Abstract
Background
The patient and observer scar assessment scale (POSAS) recently emerged as a promising method, reflecting both observer's and patient's opinions in evaluating scar. This tool was shown to be consistent and reliable in burn scar assessment, but it has not been tested in the setting of skin graft scar in skin cancer patients.
Objective
To evaluate facial skin graft scar applied to POSAS and to compare with objective scar assessment tools.
Methods
Twenty three patients, who diagnosed with facial cutaneous malignancy and transplanted skin after Mohs micrographic surgery, were recruited. Observer assessment was performed by three independent rates using the observer component of the POSAS and Vancouver scar scale (VSS). Patient self-assessment was performed using the patient component of the POSAS. To quantify scar color and scar thickness more objectively, spectrophotometer and ultrasonography was applied.
Results
Inter-observer reliability was substantial with both VSS and the observer component of the POSAS (average measure intraclass coefficient correlation, 0.76 and 0.80, respectively). The observer component consistently showed significant correlations with patients' ratings for the parameters of the POSAS (all p-values<0.05). The correlation between subjective assessment using POSAS and objective assessment using spectrophotometer and ultrasonography showed low relationship.
Conclusion
In facial skin graft scar assessment in skin cancer patients, the POSAS showed acceptable inter-observer reliability. This tool was more comprehensive and had higher correlation with patient's opinion.
Keywords: Cicatrix, Patient and observer scar assessment scale, Skin graft
INTRODUCTION
Mohs micrographic surgery is preferred treatment option for facial malignant tumor due to assurance tumor removal and minimal loss of surrounding normal tissue1. Skin grafting is simple and better reconstruction procedure according to anatomic site, but it can lead to poor aesthetic results due to mismatch of thickness, texture and scar contraction2. The development of a skin graft scar is inevitable. In particularly, facial skin graft scar might be associated with adverse physical and psychological disturbances in patients undergoing treatment for cutaneous malignancy.
The ideal scar assessment tool should contain the following parameters: noninvasiveness, painlessness, easiness of work and reliability. The objective measurement parameters to evaluate the scar include color, thickness, surface texture, suppleness, and surface area3. The objective measurement apparatus like computerized image capture systems and digital color analysis methods require complex equipment and experienced operators, which may limit its use in a busy clinical setting. Hence, although objective measurements for scar evaluation are essential, there is a need for subjective assessment of scars.
The patient and observer scar assessment scale (POSAS) was designed to evaluate various types of scar subjectively4,5. This tool provides both the observers' and the patient's insights and it is easy to use, proving to be more advantageous than other tools. It was used to evaluate burn scars4 and linear surgical scars5, which showed reliable and valid results for scar evaluation.
Therefore, the aim of this study was to evaluate the usefulness of POSAS in facial skin graft scars in skin cancer patients and to compare POSAS with objective scar assessment tools like spectrophotometer and ultrasonography.
MATERIALS AND METHODS
Study design
This was a prospective, single-center study conducted from June 2015 to October 2015 at the Department of Dermatology, Wonkwang University Hospital, Republic of Korea. Informed consent was obtained from all participants after providing them with written and oral information about the study. The study protocol was approved by the ethics committee of Wonkwang University Hospital (IRB no. WKUH 201506-HR-043).
Patients
Twenty-three patients who underwent Mohs micrographic surgery from October 2011 to June 2015 for facial cutaneous malignancy were assessed for inclusion. The criteria for patient selection were as follows: patients with cutaneous malignancy on the face who had undergone Mohs micrographic surgery at least 2 months after the surgery; patients must be able to provide written informed consent; and, patients must not have any severe dermatologic, mental, or physical illness.
Measures
1) The Vancouver scar scale and the patient and observer scar assessment scale
Three observers (two dermatologists and one outpatient nurse) from the department of dermatology independently assessed all facial skin graft scar, using the Vancouver scar scale (VSS) and POSAS on the same day. The VSS, which was designed by Sullivan et al.6 in 1990, rated the scars according to four parameters: vascularity, pigmentation, pliability, and height. Each parameter contained ranked subscales that may be summed to obtain a total score ranging from 0 (representing normal skin) to 13 (representing worst scar imaginable) (Table 1). In POSAS, which was developed by Draaijers et al.4 in 2004, the observer component was composed of six parameters of scars: vascularity, pigmentation, thickness, relief, pliability, and surface area. Each parameter consisted of several categories. The degree of vascularity might be difficult to measure visually when there is pigmentation of the wound. A transparent plate can be used to compress the blood vessels7 and the amount of blood return after blanching can be scored numerically3. Also, when measuring the degree of pigmentation, the plate can be used to eliminate the effect of vascularity. According to Draaijers et al.4 a Plexiglas tool was used to evaluate vascularity and pigmentation in their study. However, this study used slide glass as a substitute. The patients, who were blinded from observers' scores, rated their own scars using the patient component of the POSAS during the same day. The patient component consisted of six parameters: scar-related pain, itchiness, color, stiffness, thickness, and irregularity. Each parameter used a 10-point scoring system, with 1 representing normal skin and 60 representing the worst scar imaginable (Table 2, 3).
