Abstract
Treatment of perianal and vulvar extramammary Paget disease (EMPD), rare intraepithelial malignancies, is often challenging because of its potential to spread into the anal canal. However, there is still no consensus regarding the optimal resection margin within the anal canal. Between 2004 and 2014, six patients (three with perianal EMPD and three with vulvar EMPD) in which the spread of Paget cells into the anal canal was highly suspected were referred to our department. To evaluate the disease extent within the anal canal, preoperative mapping biopsy of the anal canal was performed in five out of six patients. Two patients were positive for Paget cells within the anal canal (one at the dentate line and the other at 0.5 cm above the dentate line), whereas in three patients, Paget cell were present only in the skin of the anal verge. Using 1 cm margin within the anal canal from the positive biopsy sites, we performed anal-preserving wide local excision (WLE), and negative resection margins within the anal canal were confirmed in all five patients. The remaining one patient with perianal EMPD did not undergo mapping biopsy of the anal canal because preoperative colonoscopy revealed that the Paget cells had spread into the lower rectum. Therefore, WLE with abdominoperineal resection was performed. During the median follow-up period of 37.3 months, no local recurrence was observed in all patients. Our small case series suggest the usefulness of mapping biopsy of the anal canal for the treatment of perianal and vulvar EMPD.
Keywords: Anal canal, Biopsy, Extramammary Paget disease
INTRODUCTION
Extramammary Paget disease (EMPD) is a rare intraepithelial malignancy affecting apocrine glands, most commonly in the vulva and perianal regions1,2. According to a recent literature review, perianal EMPD accounts for 20% of all EMPDs and 6.5% of all Paget disease cases3.
The mainstay of EMPD treatment is wide local excision (WLE) with lateral margins extending from 2 to 3 cm beyond the clinically affected area4,5. However, owing to the multicentricity of the disease and irregular histological margins that often extend beyond the visible limits of the lesion6,7, the recurrence rates after WLE was reported as 33%~60%, making surgical management difficult8,9,10. To improve surgical outcomes, various methods, such as Mohs micrographic surgery11 and intraoperative frozen section analysis, used to evaluate the resection margins12 have been suggested. However, these methods are complicated and time-consuming because they require multiple biopsies during the operation. Previous studies have also reported the advantages of preoperative mapping biopsies. Kim et al.13 reported that the local recurrence rate was 4.8% in 21 patients with EMPD who underwent preoperative mapping biopsy 2 cm from the tumor margin. Kato et al.14 examined 17 patients with EMPD who received mapping biopsy 1 cm from the defined border and 3 cm from the ill-defined border, according to the Japanese guidelines, and reported a recurrence rate of 5.9%. However, these reports mainly focused on mapping biopsy of the skin for evaluation of the lateral spread of the lesion.
In case of perianal and vulvar EMPD, Paget cells often spread into the anal canal mucosa making treatment challenging. Because excessive resection can easily impair the postoperative anorectal function, it is of great importance to appropriately identify the resection margins within the anal canal during treatment of perianal and vulvar EMPD.
Here we report our experience of treating six patients with perianal or vulvar EMPD who were suspected of exhibiting spread of Paget cells into the anal canal. We performed preoperative mapping biopsy of the anal canal under spinal anesthesia in five out of six patients and observed good results when evaluating the disease extent within the canal. We believe that this case report may add a new perspective to the treatment strategy for perianal and vulvar EMPD.
CASE REPORT
Six patients with perianal or vulvar EMPD were referred to the Department of Surgical Oncology, The University of Tokyo Hospital between December 2004 and April 2014. All six patients were suspected of exhibiting spread of Paget cells into the anal canal. Written informed consent was obtained from all participating patients.
