In a multisite birth cohort study, we document a high burden of Campylobacter infection using enzyme immunoassay, demonstrate an association between Campylobacter and linear growth shortfalls and both increased intestinal permeability and intestinal and systemic inflammation, and identify potential interventions.
Keywords: Campylobacter, children, risk factors, growth, inflammation
Abstract
Background. Enteropathogen infections have been associated with enteric dysfunction and impaired growth in children in low-resource settings. In a multisite birth cohort study (MAL-ED), we describe the epidemiology and impact of Campylobacter infection in the first 2 years of life.
Methods. Children were actively followed up until 24 months of age. Diarrheal and nondiarrheal stool samples were collected and tested by enzyme immunoassay for Campylobacter. Stool and blood samples were assayed for markers of intestinal permeability and inflammation.
Results. A total of 1892 children had 7601 diarrheal and 26 267 nondiarrheal stool samples tested for Campylobacter. We describe a high prevalence of infection, with most children (n = 1606; 84.9%) having a Campylobacter-positive stool sample by 1 year of age. Factors associated with a reduced risk of Campylobacter detection included exclusive breastfeeding (risk ratio, 0.57; 95% confidence interval, .47–.67), treatment of drinking water (0.76; 0.70–0.83), access to an improved latrine (0.89; 0.82–0.97), and recent macrolide antibiotic use (0.68; 0.63–0.74). A high Campylobacter burden was associated with a lower length-for-age Z score at 24 months (−1.82; 95% confidence interval, −1.94 to −1.70) compared with a low burden (−1.49; −1.60 to −1.38). This association was robust to confounders and consistent across sites. Campylobacter infection was also associated with increased intestinal permeability and intestinal and systemic inflammation.
Conclusions. Campylobacter was prevalent across diverse settings and associated with growth shortfalls. Promotion of exclusive breastfeeding, drinking water treatment, improved latrines, and targeted antibiotic treatment may reduce the burden of Campylobacter infection and improve growth in children in these settings.
Enteropathogen infection has been associated with impaired growth in young children in low-resource settings [1–4], which in turn has been associated with long-term sequelae, with significant implications for health and human capital [5–7]. Recent work has implicated Campylobacter [2, 3], with the putative mechanism being environmental enteric dysfunction, a condition characterized by altered intestinal function and inflammation [8–10]. In high-resource settings, Campylobacter infection is sporadic and associated with exposure to undercooked chicken [11, 12], or less frequently common-source outbreaks, often due to contaminated dairy products [13]. In contrast, in low-resource settings, Campylobacter infection is frequently endemic [2, 14]. Although exposure to poultry may be important, identified determinants are varied [11, 15–17].
The Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) is a birth cohort study performed at 8 sites in South America, sub-Saharan Africa, and Asia [18]. Notably, MAL-ED used an enzyme immunoassay (EIA) to detect Campylobacter in stool samples, which is substantially more sensitive than stool culture [19]. Previous work found Campylobacter to have the highest attributable burden of diarrhea in this study [20]. Finally, asymptomatic infection with enteropathogens was strongly associated with linear growth shortfalls, with a uniquely strong association between Campylobacter and growth (MAL-ED investigators, manuscript in preparation). In the present work, we aimed to describe the burden and impact of Campylobacter and identify potential interventions to reduce infection with this pathogen.
METHODS
Study Design and Procedures
The MAL-ED study design and methodology have been described elsewhere [18]. The study was conducted at 8 sites: Dhaka, Bangladesh; Vellore, India; Bhaktapur, Nepal; Naushero Feroze, Pakistan; Venda, South Africa; Haydom, Tanzania; Fortaleza, Brazil; and Loreto, Peru. Children were enrolled from November, 2009 to February, 2012 and followed up through 24 months of age. Monthly anthropometry was performed [21]. Stool samples were collected in the absence of diarrhea at 1–12, 15, 18, 21, and 24 months of age as well as from each diarrhea episode, defined as maternal report of ≥3 loose stools in 24 hours or visible blood in stool and identified through twice-weekly home visits.
Caregivers were surveyed biannually from 6 months of age, including questions about maternal income and education, the home environment, drinking water source and treatment, and the presence of animals. Crowding was defined as >2 persons per room living in the home. An improved latrine and water source were defined following World Health Organization guidelines [22]. Treatment of drinking water was defined as boiling, filtering, or adding bleach. Poor access to water was defined as having a primary drinking water source more than a 10-minute walk from the home. Twice-weekly home surveillance assessed breastfeeding in the prior day as exclusive (no consumption of other food or liquid), partial, or none, identified the introduction of specific foods, and recorded antibiotic use. All sites received ethical approval from their respective governmental, local institutional, and collaborating institutional review boards. Written informed consent was obtained from the parent or guardian of each child.
Laboratory Testing
The laboratory methods used in the MAL-ED study have been described elsewhere [10, 23]. Pertinently, EIA was performed for Campylobacter (ProSpecT) as well as Giardia and Cryptosporidium (TechLab). Monthly surveillance stool samples were also tested for myeloperoxidase (MPO; measured in nanograms per milliliter), a marker of neutrophil activity in the intestinal mucosa (Alpco); neopterin (NEO; measured in nanomoles per liter), a marker of T-helper cell 1 activity (GenWay Biotech); and α-1-antitrypsin (AAT; measured in milligrams per gram), a marker of intestinal permeability (Biovendor). Blood samples collected at 7, 15 and 24 months were tested for α-1-acid glycoprotein (AGP; measured in milligrams per deciliter), a marker of systemic inflammation.