Table 1. The Vancouver scar scale.
| Scar characteristic | Score |
|---|---|
| Vascularity | |
| Normal | 0 |
| Pink | 1 |
| Red | 2 |
| Purple | 3 |
| Pigmentation | |
| Normal | 0 |
| Hypopigmentation | 1 |
| Hyperpigmentation | 2 |
| Pliability | |
| Normal | 0 |
| Supple | 1 |
| Yielding | 2 |
| Firm | 3 |
| Ropes | 4 |
| Contracture | 5 |
| Height (mm) | |
| Flat | 0 |
| <2 | 1 |
| 2~5 | 2 |
| >5 | 3 |
| Total score | 13 |
Table 2. The patient and observer scar assessment scale.
| Observer component* | Normal skin | Worst scar imaginable | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Vascularity | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Pigmentation | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Thickness | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Relief | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Pliability | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Surface area | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Overall opinion | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
*In observer component, all parameters consisted of additional category: Vascularity: pale, pink, red, purple or mix; Pigmentation: hypopigmentaion, hyperpigmentaion or mix; Thickness: thicker or thinner; Relief: more, less or mix; Pliability: supple, stiff or mix; Surface area: expansion, contraction or mix.
Table 3. The definitions of terms used in the patient and observer scar assessment scale.
| Category | Definition |
|---|---|
| Vascularity | Presence of vessels in scar tissue assessed by the amount of redness, tested by the amount of blood return after blanching with a slide glass |
| Pigmentation | Brownish coloration of the scar by pigment (melanin); apply slide glass to the skin with moderate pressure to eliminate the effect of vascularity |
| Thickness | Average distance between the subcutical-dermal border and the epidermal surface of the scar |
| Relief | The extent to which surface irregularities are present |
| Pliability | Suppleness of the scar tested by wrinkling the scar between the thumb and index finger |
| Surface area | Surface area of the scar in relation to the original wound area |
2) Spectrophotometer
To quantify the scar color objectively, Minolta Spectrophotometer® CM-700d (Minolta Camera Co., Osaka, Japan) was used with identical room lighting. The measurement of scar color was compared to normal skin color in the same cosmetic units. The face could be divided into six cosmetic units, (i.e., forehead, eyes and periorbital area, cheeks, nose, lips and perioral area, and chin) according to skin texture, color, and contour8. The Minolta Spectrophotometer® is a tri-stimulus colorimeter that contains a Xenon lamp as a light source. The light that is reflected perpendicular to the skin is collected by photodetectors with color filter for a tristimulus color analysis at 450, 560, and 600 nm. This equipment uses the L*a*b* system, where L* signifies brightness (scored from 0 for white to 100 for black), a* indicates color values from green to red (negative values indicated green and positive values indicated red), and b* indicates color values from blue to yellow (negative values indicated blue and positive values indicated yellow)9. The a* parameter used to evaluate scar vascularity, while the L* and b* parameters were used to evaluate scar pigmentation10.
3) Ultrasonography
When evaluating scar thickness objectively, ultrasonography provided reliable and accurate quantitative information11. The average distance between the subcutical-dermal border and epidermal surface of the scar was measured with LOGIQ® 9 (General Electric Company, Niskayuna, NY, USA) ultrasonography system. Scar thickness was compared to normal skin using in the same cosmetic units.
