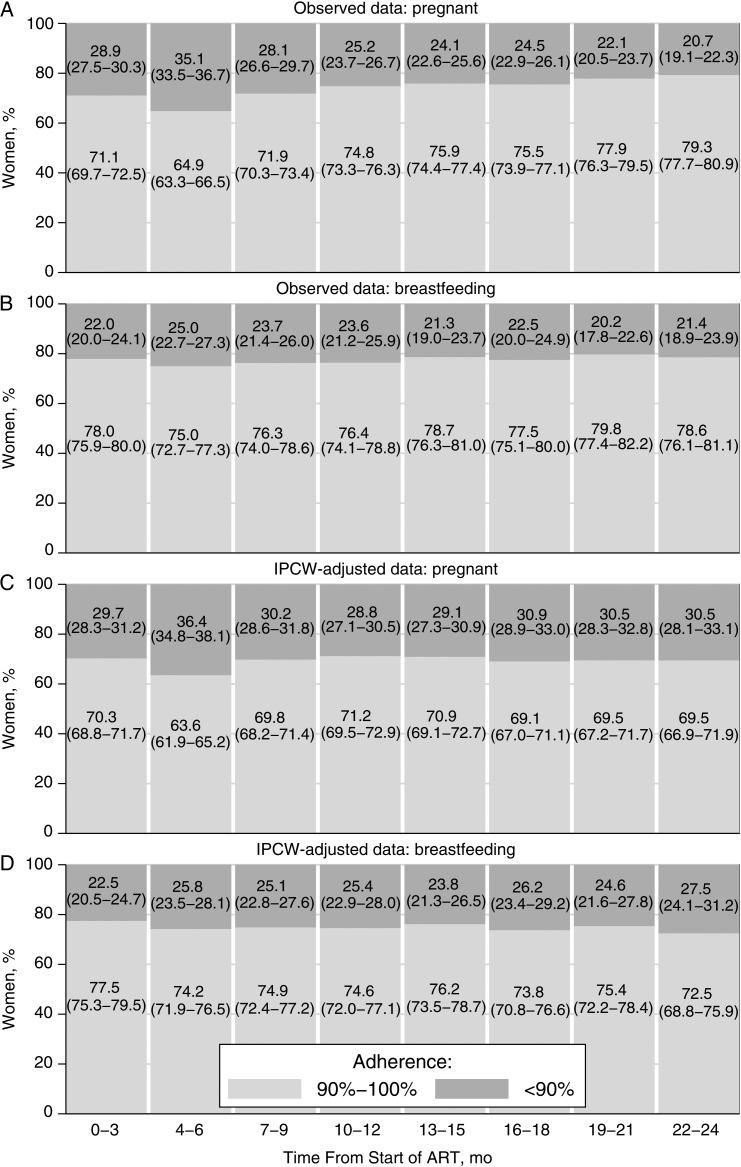
Figure 3 shows observed and IPCW-adjusted adherence estimates for 3-month intervals after ART initiation. Observed data (Figure 3A and 3B) show proportions of women with adequate and inadequate adherence among women retained in care by the end of the time interval. The proportion of adequately adhering women who started ART during pregnancy dropped from 71.1% (95% CI, 69.7%–72.5%) during the first 3 months of ART to 64.9% (63.3%–66.5%) during months 4–6. Thereafter, the proportion of women who adhered adequately increased to 79.3% (77.7%–80.9%) (Figure 3A). Among women who started ART while breastfeeding, between 75.0% (95% CI, 72.7%–77.3%) and 79.8% (95% CI, 77.4%–82.2%) adhered adequately (Figure 3B). Between 80.8% (95% CI, 78.7%–82.9%) and 86.3% (84.2%–88.4%) of women who started ART when they were neither pregnant nor breastfeeding adhered adequately (Supplementary Appendix 1).
Figure 3.