Data Analysis
To identify factors associated with Campylobacter detection in surveillance stool samples, we used generalized estimating equations to fit a generalized linear model with a first-order autoregressive working correlation matrix and robust variance to account for nonindependence of stool testing within each child. To estimate risk ratios for Campylobacter detection, we used Poisson regression as an approximation of log-binomial regression since the log-binomial models did not converge [24]. First, we estimated the association for each factor of interest with Campylobacter detection, adjusting for age (using a natural spline with knots at 6, 12, and 18 months), sex, site, and season via the terms , where m is the month of the year as well as—given possible variation in seasonality between sites—an interaction between these terms and site [25]. Then, based on statistical significance, model fit based on the quasi-likelihood information criterion and an assessment of covariance between individual factors, we fit a multivariable model. We also fit site-specific models, excluding those variables from the multivariable model that did not vary within specific sites. Finally, to further describe any association with recent antibiotic use, we fit 2 multivariable models, adjusted as described above, which included (1) class-specific antibiotic use in the prior month and (2) class-specific use in 15-day windows over the prior 60 days.
For the analysis of linear growth, included individuals were required to have a length-for-age Z (LAZ) score at 24 months of age. We excluded children from the Pakistan site, owing to bias noted in a subset of length measurements at this site. To estimate the association between the burden of detection of an individual pathogen and 24-month LAZ score, we calculated the enteropathogen burden for each subject using the surveillance stool samples (namely, samples positive/samples tested). Similar burden indices were calculated for the 0–6, 7–12, and 13–24 month intervals. Persistent infection was defined as detection of Campylobacter from all surveillance stool samples tested during a 3-month period. We then fit a multiple linear regression, including enrollment LAZ score, sex, site, and Campylobacter burden and further adjusted for possible confounders, including factors associated with Campylobacter infection in the multivariable model as well as highly-correlated pathogens. To calculate model-predicted 24-month LAZ scores, we calculated predicted population marginal effects using least-squares means [26]. Overall and site-specific high and low burdens of Campylobacter were defined as the 90th and 10th percentile of Campylobacter burden, respectively.
To estimate the association between Campylobacter detection and fecal markers of intestinal permeability and inflammation collected in surveillance stool samples (MPO, NEO, and AAT), we used generalized estimating equations to fit a generalized linear model, as described previously but using a gaussian distribution. These models adjusted for age, sex and site, as previously described, and we also fit site-specific models. Finally, to describe the association between Campylobacter burden and systemic inflammation, we fit multiple linear regression models and calculated predicted population marginal effects using least-squares means, both for the entire cohort and for each site, as described for the analysis of linear growth, but with the mean AGP value for each individual as the response variable instead of 24-month LAZ score. All statistical analysis was performed using R software, version 3.2.2 (Foundation for Statistical Computing).
RESULTS
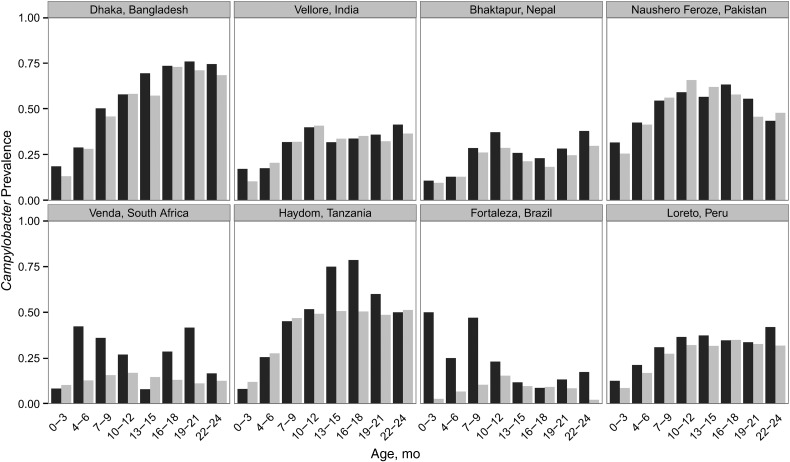
A total of 2145 children were enrolled, of whom 2087 had ≥1 surveillance stool sample tested for Campylobacter, and 1892 additionally had ≥1 biannual survey performed. These 1892 children had 7601 diarrheal and 26267 surveillance stool samples tested by Campylobacter EIA (Supplementary Table 1). Campylobacter prevalence in diarrheal and surveillance stool samples varied substantially by site (Figure 1). Generally, Campylobacter was frequently detected in surveillance stool samples, with an increasing prevalence over the first year of life and with peak prevalence varying by site, ranging from approximately 10% of surveillance stool samples in Brazil and South Africa to more than half in Bangladesh, Pakistan, and Tanzania. Approximately half of the children had a Campylobacter-positive stool sample by 6 months of age, and most (n = 1606; 84.9%) had a Campylobacter-positive stool sample by 1 year of age (Supplementary Figure 1). For some sites, exposure was much earlier (eg, in Pakistan, the majority of children had a Campylobacter-positive stool sample by 3 months of age). The median proportion of surveillance stool samples positive for Campylobacter from 0–24 months was 0.20 (interquartile range, 0.11–0.38).
Figure 1.
Campylobacter prevalence in diarrheal and nondiarrheal surveillance stool samples. Histogram shows proportions of diarrheal (black) and surveillance (gray) stool samples positive for Campylobacter by enzyme immunoassay by age at each site.