4) Statistical analysis
Internal consistency was defined as "the homogeneity of a set of categories and the degree to which they all share the same characteristics." It was assessed by using Cronbach's alpha statistics which considered the values greater than or equal to 0.70 to be acceptable12. Interobserver reliability was defined as "the extent of agreement between three observers" and was assessed by computing the intraclass correlation coefficient (ICC) using a two-way mixed model with measures of consistency. An ICC within the range of 0 to 0.20 was considered as "slight", 0.21 to 0.40 as "fair", 0.41 to 0.60 as "moderate", 0.61 to 0.80 as "substantial", and 0.81 to 1.0 as "almost perfect"13. Convergent validity, which refers to the correlation among independently gathered rating, was evaluated using Pearson's correlation statistics. Simple linear regression analysis was used to identify variables that significantly influenced the patients' overall opinions of their scars. The differences in skin color and thickness between the skin graft scar and normal skin, which were measured with spectrophotometer and ultrasonography, were analyzed statistically by using t-test. All statistical analyses were performed by using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) and p-values of <0.05 were considered significant.
RESULTS
Patient characteristics
Twenty-three patients (11 men, 12 women) were recruited for the study (Table 4). The median age was 70.9 years (range, 33~86 years). The mean time that had passed from Mohs micrographic surgery to the study was 23.9 months (range, 2~48 months). The most common type of malignant cutaneous neoplasm was basal cell carcinoma (73.9%) and the most common skin graft recipient site was the nose (56.5%).
Table 4. Characteristics of 23 patients with facial skin graft scar.
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 11 (47.8) |
| Female | 12 (52.2) |
| Age (yr) | |
| 30~40 | 1 (4.4) |
| 41~50 | 0 |
| 51~60 | 3 (13.0) |
| 61~70 | 4 (17.4) |
| 71~80 | 9 (39.1) |
| 81~90 | 6 (26.1) |
| Type of malignant skin cancer | |
| Basal cell carcinoma | 17 (73.9) |
| Squamous cell carcinoma | 4 (17.4) |
| Malignant melanoma | 2 (8.7) |
| Site of malignant skin tumor | |
| Forehead | 3 (13.0) |
| Eyes and periorbital | 2 (8.7) |
| Cheek | 3 (13.0) |
| Nose | 13 (56.5) |
| Lips and perioral | 1 (4.4) |
| Chin | 1 (4.4) |
| Location of skin donor site | |
| Forehead | 9 (39.1) |
| Cheek | 2 (8.7) |
| Postauricular | 10 (43.4) |
| Supraclavicular | 1 (4.4) |
| Thigh | 1 (4.4) |
Scar assessment using VSS and POSAS
1) Overall opinion of the VSS and POSAS
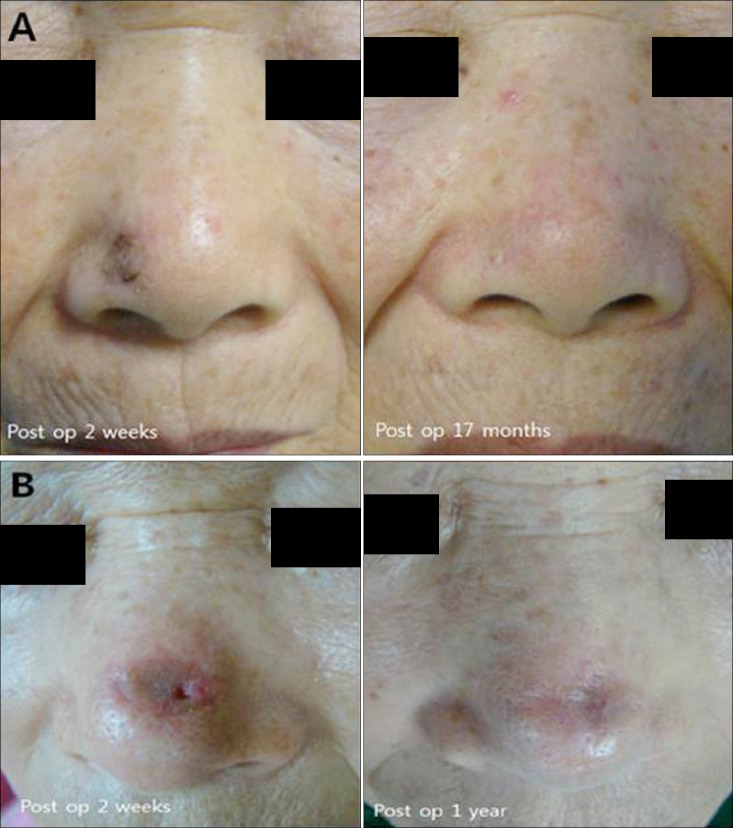
The mean total score using the VSS for facial skin graft scar was 3.4±1.8. The mean total score using the observer component of POSAS for facial skin graft scar was 15.8±7.0 and that using the patient component of POSAS was 18.4±10.3 (p=0.09) (Fig. 1).