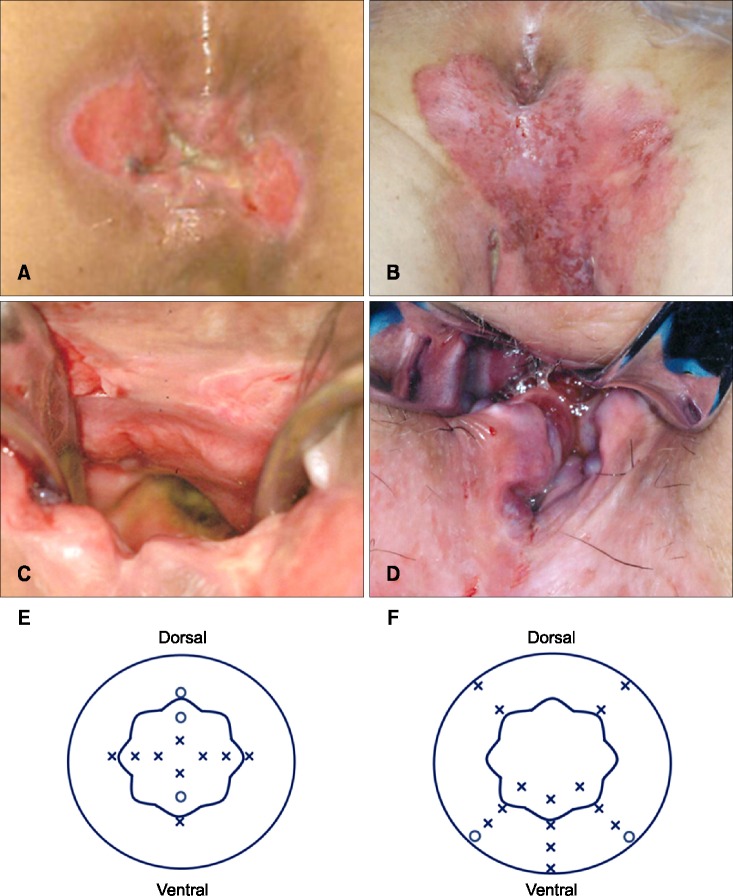
The clinical findings and results of mapping biopsy of the anal canal were summarized in Table 1. Preoperative mapping biopsy of the anal canal was performed in five out of six patients. Fig. 1 demonstrates the procedure of this method in the second patient with perianal EMPD and the sixth patient with vulvar EMPD. Two patients with perianal EMPD were positive for Paget cells within the anal canal, one at the dentate line (the first patient) and the other at 0.5 cm above the dentate line (the second patient). In the remaining three patients with vulvar EMPD, though the spread of Paget cells into the anal canal was highly suspected, mapping biopsy showed that they were present only in the skin of the anal verge and not within the canal itself. The fourth patient with perianal EMPD did not undergo this procedure because preoperative biopsy of the rectal mucosa under colonoscopy revealed that the Paget cells had spread into the lower rectum. Therefore, the mapping biopsy of the anal canal was abbreviated and WLE with abdominoperineal resection was performed.
Table 1. Summaries of six patients with perianal or vulva EMPD.
| Clinicopathological factor | Patient number | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Clinical findings | ||||||
| Tumor location | Perianal | Perianal | Vulva | Perianal | Vulva | Vulva |
| Sex | Male | Female | Female | Male | Male | Male |
| Age at diagnosis (yr) | 74 | 69 | 81 | 78 | 75 | 74 |
| Chief complaint | Eczema | Eczema | Eczema and itching | Eczema | Eczema | Eczema and itching |
| Duration of symptom (mo) | 72 | 36 | 30 | 2 | 84 | 120 |
| Mapping biopsy of the anal canal | ||||||
| Number of biopsies | 12 | 12 | 21 | Not performed | 16 | 12 |
| Biopsy-positive site | DL | 0.5-cm above DL | Anal verge | - | Anal verge | Anal verge |
| Surgical findings | ||||||
| Surgical procedure | WLE | WLE | WLE | WLE+APR | WLE | WLE |
| Reconstruction method | STSG | STSG | STSG | STSG | STSG | STSG |
| Lymph node dissection | No | No | Yes | No | Yes | No |
| Covering colostomy | Yes | Yes | No | - | No | No |
| Pathological findings | ||||||
| Resection margin | Negative | Negative | Negative | Positive | Negative | Negative |
| Histological stage | Intraepithelial | Intraepithelial | Invasive | Intraepithelial | Invasive | Intraepithelial |
| Lymph node metastasis | No | No | Yes | No | Yes | No |
| Outcomes | ||||||
| Graft infection | Yes | No | Yes | No | Yes | No |
| Postoperative hospital stay (d) | 86 | 51 | 72 | 24 | 82 | 22 |
| Tumor recurrence | No | No | Yes | No | Yes | No |
| Prognosis | Alive | Alive | Died | Alive | Alive | Alive |
| Disease free survival (mo) | 37.2 | 112 | 2.9 | 50.2 | 13.1 | 5.8 |
| Overall survival (mo) | 37.2 | 112 | 12.8 | 50.2 | 37.4 | 5.8 |
EMPD: extramammary Paget disease, DL: dentate line, WLE: wide local excision, APR: abdominoperineal resection, STSG: split thickness skin graft.