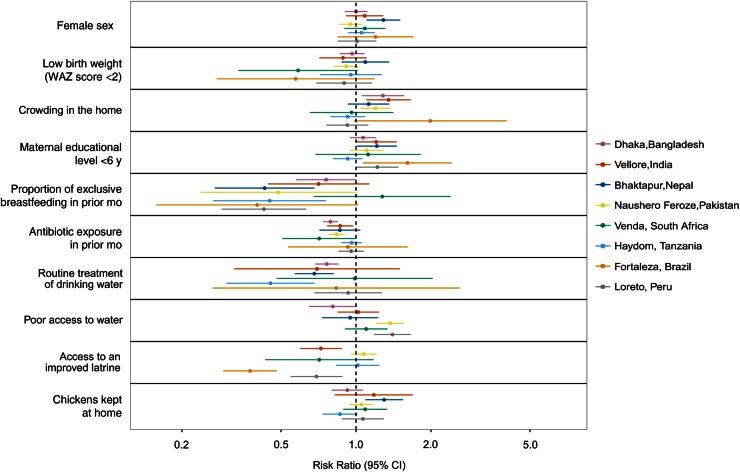
Possible sociodemographic, environmental, and behavioral determinants of Campylobacter infection varied substantially between sites, including crowding in the home (4.7%–83.2%), routine treatment of drinking water (0%–56.5%), and access to an improved latrine (15.1%–100%) (Supplementary Table 1). Overall, the duration of exclusive breastfeeding was short (median, 3.2 months; interquartile range, 2.0–5.3 months) (Supplementary Table 1), and fewer than half of children were exclusively breastfed to 6 months [27]. Nonexclusive breastfeeding generally continued beyond 12 months, with variable cessation in the second year. We identified factors associated with Campylobacter detection in surveillance stool samples across all sites (Table 1) and for individual sites (Figure 2). Despite substantial heterogeneity between sites, exclusive breastfeeding in the prior month, routine treatment of drinking water, and access to an improved latrine were most consistently associated with a reduced risk of Campylobacter detection. Keeping chickens was marginally associated with increased Campylobacter detection, and consumption of chicken was not common in these children (data not shown).
Table 1.
Risk Factors for Campylobacter Detection in Surveillance Stool Samples
| Risk Factors by Category | Risk Ratio (95% CI) |
|
|---|---|---|
| Univariable Analysisa | Multivariable Analysisb | |
| Sociodemographic/maternal | ||
| Female sex | 1.06 (1.01–1.12) | 1.05 (1.00–1.11) |
| Low birth weight (WAZ score <2) | 0.95 (.89–1.03) | 0.93 (.87–1.00) |
| Crowding in the home | 1.19 (1.11–1.27) | 1.13 (1.05–1.21) |
| Maternal educational level <6 y | 1.16 (1.09–1.24) | 1.08 (1.01–1.15) |
| Monthly income <$150 | 1.14 (1.07–1.22) | 1.04 (.97–1.11) |
| Breastfeeding/diet | ||
| Proportion of breastfeeding in prior mo | ||
| Exclusive | 0.54 (.46–.62) | 0.56 (.47–.67) |
| Nonexclusive | 1.22 (1.14–1.30) | 1.02 (.95–1.10) |
| Consumption in prior mo | ||
| Water | 1.24 (1.13–1.36) | 1.06 (.96–1.17) |
| Animal milk | 1.06 (1.01–1.12) | … |
| Solid food | 1.21 (1.09–1.34) | … |
| Child observed to eat nonfood items | 1.08 (1.01–1.16) | … |
| Antibiotics in prior mo | 0.86 (.83–.89) | 0.86 (.83–.90) |
| Water | ||
| Routine treatment of drinking water | 0.72 (.66–.78) | 0.75 (.69–.82) |
| Improved source of drinking water | 0.93 (.82–1.04) | 0.96 (.85–1.07) |
| Poor access to water | 1.22 (1.10–1.34) | 1.16 (1.06–1.28) |
| Sanitation: access to an improved latrine | 0.85 (.78–.92) | 0.89 (.81–.97) |
| Environment | ||
| Dirt floor in home | 1.16 (1.07–1.25) | 1.04 (.96–1.13) |
| Chickens kept at home | 1.07 (.99–1.15) | 1.06 (.99–1.13) |
| Cattle kept at home | 1.04 (.96–1.12) | … |
| Agricultural land ownership | 0.99 (.92–1.07) | … |
Abbreviations: CI, confidence interval; WAZ score, weight-for-age Z score.
a Adjusted for age, sex, site, and season.
b Adjusted for age, site, season, and all variables included in multivariable model.
Figure 2.
Site-level sensitivity analysis of risk factors for Campylobacter detection in surveillance stool samples. Risk ratios with 95% confidence intervals (CIs) are shown for risk factors of interest as identified in the overall model (Table 1). All estimates are adjusted for age, sex, season, and the factors shown in the figure. Factors that did not vary at each site were excluded. Abbreviation: WAZ score, weight-for-age Z score.