Fig. 1. Clinical images of skin graft scar. (A) A 73-year-old woman who underwent skin graft because of basal cell carcinoma on the ala of nose. Evaluating skin graft scar using patient and observer scar assessment scale (POSAS) 17 months later, patient scar score was 13 and the mean observer scar score was 8.6. The patient score of POSAS: pain (2), itchiness (3), color (2), stiffness (2), thickness (2), irregularity (2). The mean observer score of POSAS: vascularity (1.3), pigmentation (1.3), thickness (2.0), relief (1.0), pliability (1.3), surface area (1.3). (B) The patient score of skin graft scar who had Moh's micrographic surgery on dorsum of nose 1 year ago was 22. The observer score was 15.3. The patient score of POSAS: pain (1), itchiness (1), color (5), stiffness (5), thickness (5), irregularity (2). The mean observer score of POSAS: vascularity (1.7), pigmentation (3.0), thickness (3.0), relief (2.3), pliability (3.3), surface area (2.0) (presented with permission patients). Preop: preoperative, postop: postoperative.
2) Internal consistency
The internal consistency (Table 5) was acceptable for the VSS and observer and patient components of the POSAS, with Cronbach's alpha values of 0.76, 0.84, and 0.88, respectively.
Table 5. The reliability of the Vancouver scar scale and patient and observer scar assessment scale (POSAS).
| Items | Cronbach's alpha | Number of items |
|---|---|---|
| Vancouver scar scale | 0.76 | 4 |
| Observer component of POSAS | 0.84 | 6 |
| Patient component of POSAS | 0.88 | 6 |
3) Interobserver reliability
The interobserver reliability (Table 6) was substantial for both the VSS and the observer component of the POSAS in terms of total score (the average measures of ICC were, 0.76 and 0.80, respectively). For the individual VSS categories, the interobserver reliability was substantial for vascularity (0.73) and, pliability (0.69), moderate for height (0.43), and fair for pigmentation (0.27). For the individual observer component of the POSAS, interobserver reliability was substantial for pliability, thickness, surface area, and relief (0.74, 0.70, 0.66, and 0.62, respectively), and moderate for pigmentation and vascularity (0.56 and 0.50, respectively).
Table 6. Interobserver reliability of the Vancouver scar scale (VSS) and the observer component of the patient and observer scar assessment scale (POSAS).
| Single measure ICC (95% CI) | Average measure ICC (95% CI) | |
|---|---|---|
| VSS | ||
| Vascularity | 0.48 (0.23~0.70) | 0.73 (0.47~0.88) |
| Pigmentation | 0.11 (−0.11~0.40) | 0.27 (−0.44~0.67) |
| Pliability | 0.42 (0.17~0.67) | 0.69 (0.38~0.86) |
| Height | 0.20 (−0.04~0.48) | 0.43 (−0.13~0.74) |
| Total | 0.51 (0.26~0.73) | 0.76 (0.52~0.89) |
| Observer component of the POSAS | ||
| Vascularity | 0.25 (0.00~0.53) | 0.50 (0.00~0.77) |
| Pigmentation | 0.30 (0.04~0.57) | 0.56 (0.13~0.80) |
| Thickness | 0.44 (0.19~0.68) | 0.70 (0.41~0.86) |
| Relief | 0.35 (0.09~0.61) | 0.62 (0.24~0.82) |
| Pliability | 0.48 (0.23~0.71) | 0.74 (0.47~0.88) |
| Surface area | 0.39 (0.14~0.65) | 0.66 (0.33~0.84) |
| Total | 0.57 (0.34~0.77) | 0.80 (0.60~0.91) |
CI: confidence interval, single measure ICC: intraclass correlation coeficient for a single observer, average measure ICC: intraclass correlation coefficient for the group of three observer.
4) Convergent validity
The correlations between the observer ratings of VSS and the observer component of POSAS were found to be significant (all p-values<0.05; Table 7). The observer component consistently showed significant correlations with the patients' ratings for the individual categories (all p-values< 0.05; Table 8). In VSS, pliability, height, and total score correlated significantly with the patient components of stiffness, thickness, and total scores.