Adherence during 3-month intervals after antiretroviral therapy (ART) initiation. Adherence is compared according to pregnancy and breastfeeding status at start of ART: pregnant (A, C) or breastfeeding (B, D). Observed and inverse probability of censoring weighted (IPCW) estimates are shown for adherence levels during 3-month intervals after ART initiation. Data represent percentages (with 95% confidence intervals) of women with adherence of <90% or ≥90%. Observed data include women retained in care by the end of the interval. IPCW-adjusted data represent a pseudopopulation that would have been observed without censoring.
IPCW-adjusted estimates (Figure 3C and 3D) are adjusted for censoring. After adjustment, adherence among women who started ART during pregnancy was constant over time, except during months 4–6, when it was significantly lower (Figure 3C). In women who started ART while breastfeeding, adherence remained constant over time (Figure 3D), and in women who were not pregnant or breastfeeding, adherence dropped after the first 3 months and remained constant thereafter (Supplementary Appendix 1).
Adherence Before and After Delivery
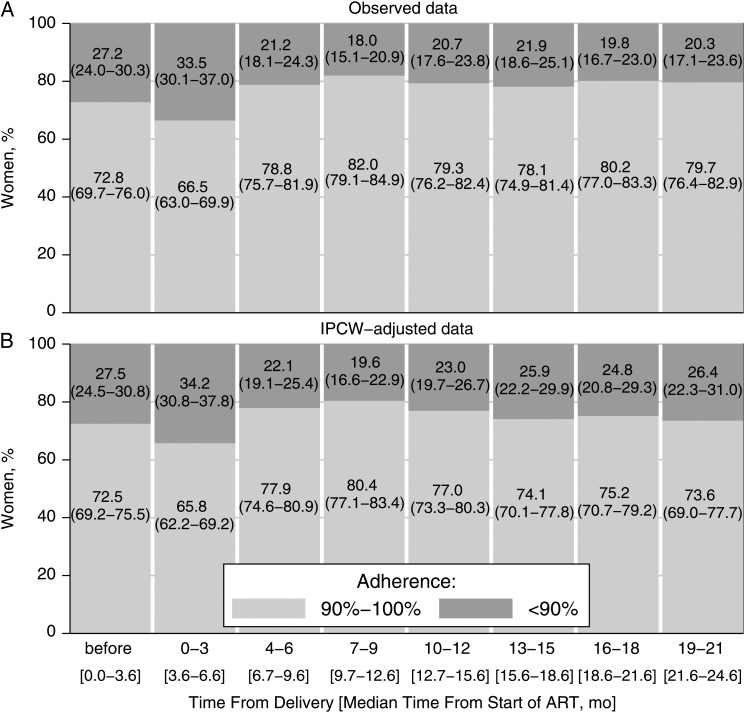
We linked maternal and infant records to determine the date of delivery in 916 (22%) of the 4248 women who started ART during pregnancy. Of those women, 765 were eligible for analysis (Figure 1) in which we compared adherence before and after delivery. Women who could be linked to their infants had higher mean adherence during the first 2 years of ART than those who could not be linked (89.8% vs 87.1%; P = .002). The populations also differed as to year of ART initiation and facility type (Supplementary Appendix 2). The median time from start of ART to delivery was 3.6 months. IPCW-adjusted analysis showed that, during pregnancy, 72.5% (95% CI, 69.2%–75.5%) of the women adhered adequately. In the first 3 months after delivery, the proportion of women with adequate adherence dropped to 65.8% (95% CI, 62.2%–69.2%). In the 6 subsequent 3-month intervals (months 4–21 post partum), the proportion of women with adequate adherence was similar to that during pregnancy (Figure 4).
Figure 4.
Adherence before and after delivery. Observed (A) and inverse probability of censoring weighted (IPCW) (B) estimates are shown; data represent percentages (95% confidence intervals) of women with adherence of <90% or ≥90% during pregnancy and in 3-month intervals after delivery. IPCW-adjusted estimates are adjusted for censoring, representing a pseudopopulation that would have been observed without censoring. Data are from 765 women who started antiretroviral therapy (ART) during pregnancy and could be linked to infant records (see Figure 1).