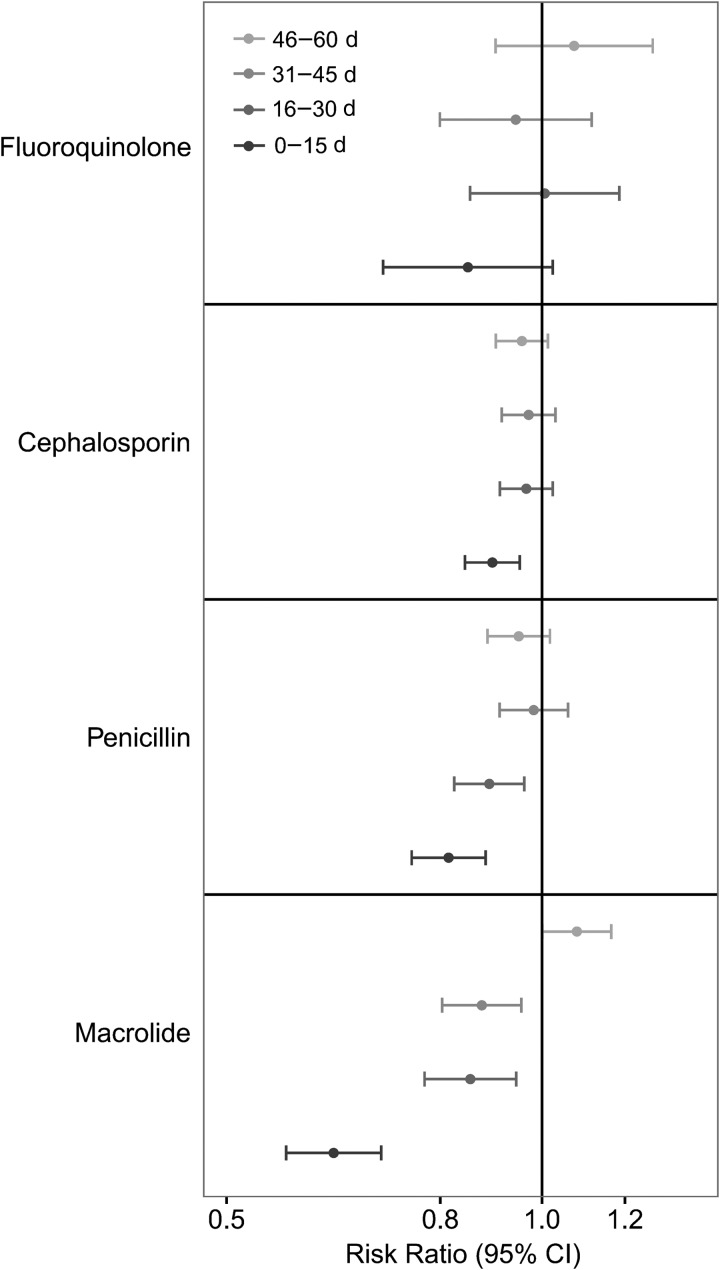
Antibiotic use varied between sites from on average <1 to almost 10 courses of antibiotics per child-year (Supplementary Table 1). Antibiotic use in the prior month was consistently associated with a reduced risk of Campylobacter detection (Table 1, Figure 2). Among all classes, macrolide, cephalosporin, penicillin, and fluoroquinolone use were associated with a reduced risk of detection (Table 2), and macrolide use was associated with the most prolonged clearance of Campylobacter from stool, with a significant association for macrolide use in the previous 45 days (Figure 3).
Table 2.
Association Between Antibiotic Class Administration in the Prior Month and Campylobacter Detection in Surveillance Stool Samples
| Antibiotic Class | Antibiotic Courses, No. | Risk Ratio (95% CI) |
|---|---|---|
| Macrolide | 2820 | 0.68 (.63–.74) |
| Cephalosporin | 3534 | 0.80 (.76–.85) |
| Fluoroquinolone | 692 | 0.89 (.78–1.02) |
| Penicillin | 6577 | 0.92 (.88–.96) |
| Metronidazole | 2289 | 0.95 (.87–1.02) |
| Other/unknown | 2761 | 0.95 (.89–1.02) |
| Sulfonamide | 1560 | 1.02 (.94–1.10) |
| Tetracycline | 42 | 1.03 (.58–1.83) |
Abbreviation: CI, confidence interval.
Figure 3.
Timing and class of antibiotic use and Campylobacter detection in surveillance stool samples. Risk ratios and 95% confidence intervals (CIs) are derived from a single model adjusted for age, sex, site, season, and all shown windows for antibiotic use.
To examine whether Campylobacter detection served as a marker of infection with other enteropathogens, we examined the correlation between the total Campylobacter burden from both surveillance and diarrheal stool samples for each individual with that of non-Campylobacter bacterial, viral, and parasitic enteropathogens (Supplementary Figure 2). At all sites, Campylobacter burden was more strongly correlated with the burden of parasitic enteropathogens (Pearson correlation coefficient, 0.35; P < .001) than with non-Campylobacter bacterial (0.01; P = .55) or viral enteropathogens (0.25; P < .001). Protozoal parasites were more strongly associated than helminths (0.35 vs 0.09; both P < .001). The majority of protozoal enteropathogen detections were either Giardia or Cryptosporidium, and Giardia correlated most strongly with Campylobacter (0.32 vs 0.21; both P < .001).