Table 7. Correlations between the VSS and the observer component of the patient and observer scar assessment scale.
| Pearson's correlation coefficient | p-value | |
|---|---|---|
| VSS vascularity score vs. OSAS vascularity score | 0.56 | 0.005 |
| VSS pigmentation score vs. OSAS pigmentation score | 0.57 | 0.004 |
| VSS pliability score vs. OSAS pliability score | 0.83 | <0.001 |
| VSS height score vs. OSAS thickness score | 0.82 | <0.001 |
| VSS total score vs. OSAS total score | 0.80 | <0.001 |
VSS: Vancouver scar scale, OSAS: observer scar assessment scale.
Table 8. Correlation between observer scores using the VSS and the observer component of the patient and observer scar assessment scale (POSAS) and patient scores using the POSAS.
| Pearson's correlation coefficient | p-value | |
|---|---|---|
| VSS vascularity score vs. PSAS color score | 0.28 | 0.181 |
| VSS pigmentation score vs. PSAS color score | 0.35 | 0.097 |
| VSS pliability score vs. PSAS stiffness score | 0.65 | 0.001 |
| VSS height score vs. PSAS thickness score | 0.62 | 0.001 |
| VSS total score vs. PSAS total score | 0.61 | 0.002 |
| OSAS vascularity score vs. PSAS color score | 0.47 | 0.022 |
| OSAS pigmentation score vs. PSAS color score | 0.47 | 0.024 |
| OSAS pliability score vs. PSAS stiffness score | 0.56 | 0.005 |
| OSAS thickness score vs. PSAS thickness score | 0.63 | 0.001 |
| OSAS relief score vs. PSAS irregularity score | 0.59 | 0.003 |
| OSAS total score vs. PSAS total score | 0.49 | 0.015 |
VSS: Vancouver scar scale, PSAS: patient scar assessment scale, OSAS: observer scar assessment scale.
5) Patient self-assessment and linear regression analysis
On simple linear regression analysis, the patients' overall opinion regarding their own scars was significantly influenced by scar-related itchiness, color, stiffness, thickness, and irregularity (p<0.05; Table 9).
Table 9. Simple linear regression analysis of variables associated with patient scar assessment scale scores.
| Items | Slope coefficient | p-value |
|---|---|---|
| Pain | 0.79 | 0.096 |
| Itchness | 0.74 | 0.006 |
| Color | 0.73 | <0.001 |
| Stiffness | 0.73 | <0.001 |
| Thickness | 0.71 | <0.001 |
| Irregularity | 0.67 | <0.001 |
Scar evaluation using spectrophotometer and ultrasonography
1) Scar color
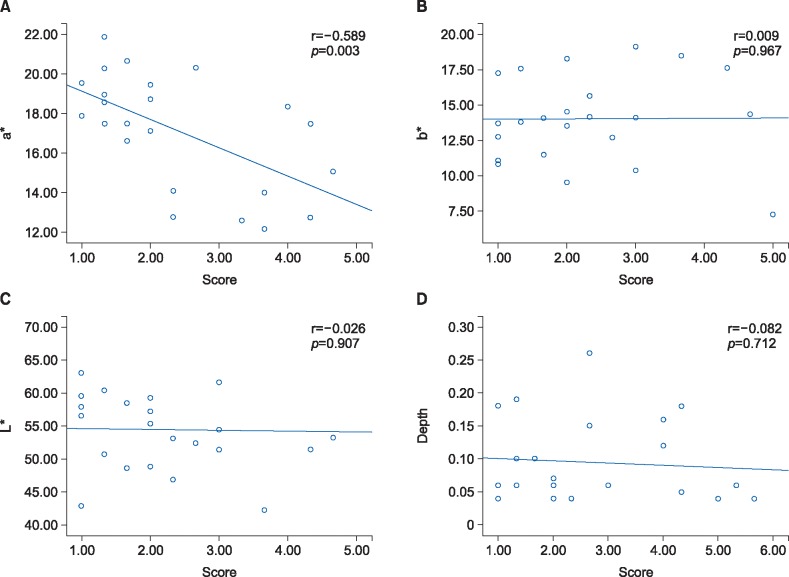
The interobserver reliability of the observer component of POSAS was moderate in terms of vascularity and pigmentation (0.50 and 0.56, respectively). When measuring scar color using spectrophotometer, there was no significant difference between the scar and normal skin. The value of L* in the facial skin graft scar was 54.4±6.1 and that of the normal facial skin was 55.7±4.4 (p=0.44). The value of a* in the facial skin graft scar was 14.0±3.1 and that of the normal skin was 13.9±2.3 (p=0.87). The value of b* in the facial skin graft scar was 17.1±2.9 and that of the normal skin was 18.2±2.7 (p=0.21). The correlation between the subjective assessment using the observer component of POSAS and the objective assessment using spectrophotometer showed moderate but significant relationship in the vascularity subscale. However, the degree of correlation about pigmentation showed insignificant results between two different scar evaluation tools (Fig. 2).