Risk Factors for Inadequate Adherence
Women who started ART during pregnancy (aHR, 1.66; 95% CI, 1.43–1.93) or while breastfeeding (1.45, 1.24–1.70) were more likely to adhere inadequately (<90%) in the first 2 years of ART than women who were not pregnant or breastfeeding when they started ART. Women aged 15–19 years (aHR, 1.78; 95% CI, 1.51–2.11), 19–24 years (1.47; 1.32–1.63), or 25–29 years (1.12; 1.03–1.22) were more likely to adhere inadequately than those aged ≥30 or older. Women managed in district hospitals (aHR, 2.37; 95% CI, 2.06–2.72) and urban health centers (2.20; 1.89–2.56) were at higher risk of inadequate adherence than those managed in faith-based health facilities (1.21; 1.02–1.44) or central hospitals (reference). Women in WHO clinical stage 3 were more likely to adhere inadequately (aHR, 1.63; 95% CI, 1.23–2.16) than those in WHO clinical stage 1 (1.18; 0.89–1.56), 2 (1.02; 0.72–1.43), or 4 (reference).
DISCUSSION
About 70% of the Option B+ patients retained after 2 years adhered adequately (≥90%), but only about a third of women maintained adequate adherence at every visit during the first 2 years of ART. Women who started ART under Option B+ were about 1.5 times more likely to adhere inadequately than those who were not pregnant or breastfeeding. Other risk factors for inadequate adherence were younger age and receiving care at district hospitals or health centers. We examined adherence during and after pregnancy in a subgroup of women and found it to be constant during pregnancy and breastfeeding; slightly more than 70% of these women adhered adequately, except during the first 3 months post partum, when fewer women adhered. In a subgroup analysis, we showed that women who adhered adequately were much more likely to be virologically suppressed than those who adhered inadequately.
Based on pharmacy records, we compared adherence to ART for a large group of women enrolled in Malawi's Option B+ program. We validated our adherence measure and determined the threshold for adequate adherence against virological outcomes [22]. The ≥90% threshold aligns with a recent systematic review that found a threshold of slightly lower than 95% predicted virological suppression [23]. Pharmacy-based adherence indicators are widely used and considered superior to self-reported data, because self-reports are subject to recall and social desirability bias [22]. Pharmacy-based adherence measures do have limitations and may be biased: adherence may be overestimated in patients who do not take all the pills they collect. Adherence will be underestimated if patients received drugs from other sources or without documentation [24].
To minimize the risk of incorrectly documented drug dispensation, healthcare workers used barcode scanners and recorded drug dispensation prospectively at the point of care [18], except during occasional outages of the electronic system, when data were collected on paper forms and entered into the system retrospectively. Data entry may not always have been complete, but it is unlikely that we seriously underestimated adherence because retrospective data entry was rare. Our earlier analysis of data from the same cohort revealed that women with poor adherence have a higher risk of LTF [7]. We used IPCW to adjust for informative censoring and enable a valid comparison of antepartum and postpartum adherence [25, 26]. The date of delivery was not recorded in maternal ART records, so we could not calculate antepartum and postpartum adherence for all women. By linking maternal and infant records, we determined date of delivery for about 20% of the women. This subset was not representative of all women who started ART during pregnancy; those in the subset had slightly better adherence, probably because women who adhere to ART are more likely to enroll their infants in care. Adherence levels for the antepartum and postpartum period may therefore be slightly overestimated.