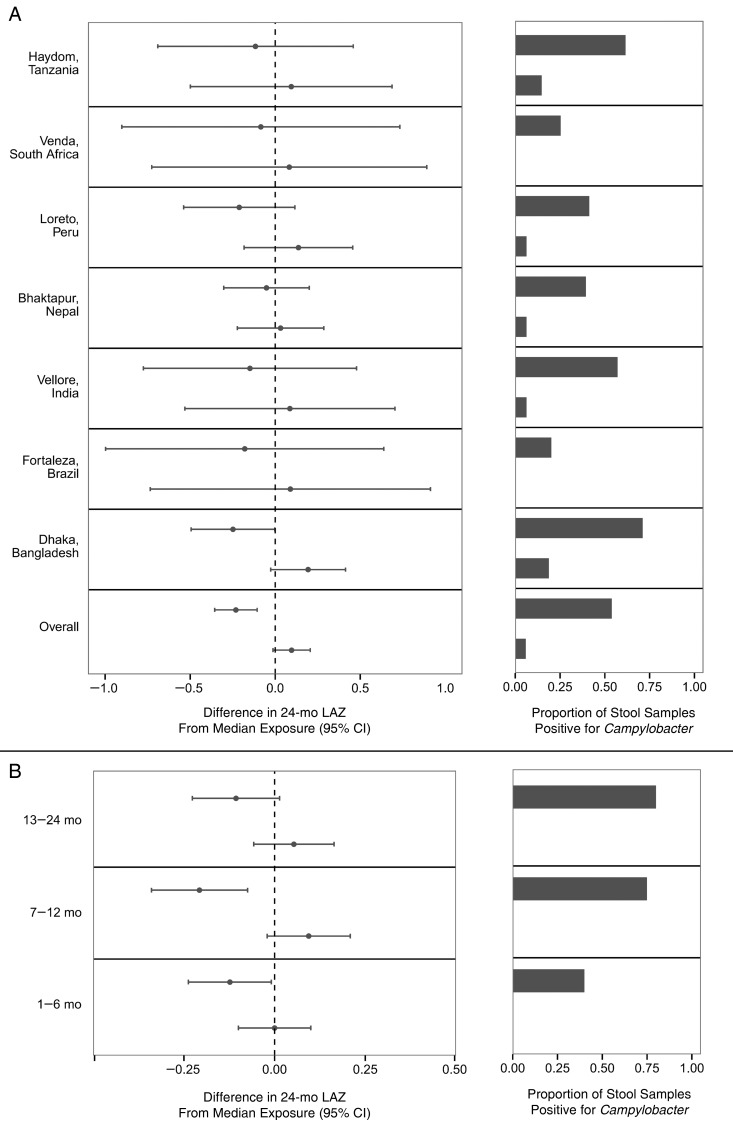
For the 7 sites included in the growth analysis, 1426 of 1892 children had anthropometry performed at enrollment and 24 months. The predicted 24-month LAZ score, adjusted for LAZ score at enrollment and site, was −1.90 (95% confidence interval [CI], −1.99 to −1.80) for children with high Campylobacter burden and −1.49 (−1.56 to −1.42) for those with low Campylobacter burden. The association persisted after adjustment for potential confounders, specifically sex, crowding in the home, maternal education, monthly income, duration of exclusive breastfeeding, antibiotic use, routine treatment of drinking water, poor access to drinking water, access to an improved latrine, and keeping chickens (high burden, −1.82 [95% CI, −1.94 to −1.70]; low burden, −1.49 [−1.60 to −1.38]).
In site-specific analyses, despite substantial variation in both Campylobacter prevalence and associated determinants, the effect was consistent (Figure 4A). Additionally adjusting for parasite burden did not substantially change the effect size (0.29 difference in 24-month LAZ score between high and low Campylobacter burden after adjustment vs 0.33), and parasite burden was not significantly associated with 24-month LAZ score. We also assessed whether the timing of the Campylobacter infection modified the association between Campylobacter burden and length attainment, and although the association was seen across all age ranges, it was most notable in children aged 7–12 months (Figure 4B). A total of 412 children (28.9%) had ≥1 persistent Campylobacter infection during the first year of life. After adjustment for overall burden, having a persistent infection was not associated with 24-month LAZ score (change in 24-month LAZ score, 0.01; 95% CI, −.13 to .15).
Figure 4.
Association between Campylobacter burden and length attainment at 24 months. A, Left, Difference in model-predicted 24-month length-for-age Z (LAZ) score between 10th and 90th percentiles of Campylobacter burden and median Campylobacter burden, overall and at each site. Right, Campylobacter burden expressed as proportion of surveillance stool samples tested that were positive for Campylobacter. B, Same estimates for overall 10th and 90th percentiles of Campylobacter burden by age interval. Abbreviation: CI, confidence interval.
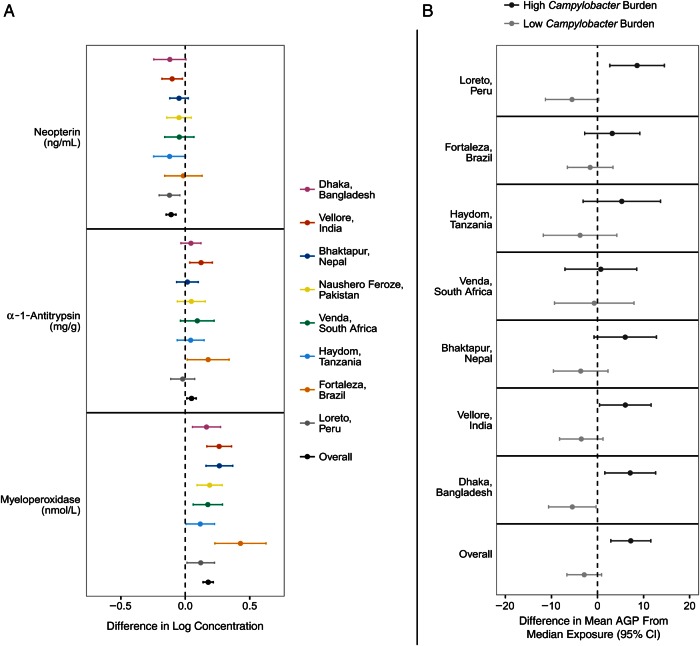
We then estimated the association between Campylobacter infection and fecal markers of intestinal permeability and inflammation as well as systemic inflammation. Campylobacter was detected in ≥1 surveillance stool sample in 1680 of 1892 children (88.8%). With adjustment for age, sex, and site, Campylobacter-positive stool samples were associated with higher MPO (difference in log concentration, 0.18 ng/mL; 95% CI, .13–.22 ng/mL) and AAT (0.05 mg/g; 0.02–0.09 mg/g) and lower NEO (−0.12 nmol/L; −0.16 to −0.08 nmol/L) concentrations (Figure 5A). The concentration of MPO was higher at the time of the first Campylobacter detection (mean [standard deviation], 8.94 [1.24]) than in the month prior (8.86 [1.27]; paired t test P < .001) or in the month after (8.84 [1.25]; P < .001). No such differences were observed for AAT and NEO. Of the 1426 children included in the growth analysis, 1383 had ≥1 blood sample tested for AGP. A high Campylobacter burden was associated with a higher mean AGP concentration (difference between high and median burden, 7.1 mg/dL [95% CI, 2.7–11.4]; difference between low and median burden −2.8 [95% CI, −6.6–.9)] (Figure 5B). This association was consistent across sites.