Fig. 2. The correlation between patient and observer scar assessment scale (POSAS) to objective scar assessment tool. (A) a* indicated color values from green to red and it was applied to scar vascularity. The correlation between POSAS and spectrometer was significant but moderate relationship. (B, C) L* expressed brightness and it was used for the evaluation of scar pigmentation. b* designated values from blue to yellow and it was used for the measurement of scar pigmentation along with L*. The degree of correlation about pigmentation showed insignificant results between two different scar evaluation tool. (D) The correlation between POSAS and objective assessment using ultrasonography showed low relationship.
2) Scar thickness
The interobserver reliability of the observer component of POSAS was substantial in the thickness category (0.70). When measuring scar thickness using ultrasonography, a significant difference between the scar and normal control group was observed. The mean depth of the facial skin graft scar was 0.10±0.01 cm, while that of the normal facial skin was 0.09±0.06 cm (p<0.001). The correlation between the subjective assessment using the observer component of POSAS and the objective assessment using ultrasonography showed low relationship (Fig. 2).
DISCUSSION
In evaluating the facial skin graft scars by using subjective methods, both VSS and POSAS had acceptable internal consistency and interobserver reliability. This indicated that both VSS and POSAS had good feasibility as a scar assessment tool for clinical follow-up and research purposes in skin graft scar. The reliability of the VSS for facial skin graft scar (0.76) was similar to linear scars as previously reported (0.78)14. The score of the interclass correlation coefficient of the observer component of POSAS (0.80) was higher than that of the linear surgical scar (0.60)14. The potential factors that contribute to the variations in the reliability test results were attributed to the differences in the type of scars and observer's training.
In the analysis of the individual components of the VSS and POSAS, the results of our study suggested that the observer component of POSAS had an advantage over the VSS. The observer component of the POSAS subscale exhibited significant correlations with the patient component of POSAS for color, stiffness, thickness, and irregularity. However, the VSS subscale showed significant correlations with the patient component of POSAS for stiffness and thickness only. Also, the POSAS reflected the patients' subjective scar-related symptoms. This study showed that color, stiffness, irregularity, and itchiness significantly affected the patients' overall opinion. This suggests that not does the visibility of scars as an important factor, but also the scar symptoms, which needed to be monitored more closely in scar evaluation.
Scars distorted the physical appearance, especially when found on the head and neck, which could cause a negative impact on quality of life15,16. Nose is the most common site of skin cancer in the head and neck, which is related to sun exposure and ultraviolet damage17. Because of its complex subunit, with intersecting concavities and convexities, the nose is complicated aesthetic units to operate. Full-thickness skin graft takes large portion of nasal reconstruction along with nasolabial flap, especially in nasal sidewall and nasal dorsum skin defect18. In our study, nose is the most common site of operation (56.5%). In nasal subunit, the ala of nose (46.1%), dorsum (30.7%) and tip (23.0%) are the common recipient site of operation and forehead is the most common donor site (61.5%). The mean total score using the observer component of POSAS for nasal skin graft scar was 15.5±7.5 and that using the patient component of POSAS was 17.4±9.6 (p=0.19). In observer component of POSAS, the relief, pliability and thickness had a high score. The irregularity, thickness and color were a high proportion than subjective symptom like pain and itchiness in patient component of POSAS. These results showed that scar irregularity was a major considering factor in nasal skin graft scar. It is very important that the operating surgeon should be considered postoperative care for patients undergoing nasal reconstruction. In this regard, the POSAS is easy and efficient evaluation tool in scar assessment.