A meta-analysis of studies published before 2012 compared maternal antepartum and postpartum adherence and found poor and deteriorating adherence after delivery [8]. More recent data suggest high and constant levels of adherence during pregnancy and breastfeeding. The PROMOTE trial and the Kisumu Breastfeeding Study included pregnant women regardless of clinical or immunological criteria. In the PROMOTE trial, mean self-reported adherence in the antepartum and postpartum period was >97% [10]. In the Kisumu Breastfeeding Study, about 80% of women had >95% adherence during pregnancy and breastfeeding [12]. Two cohort studies from Africa included pregnant women with a low CD4 cell count or at an advanced clinical stage, and reported adherence was high during and after pregnancy [9, 11]. In general, our data are in line with these studies, but comparisons are limited by heterogeneous study designs, eligibility criteria, and adherence measures. We found overall adherence levels to be constant during pregnancy and breastfeeding but noted a drop in adherence in the first 3 months after delivery. Out-referral after delivery, postnatal depression, or difficulties travelling to the facilities after delivery may explain the temporary decline in adherence [27, 28].
Medical sociologists argue that patients may interrupt treatment or reduce doses to test the effectiveness of medication and discontinue treatment if they think it is ineffective [29, 30]. Patients' state of health before they start therapy and adverse effects of drugs can influence patient assessment of treatment effectiveness. Patients who feel sick before starting ART feel better quickly after they start treatment [30, 31]. However, patients who feel well before starting ART may see no improvement in their own health or may experience unpleasant adverse effects [30, 32]. Pregnant and breastfeeding women started ART in a less advanced stage of HIV infection than women who were not pregnant or breastfeeding, which may explain their worse adherence. This hypothesis is supported by a qualitative study showing that feeling healthy and adverse effects are reasons why Option B+ women stop taking their medication [33] and by quantitative studies showing that high CD4 cell counts are associated with treatment interruptions or defaulting [34, 35]. We examined the association between clinical staging and adherence and found no support for the hypothesis that starting at an advanced clinical stage leads to better adherence. Inaccurate WHO staging in routine care may explain our unexpected finding.
About a quarter of the women who started ART with an Option B+ indication were LTF during the first 2 years of ART [7]. About 30% of those retained adhered inadequately. HIV-infected patients with inadequate adherence are at a high risk of viral rebound and drug resistance [13, 17, 36]. Programs should carefully monitor drug resistance and the long-term effectiveness of ART among women who started ART under Option B+ and should expect demand for second-line ART to increase in this population. If access to viral load count is limited, it may be useful to target testing to patients with poor adherence. Pharmacy-based or self-reported adherence measures could identify patients with adherence problems [22, 37]. Programs should consider implementing feasible, evidence-based interventions to promote adherence, including adherence clubs, adherence counseling, short message service text message reminders, and treatment supporters, and could also consider better-tolerated first-line options [38–40].
Most women enrolled in the Option B+ program adhered well during pregnancy and breastfeeding, but close to one-third adhered inadequately. We suggest that long-term virological outcomes be closely monitored and effective interventions be deployed to improve adherence and retention among pregnant and breastfeeding women.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank all patients, doctors and study nurses in the participating facilities. We thank Marcel Zwahlen from the Institute of Social and Preventive Medicine and Constantin T. Yiannoutsos from Indiana University for advice on statistical analysis.
Author contributions. A. D. H. wrote the first draft of the study protocol; all authors critically reviewed the study protocol and contributed to its final version. A. D. H. performed statistical analyses, with interpretation of results by all authors. A. D. H. wrote the first draft of the report, which was revised by M. E., H. T., A. J., K. T., L. S.-V., J. E., N. P., J. J. v. O., and O. K. A. D. H. and M. T. M. extracted the data and did data management. M. T. M. and A. S. did probabilistic record linkage. A. J. coordinates monitoring and evaluation of the Malawian ART/prevention of mother-to-child transmission program and implemented data collection. O. J. G. coordinates electronic data collection. F. C. heads the HIV and AIDS Department of Malawi's Ministry of Health. F. C. and O. K. are the principal investigators of the study. All authors reviewed and approved the final version of the manuscript for submission.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors.