Figure 5.
Association between Campylobacter detection and fecal markers of intestinal permeability and inflammation (A) and systemic inflammation (B). A, Difference in the log concentrations of neopterin, α-1-antitrypsin, and myeloperoxidase from surveillance stool samples associated with Campylobacter detection. B, Difference in model-predicted mean α-1-acid glycoprotein (AGP) concentrations between 10th and 90th percentiles of Campylobacter burden and median Campylobacter burden, both overall and at each site; blood samples were obtained at 7, 15, and 24 months of age. Abbreviation: CI, confidence interval.
DISCUSSION
As a prospective, multisite birth cohort study, MAL-ED is the first to use a culture-independent diagnostic for Campylobacter, which affords a substantial increase in sensitivity over selective culture [19]. We describe an astonishingly high prevalence of Campylobacter infection beginning early in life and a negative association between Campylobacter burden and linear growth that is consistent across sites and persists after adjusting for potential confounders. The second half of the first year of life seems the most critical period for Campylobacter-associated growth shortfalls, with a large increase in Campylobacter prevalence during that time period. These findings build on evidence from the MAL-ED study that subclinical infections with enteropathogens were more strongly linked to linear growth shortfalls than overt diarrhea (MAL-ED investigators, manuscript in preparation).
Campylobacter was associated with increased intestinal permeability and local and systemic inflammation, suggesting a mechanism for the association with reduced linear growth [8]. This is consistent with ample evidence that Campylobacter can drive intestinal inflammation, in part by altering the composition of the intestinal microbiota, impairing the intestinal barrier, and priming the intestine for chronic inflammatory responses [9, 28–32]. Fecal NEO concentrations were lower in the setting of Campylobacter infection, which may support that Campylobacter drives inflammation primarily via the innate immune system. A preliminary analysis of a subset of infants aged 0–9 months showed an association between fecal NEO and growth [33], but this association was not seen in the final analysis of the complete cohort.
Despite heterogeneity between sites, factors associated with reduced Campylobacter detection included exclusive breastfeeding, treatment of drinking water, access to an improved latrine, and recent use of antibiotics, particularly macrolides. These findings are consistent with other studies which have shown exclusive breastfeeding, water quality, and sanitation to be important determinants of Campylobacter infection [34, 35]. These observations, along with the correlation between infection with Campylobacter and intestinal parasites, are suggestive of diffuse environmental exposure to Campylobacter.
Breastfeeding is thought to reduce the risk of Campylobacter infection in infants via passive immunity [36] as well as reducing exposure to the environment, although it has not been shown to improve growth [37]. In the present study, protection was associated with exclusive breastfeeding but not with nonexclusive breastfeeding. However, the observed burden and impact of Campylobacter infection extends well beyond the commonly recommended duration of exclusive breastfeeding. The impact of water and sanitation interventions on Campylobacter infection has not been evaluated. Clinical trials of water treatment [38] and latrine promotion and construction [39] in India failed to reduce all-cause diarrheal incidence but did not interrogate for specific enteropathogens. A trial of a community-led sanitation program in Mali showed a significant improvement in linear growth [40], an effect that was largest for children <2 years of age, despite no decrease in diarrhea. Intriguingly, the prevalence of bloody diarrhea was reduced, and Campylobacter was the primary etiology of bloody diarrhea in infants in the MAL-ED cohorts [20]. Further studies are needed to assess the impact of these interventions on enteropathogen infection and child growth [41].
The observed association between antibiotic use, in particular of the macrolide class, and clearance of Campylobacter from surveillance stool samples suggests that antibiotic use could reduce Campylobacter burden and possibly improve growth outcomes in these settings. There is evidence from mass treatment trials for trachoma that macrolide administration reduced all-cause mortality, although it is unclear whether undernutrition mediated this effect [42]. Estimates from similar trials that compared the frequency of azithromycin administration for trachoma control have suggested some effect of more frequent azithromycin administration on linear growth [43]. Any observed benefit would have to be weighed against the potential harms of early antibiotic use, in particular selection for drug resistance and collateral effects on the gut microbiota [44–46].