The color of the skin was affected by the distribution of the blood vessels and the pigmentation of the skin. The correlation between subjective assessment using POSAS and objective assessment using Spectrophotometer showed significant and moderate relationships in terms of vascularity and low relationships in pigmentation. POSAS has a limitation in that it may assess hypopigmented scar as high as a hyperpigmented scar because of pigmentation was compared to normal skin. In burn scar evaluation, the correlation between the vascularity score in POSAS and objective assessments in chromameter was moderate. On the other hand, the correlation coefficient in pigmentation was varied enormous per observer9. It is difficult to rate the pigmentation of the scar reliably. Wei et al.19 suggested the use of dermoscopy in assessment of vascularity and pigmentation of scar. The dermoscopy is a non-invasive method that allows the visualization of dilated capillaries and pigments in the dermal and epidermal layers of scar. It expresses great advantage in assessing the vascularity and pigmentation accurately. Also, he applied transformed VSS pigmentation score when correlating with objective scar assessment tool. The negative value represented hyperpigmentation and positive value implied hypopigmentation. This method showed a significant correlation between scar evaluation in objective tools and the subjective assessment.
To evaluate scar thickness objectively, ultrasound techniques can be used. It can produce images that are reflected from the interfaces of different tissues7. The interobserver reliability of the POSAS among three observers was substantial in scar thickness. The correlation between subjective assessment using POSAS and objective assessment using ultrasonography showed poor relationships. When we evaluate scar thickness by POSAS, there is no difference in scores between hypertrophic scar and hypotrophic scar.
Over the years, a lot of efforts have tried in exploring more objective and accurate assessment tools for scar measurement with the development in electronics and information technology. Objective tools, such as Laser Doppler can be used to measure the blood perfusion of scars which is also an important indicator of vascularity20. However, the measurements are difficult in the border of scar, and pigmentation characteristics cannot be measured with Laser Doppler. A non-contact 3D digitiser (Kinoca-Minolta Vivid 900®; Konica Minolta Co., Tokyo, Japan) can measure the volume of a scar21. This tool used initially in industry to scan a 3D image of an object. When evaluating a scar, this method showed a significant positive correlation between the volume of scar and the clinical severity according to scar scale. An optical system that produces three-dimensional measurement of the skin surface, the PRIMOS® system, can be used to evaluate the scar surface22. It is available to measure skin surface level difference up to 10 mm with a resolution over 0.004 mm. However, these kinds of instruments are usually extremely expensive and difficult to be carried around, thus it is not easily applicable tools to be widely used for clinical practice.
The POSAS is a standardized, validated, and comprehensive scar assessment tool in clinical care. To our knowledge, this is the first attempt to evaluate facial skin graft scar by POSAS. The POSAS had more advantage as its observer component showed better correlation to the patient's rating. Additionally, the POSAS reflected the patient's perspective about scar-related symptoms like pain and itchiness, which were not considered in previous scar assessment tool4,5. These findings support the use of POSAS as a reliable, valid, and comprehensive tool to assess facial skin graft scar. Even though there are many advantages of POSAS as scar evaluation tool, the correlation between the POSAS and objective scar evaluation tools shows low relationships. To compensate these limitations, there are several things to complement. First, it is insufficient to evaluate scar color with naked eye, so additional tools like dermoscopy could increase the exactitude of scar evaluation. Second, the POSAS lacks the score system in the category of parameters. It cannot make distinction between hyperpigmentation and hypopigmentation because of pigmentation was compared to normal skin. The transformed POSAS scoring system which included positive and negative value will enable accurate scar evaluation. Our study has some limitation that we included a limited number of patients in single center. Future study should contain large sample size and explore the improvement of facial skin graft scar through the management of scar.
ACKNOWLEDGMENT
This work was supported by Wonkwang University in 2014.