Financial support. This work was supported by the Bill and Melinda Gates Foundation (Global Health grant OPP1090200), the United States Agency for International Development–National Institutes of Health initiative Partnerships for Enhanced Engagement in Research Health (grant AID-OAA-A-11-00012), and the National Institute of Allergy and Infectious Diseases (grant 5U01-AI069924). The Malawi Ministry of Health's HIV/AIDS Programme is funded by the Fight AIDS, Tuberculosis and Malaria and President's Emergency Plan for AIDS Relief. Baobab Health Trust is funded by the Centers for Disease Control and Prevention. O. K. was supported by a PROSPER fellowship grant from the Swiss National Science Foundation (grant 3233B-150934).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Schouten EJ, Jahn A, Midiani D et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet 2011; 378:282–4. [DOI] [PubMed] [Google Scholar]
- 2.Ministry of Health Malawi. Clinical management of HIV in children and adults, 2014. Available at: http://cms.medcol.mw/cms_uploaded_resources/18381_16.pdf Accessed 2 August 2016.
- 3.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2013. Available at: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf Accessed 26 June 2015. [PubMed]
- 4.Tenthani L, Haas AD, Tweya H et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. AIDS 2014; 28:589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Impact of an innovative approach to prevent mother-to-child transmission of HIV—Malawi, July 2011-September 2012. MMWR Morb Mortal Wkly Rep 2013; 62:148–51. [PMC free article] [PubMed] [Google Scholar]
- 6.Coutsoudis A, Goga A, Desmond C, Barron P, Black V, Coovadia H. Is Option B+ the best choice? Lancet 2013; 381:269–71. [DOI] [PubMed] [Google Scholar]
- 7.Haas AD, Tenthani L, Msukwa MT et al. Retention in care during the first 3 years of antiretroviral therapy for women in Malawi's option B+ programme: an observational cohort study. Lancet HIV 2016; 3:e175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nachega JB, Uthman OA, Anderson J et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS 2012; 26:2039–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henegar CE, Westreich DJ, Maskew M, Miller WC, Brookhart MA, Van Rie A. Effect of pregnancy and the postpartum period on adherence to antiretroviral therapy among HIV-infected women established on treatment. J Acquir Immune Defic Syndr 2015; 68:477–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koss CA, Natureeba P, Mwesigwa J et al. Hair concentrations of antiretrovirals predict viral suppression in HIV-infected pregnant and breastfeeding Ugandan women. AIDS 2015; 29:825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews LT, Ribaudo HB, Kaida A et al. HIV-infected Ugandan women on antiretroviral therapy maintain HIV-1 RNA suppression across periconception, pregnancy, and postpartum periods. J Acquir Immune Defic Syndr 2016; 71:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okonji JA, Zeh C, Weidle PJ et al. CD4, viral load response, and adherence among antiretroviral-naive breast-feeding women receiving triple antiretroviral prophylaxis for prevention of mother-to-child transmission of HIV in Kisumu, Kenya. J Acquir Immune Defic Syndr 2012; 61:249–57. [DOI] [PubMed] [Google Scholar]
- 13.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor – based HIV therapy and virologic outcomes. Ann Intern Med 2007; 146:564–73. [DOI] [PubMed] [Google Scholar]
- 14.Garcia PM, Kalish LA, Pitt J et al. Women and Infants Transmission Study Group. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N Engl J Med 1999; 341:394–402. [DOI] [PubMed] [Google Scholar]
- 15.Chi BH, Cantrell RA, Zulu I et al. Adherence to first-line antiretroviral therapy affects non-virologic outcomes among patients on treatment for more than 12 months in Lusaka, Zambia. Int J Epidemiol 2009; 38:746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nachega JB, Hislop M, Dowdy DW et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr 2006; 43:78–84. [DOI] [PubMed] [Google Scholar]
- 17.Sethi AK, Celentano DD, Gange SJ, Moore RD, Gallant JE. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis 2003; 37:1112–8. [DOI] [PubMed] [Google Scholar]