Our work has several limitations. First, Platts-Mills et al [19] have shown that the Campylobacter EIA detects a broad range of Campylobacter species, including species of nonavian origin and unclear pathogenicity. This may have reduced the association between some risk factors (eg, keeping chickens) and Campylobacter infection as well as the association between pathogenic Campylobacter species and inflammation and growth. Nucleic acid–based detection may provide a more granular understanding of specific Campylobacter species [47]. In the absence of culture isolates and molecular typing, our ability to distinguish persistent infection from reinfection was limited. However, the high prevalence and relatively transient clearance after antibiotic use suggest that reinfection is common. Third, measurement of environmental exposures is difficult, which may preclude the identification of risk factors for Campylobacter infection. Finally, it is possible that Campylobacter infection is a marker or sequela rather than a cause of reduced linear growth. An assessment of pathogen carriage and growth in a study designed to reduce exposure to Campylobacter may better distinguish these 2 possibilities.
In sum, this work provides strong evidence to suggest that Campylobacter infection is an important contributor to linear growth shortfalls in children in low-resource settings, given the strength, consistency across diverse settings, and persistence of the observed association with adjustment for potential confounders, the high burden of infection, and the presence of a plausible mechanism. Promotion of exclusive breastfeeding, routine treatment of drinking water, access to improved latrines, and judicious antibiotic administration may reduce Campylobacter infection and improve linear growth in children in these settings. We recommend that, in addition to vaccine development, clinical trials be undertaken to reduce Campylobacter infections via such interventions, with both subclinical pathogen infection and linear growth as outcomes of interest.
Supplementary Material
Notes
Acknowledgments. We thank the staff and participants of the MAL-ED Network for their important contributions.
Financial support. The Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) is carried out as a collaborative project supported by the Bill & Melinda Gates Foundation, the Foundation for the National Institutes of Health, and the National Institutes of Health, Fogarty International Center. J. A. P. receives support from the National Institutes of Health (5K23-AI114888-02).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Checkley W, Gilman RH, Epstein LD et al. . Asymptomatic and symptomatic cryptosporidiosis: their acute effect on weight gain in Peruvian children. Am J Epidemiol 1997; 145:156–63. [DOI] [PubMed] [Google Scholar]
- 2. Lee G, Pan W, Penataro Yori P et al. . Symptomatic and asymptomatic Campylobacter infections associated with reduced growth in Peruvian children. PLoS Negl Trop Dis 2013; 7:e2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee G, Paredes Olortegui M, Penataro Yori P et al. . Effects of Shigella-, Campylobacter- and ETEC-associated diarrhea on childhood growth. Pediatric Infect Dis J 2014; 33:1004–9. [DOI] [PubMed] [Google Scholar]
- 4. Black RE, Brown KH, Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics 1984; 73:799–805. [PubMed] [Google Scholar]
- 5. Adair LS, Fall CH, Osmond C et al. . Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet 2013; 382:525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Victora CG, Adair L, Fall C et al. . Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008; 371:340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sudfeld CR, McCoy DC, Danaei G et al. . Linear growth and child development in low- and middle-income countries: a meta-analysis. Pediatrics 2015; 135:e1266–75. [DOI] [PubMed] [Google Scholar]
- 8. Mbuya MN, Humphrey JH. Preventing environmental enteric dysfunction through improved water, sanitation and hygiene: an opportunity for stunting reduction in developing countries. Matern Child Nutr 2015; 12(suppl 1):106–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol 2007; 5:665–79. [DOI] [PubMed] [Google Scholar]
- 10. Kosek M, Guerrant RL, Kang G et al. . Assessment of environmental enteropathy in the MAL-ED cohort study: theoretical and analytic framework. Clin Infect Dis 2014; 59(suppl 4):S239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaakoush NO, Castano-Rodriguez N, Mitchell HM, Man SM. Global epidemiology of Campylobacter infection. Clin Microbiol Rev 2015; 28:687–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nichols GL, Richardson JF, Sheppard SK, Lane C, Sarran C. Campylobacter epidemiology: a descriptive study reviewing 1 million cases in England and Wales between 1989 and 2011. BMJ Open 2012; 2:e001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor EV, Herman KM, Ailes EC et al. . Common source outbreaks of Campylobacter infection in the USA, 1997–2008. Epidemiol Infect 2013; 141:987–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rao MR, Naficy AB, Savarino SJ et al. . Pathogenicity and convalescent excretion of Campylobacter in rural Egyptian children. Am J Epidemiol 2001; 154:166–73. [DOI] [PubMed] [Google Scholar]
- 15. Zaki AM, DuPont HL, el Alamy MA et al. . The detection of enteropathogens in acute diarrhea in a family cohort population in rural Egypt. Am J Trop Med Hyg 1986; 35:1013–22. [DOI] [PubMed] [Google Scholar]
- 16. Lengerh A, Moges F, Unakal C, Anagaw B. Prevalence, associated risk factors and antimicrobial susceptibility pattern of Campylobacter species among under five diarrheic children at Gondar University Hospital, Northwest Ethiopia. BMC Pediatrics 2013; 13:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molbak K, Hojlyng N, Gaarslev K. High prevalence of Campylobacter excretors among Liberian children related to environmental conditions. Epidemiol Infect 1988; 100:227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. MAL-ED Network Investigators. The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis 2014; 59(suppl 4):S193–206. [DOI] [PubMed] [Google Scholar]
- 19. Platts-Mills JA, Liu J, Gratz J et al. . Detection of Campylobacter in stool and determination of significance by culture, enzyme immunoassay, and PCR in developing countries. J Clin Microbiol 2014; 52:1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Platts-Mills JA, Babji S, Bodhidatta L et al. . Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 2015; 3:e564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richard SA, McCormick BJ, Miller MA, Caulfield LE, Checkley W, MAL-ED Network Investigators. Modeling environmental influences on child growth in the MAL-ED cohort study: opportunities and challenges. Clin Infect Dis 2014; 59(suppl 4):S255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation. Progress on drinking water and sanitation: 2012 update. Available at: http://www.unicef.org/media/files/JMPreport2012.pdf Accessed 14 April 2016.