References
- 1.Viola KV, Jhaveri MB, Soulos PR, Turner RB, Tolpinrud WL, Doshi D, et al. Mohs micrographic surgery and surgical excision for nonmelanoma skin cancer treatment in the Medicare population. Arch Dermatol. 2012;148:473–477. doi: 10.1001/archdermatol.2011.2456. [DOI] [PubMed] [Google Scholar]
- 2.Sheehan JM, Kingsley M, Rohrer TE. Excisional surgery and repair, flaps, and graft. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick's dermatology in general medicine. 8th ed. New York: McGraw-Hill; 2012. pp. 2944–2949. [Google Scholar]
- 3.Idriss N, Maibach HI. Scar assessment scales: a dermatologic overview. Skin Res Technol. 2009;15:1–5. doi: 10.1111/j.1600-0846.2008.00327.x. [DOI] [PubMed] [Google Scholar]
- 4.Draaijers LJ, Tempelman FR, Botman YA, Tuinebreijer WE, Middelkoop E, Kreis RW, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg. 2004;113:1960–1965. doi: 10.1097/01.prs.0000122207.28773.56. [DOI] [PubMed] [Google Scholar]
- 5.van de Kar AL, Corion LU, Smeulders MJ, Draaijers LJ, van der Horst CM, van Zuijlen PP. Reliable and feasible evaluation of linear scars by the Patient and Observer Scar Assessment Scale. Plast Reconstr Surg. 2005;116:514–522. doi: 10.1097/01.prs.0000172982.43599.d6. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan T, Smith J, Kermode J, McIver E, Courtemanche DJ. Rating the burn scar. J Burn Care Rehabil. 1990;11:256–260. doi: 10.1097/00004630-199005000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Roques C, Teot L. A critical analysis of measurements used to assess and manage scars. Int J Low Extrem Wounds. 2007;6:249–253. doi: 10.1177/1534734607308249. [DOI] [PubMed] [Google Scholar]
- 8.Robinson JK, Anderson ER. Skin structure and surgical anatomy. In: Robinson JK, Hnke CW, Sengelmann RD, Siegel DM, editors. Surgery of the skin. Philadelphia: Elsevier Mosby; 2005. pp. 6–10. [Google Scholar]
- 9.Draaijers LJ, Tempelman FR, Botman YA, Kreis RW, Middelkoop E, van Zuijlen PP. Colour evaluation in scars: tristimulus colorimeter, narrow-band simple reflectance meter or subjective evaluation? Burns. 2004;30:103–107. doi: 10.1016/j.burns.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 10.Takiwaki H. Measurement of skin color: practical application and theoretical considerations. J Med Invest. 1998;44:121–126. [PubMed] [Google Scholar]
- 11.Beausang E, Floyd H, Dunn KW, Orton CI, Ferguson MW. A new quantitative scale for clinical scar assessment. Plast Reconstr Surg. 1998;102:1954–1961. doi: 10.1097/00006534-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Bland JM, Altman DG. Cronbach's alpha. BMJ. 1997;314:572. doi: 10.1136/bmj.314.7080.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 14.Truong PT, Lee JC, Soer B, Gaul CA, Olivotto IA. Reliability and validity testing of the Patient and Observer Scar Assessment Scale in evaluating linear scars after breast cancer surgery. Plast Reconstr Surg. 2007;119:487–494. doi: 10.1097/01.prs.0000252949.77525.bc. [DOI] [PubMed] [Google Scholar]
- 15.Sobanko JF, Sarwer DB, Zvargulis Z, Miller CJ. Importance of physical appearance in patients with skin cancer. Dermatol Surg. 2015;41:183–188. doi: 10.1097/DSS.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 16.Choi Y, Lee JH, Kim YH, Lee YS, Chang HS, Park CS, et al. Impact of postthyroidectomy scar on the quality of life of thyroid cancer patients. Ann Dermatol. 2014;26:693–699. doi: 10.5021/ad.2014.26.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans GR, Williams JZ, Ainslie NB. Cutaneous nasal malignancies: is primary reconstruction safe? Head Neck. 1997;19:182–187. doi: 10.1002/(sici)1097-0347(199705)19:3<182::aid-hed3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Weathers WM, Bhadkamkar M, Wolfswinkel EM, Thornton JF. Full-thickness skin grafting in nasal reconstruction. Semin Plast Surg. 2013;27:90–95. doi: 10.1055/s-0033-1351227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei Y, Li-Tsang CW, Luk DC, Tan T, Zhang W, Chiu TW. A validation study of scar vascularity and pigmentation assessment using dermoscopy. Burns. 2015;41:1717–1723. doi: 10.1016/j.burns.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Bray R, Forrester K, Leonard C, McArthur R, Tulip J, Lindsay R. Laser Doppler imaging of burn scars: a comparison of wavelength and scanning methods. Burns. 2003;29:199–206. doi: 10.1016/s0305-4179(02)00307-8. [DOI] [PubMed] [Google Scholar]
- 21.Taylor B, McGrouther DA, Bayat A. Use of a non-contact 3D digitiser to measure the volume of keloid scars: a useful tool for scar assessment. J Plast Reconstr Aesthet Surg. 2007;60:87–94. doi: 10.1016/j.bjps.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 22.Roques C, Téot L, Frasson N, Meaume S. PRIMOS: an optical system that produces three-dimensional measurements of skin surfaces. J Wound Care. 2003;12:362–364. doi: 10.12968/jowc.2003.12.9.26539. [DOI] [PubMed] [Google Scholar]