- 18.Douglas GP, Gadabu OJ, Joukes S et al. Using touchscreen electronic medical record systems to support and monitor national scale-up of antiretroviral therapy in Malawi. PLoS Med 2010; 7:e1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother 2006; 40:1280–8. [DOI] [PubMed] [Google Scholar]
- 20.Ranganathan P, Pramesh C. Censoring in survival analysis: potential for bias. Perspect Clin Res 2012; 3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 22.McMahon JH, Jordan MR, Kelley K et al. Pharmacy adherence measures to assess adherence to antiretroviral therapy: review of the literature and implications for treatment monitoring. Clin Infect Dis 2011; 52:493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM. Adherence to antiretroviral therapy and virologic failure: a meta-analysis. Medicine (Baltimore) 2016; 95:e3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tweya H, Gugsa S, Hosseinipour M et al. Understanding factors, outcomes and reasons for loss to follow-up among women in Option B+ PMTCT programme in Lilongwe, Malawi. Trop Med Int Health 2014; 19:1360–6. [DOI] [PubMed] [Google Scholar]
- 25.Robins JM, Rotnitzky A, Zhao LP. Analysis of semiparametric regression models for repeated outcomes in the presence of missing data. J Am Stat Assoc 1995; 90:106–21. [Google Scholar]
- 26.Rubin DB. Inference and missing data. Biometrika 1976; 63:581–92. [Google Scholar]
- 27.van Lettow M, Bedell R, Mayuni I et al. Towards elimination of mother-to-child transmission of HIV: performance of different models of care for initiating lifelong antiretroviral therapy for pregnant women in Malawi (Option B+). J Int AIDS Soc 2014; 17:18994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dow A, Dube Q, Pence BW, Van Rie A. Postpartum depression and HIV infection among women in Malawi. J Acquir Immune Defic Syndr 2014; 65:359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conrad P. The meaning of medications: another look at compliance. Soc Sci Med 1985; 20:29–37. [DOI] [PubMed] [Google Scholar]
- 30.Zhou A. The uncertainty of treatment: women's use of HIV treatment as prevention in Malawi. Soc Sci Med 2016; 158:52–60. [DOI] [PubMed] [Google Scholar]
- 31.Miller CM, Ketlhapile M, Rybasack-Smith H, Rosen S. Why are antiretroviral treatment patients lost to follow-up? a qualitative study from South Africa. Trop Med Int Health 2010; 15(suppl 1):48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sustiva (efavirenz). Available at: http://www.pdr.net/full-prescribing-information/Sustiva-efavirenz-116 Accessed 2 August 2016.
- 33.Kim MH, Zhou A, Mazenga A et al. Why did I stop? barriers and facilitators to uptake and adherence to ART in option B+ HIV care in Lilongwe, Malawi. PLoS One 2016; 11:e0149527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adakun SA, Siedner MJ, Muzoora C et al. Higher baseline CD4 cell count predicts treatment interruptions and persistent viremia in patients initiating ARVs in rural Uganda. J Acquir Immune Defic Syndr 2013; 62:317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimsrud A, Cornell M, Schomaker M et al. CD4 count at antiretroviral therapy initiation and the risk of loss to follow-up: results from a multicentre cohort study. J Epidemiol Community Health 2016; 70:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrigan PR, Hogg RS, Dong WWY et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis 2005; 191:339–47. [DOI] [PubMed] [Google Scholar]
- 37.Phillips T, Brittain K, Mellins CA et al. A self-reported adherence measure to screen for elevated HIV viral load in pregnant and postpartum women on antiretroviral therapy. AIDS Behav 2016; doi:10.1007/s10461-016-1448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson LS. ART adherence clubs: a long-term retention strategy for clinically stable patients receiving antiretroviral therapy. South Afr J HIV Med 2013; 14:48. [Google Scholar]
- 39.Mills EJ, Lester R, Thorlund K et al. Interventions to promote adherence to antiretroviral therapy in Africa: a network meta-analysis. Lancet HIV 2014; 1:e104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nachega JB, Skinner D, Jennings L et al. Acceptability and feasibility of mHealth and community-based directly observed antiretroviral therapy to prevent mother-to-child HIV transmission in South African pregnant women under Option B+: an exploratory study. Patient Prefer Adherence 2016; 10:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.