- 23. Houpt E, Gratz J, Kosek M et al. . Microbiologic methods utilized in the MAL-ED cohort study. Clin Infect Dis 2014; 59(suppl 4):S225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–6. [DOI] [PubMed] [Google Scholar]
- 25. Stolwijk AM, Straatman H, Zielhuis GA. Studying seasonality by using sine and cosine functions in regression analysis. J Epidemiol Community Health 1999; 53:235–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lenth RV. Least-squares means: the R package lsmeans. J Stat Softw 2016; 69:1–33. [Google Scholar]
- 27. Patil CL, Turab A, Ambikapathi R et al. . Early interruption of exclusive breastfeeding: results from the eight-country MAL-ED study. J Health Popul Nutr 2015; 34:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. Experimental Campylobacter jejuni infection in humans. J Infect Dis 1988; 157:472–9. [DOI] [PubMed] [Google Scholar]
- 29. Reti KL, Tymensen LD, Davis SP, Amrein MW, Buret AG. Campylobacter jejuni increases flagellar expression and adhesion of noninvasive Escherichia coli: effects on enterocytic Toll-like receptor 4 and CXCL-8 expression. Infect Immun 2015; 83:4571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Masanta WO, Heimesaat MM, Bereswill S et al. . Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis. Clin Dev Immunol 2013; 2013:526860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalischuk LD, Leggett F, Inglis GD. Campylobacter jejuni induces transcytosis of commensal bacteria across the intestinal epithelium through M-like cells. Gut Pathog 2010; 2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Riddle MS, Gutierrez RL, Verdu EF, Porter CK. The chronic gastrointestinal consequences associated with Campylobacter. Curr Gastroenterol Rep 2012; 14:395–405. [DOI] [PubMed] [Google Scholar]
- 33. Kosek M, Haque R, Lima A et al. . Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg 2013; 88:390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hassan KE, Mansour A, Shaheen H et al. . The impact of household hygiene on the risk of bacterial diarrhea among Egyptian children in rural areas, 2004-2007. J Infect Dev Ctries 2014; 8:1541–51. [DOI] [PubMed] [Google Scholar]
- 35. MacRitchie LA, Hunter CJ, Strachan NJ. A population-based exposure assessment of risk factors associated with gastrointestinal pathogens: a Campylobacter study. Epidemiol Infect 2013; 141:976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ruiz-Palacios GM, Calva JJ, Pickering LK et al. . Protection of breast-fed infants against Campylobacter diarrhea by antibodies in human milk. J Pediatrics 1990; 116:707–13. [DOI] [PubMed] [Google Scholar]
- 37. Bhandari N, Bahl R, Mazumdar S et al. . Effect of community-based promotion of exclusive breastfeeding on diarrhoeal illness and growth: a cluster randomised controlled trial. Lancet 2003; 361:1418–23. [DOI] [PubMed] [Google Scholar]
- 38. Boisson S, Stevenson M, Shapiro L et al. . Effect of household-based drinking water chlorination on diarrhoea among children under five in Orissa, India: a double-blind randomised placebo-controlled trial. PLoS Med 2013; 10:e1001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clasen T, Boisson S, Routray P et al. . Effectiveness of a rural sanitation programme on diarrhoea, soil-transmitted helminth infection, and child malnutrition in Odisha, India: a cluster-randomised trial. Lancet Glob Health 2014; 2:e645–53. [DOI] [PubMed] [Google Scholar]
- 40. Pickering AJ, Djebbari H, Lopez C, Coulibaly M, Alzua ML. Effect of a community-led sanitation intervention on child diarrhoea and child growth in rural Mali: a cluster-randomised controlled trial. Lancet Glob Health 2015; 3:e701–11. [DOI] [PubMed] [Google Scholar]
- 41. Pickering AJ, Alzua ML. Are studies underestimating the effects of sanitation on child nutrition? authors’ reply. Lancet Glob Health 2016; 4:e160. [DOI] [PubMed] [Google Scholar]
- 42. Keenan JD, Ayele B, Gebre T et al. . Childhood mortality in a cohort treated with mass azithromycin for trachoma. Clin Infect Dis 2011; 52:883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amza A, Yu SN, Kadri B et al. . Does mass azithromycin distribution impact child growth and nutrition in Niger? A cluster-randomized trial. PLoS Negl Trop Dis 2014; 8:e3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seidman JC, Coles CL, Silbergeld EK et al. . Increased carriage of macrolide-resistant fecal E. coli following mass distribution of azithromycin for trachoma control. Intern J Epidemiol 2014; 43:1105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Coles CL, Mabula K, Seidman JC et al. . Mass distribution of azithromycin for trachoma control is associated with increased risk of azithromycin-resistant Streptococcus pneumoniae carriage in young children 6 months after treatment. Clin Infect Dis 2013; 56:1519–26. [DOI] [PubMed] [Google Scholar]
- 46. Vangay P, Ward T, Gerber JS, Knights D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe 2015; 17:553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Platts-Mills JA, Kosek M. Update on the burden of Campylobacter in developing countries. Curr Opin Infect Dis 2014; 27:444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.