Fig. 1. Mapping biopsy of the anal canal. (A) A 69-year-old female with perianal extramammary Paget disease (EMPD) (the second patient). (B) The anal canal was sufficiently enlarged using a retractor in the jack-knife position under spinal anesthesia, and mapping biopsy of the anal canal was performed. (C) A scheme of the mapping biopsy of the anal canal. A total of 12 biopsies were performed (indicated by crosses and small circles): four on the dentate line, four 0.5 cm above the dentate line, and four 1.0 cm above the dentate line. The small circles demonstrate the sites that were positive for Paget cells. The large round circle indicates the anal verge, and the wavelike circle indicates the dentate line. (D) A 75-year-old female with vulvar EMPD (the sixth patient). The lesion involved the anal verge, and spread of Paget cells into the anal canal was highly suspected. (E) The anal canal was sufficiently enlarged using a retractor in the jack-knife position under spinal anesthesia. The spread of Paget cells into the anal canal were particularly suspected in the vertebral side. (F) A scheme of the mapping biopsy of the anal canal. A total of 16 biopsies were performed: five on the anal verge, three 10 mm above the anal verge, five on the dentate line, and three 10 mm above the dentate line. The small circles demonstrate the sites that were positive for Paget cells.
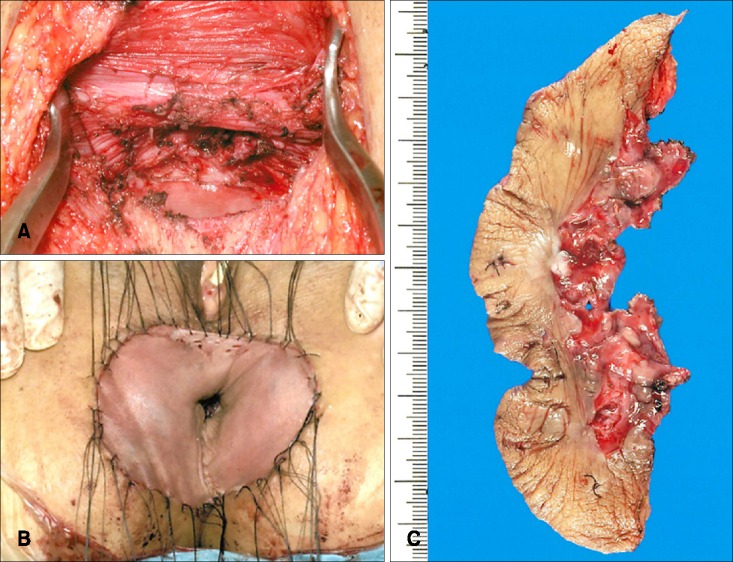
Surgical findings, pathological findings and clinical outcomes were summarized in Table 1. For the five patients who underwent mapping biopsy of the anal canal, anal-preserving WLE deep to the subcutaneous fat were performed using a 1-cm margin from the positive biopsy sites according to the results of mapping biopsy of the anal canal. Reconstructions were performed in all six patients using split-thickness skin grafts from the anterolateral thigh. Fig. 2 shows the surgical findings of the second patient. Lymph node dissection was performed in two patients (the third and fifth patient) suspected of having lymph node metastases based on preoperative computed tomography and magnetic resonance imaging.
Fig. 2. Surgical findings of a 69-year-old female with perianal extramammary Paget disease (the second patient). (A) Wide local excision deep to the subcutaneous fat was performed. (B) Reconstruction was performed with split thickness skin grafts. (C) Surgically resected specimen.
Covering colostomy was performed in two patients whose resection margins within the anal canal exceeded the dentate line and were thought to be at a high risk of infection. Despite this, one of these two patients experienced graft infection (the first patient). Graft infection was also associated with longer postoperative hospital stay (median 24 days [range, 22~51]) without infection vs. median 82 days [range, 72~86] with infection [p=0.049]).
The resection margins within the anal canal were negative for Paget cells in all patients. However, the resection margin of the skin side (lateral resection margin) was positive for Paget cells in the fifth patient, making additional skin resection necessary. During the median follow-up period of 37.3 months, no local recurrence in the anal canal was observed in all patients; however, two patients with invasive histological stages and metastasis to the inguinal lymph nodes developed recurrence. The fifth patient exhibited a recurrent lesion in the skin 13.1 months after the operation which was then resected. The third patient received only best supportive care as she experienced multiple lymph node recurrence early, and she died 12.8 months after the initial operation.
DISCUSSION
The treatment of perianal and vulvar EMPD is often challenging, particularly in case of suspected spread of Paget cells into the anal canal. The anal canal measures between 2.5 and 4.0 cm in length, beginning at the anorectal junction and ending at the anal verge. Within it, the dentate line lies approximately 2 cm proximal to the anal verge and forms the border between the anal squamous epithelium and rectal mucosa. There is still no consensus regarding the optimal resection margin within the anal canal. Previous case reports suggested that resection of the anal canal mucosa up to 1 cm above the dentate line is important to ensure inclusion of the entire anal squamous epithelium4,15. However, the resection margin within the anal canal should be carefully determined as excessive resection may easily impair the postoperative anorectal function. Our method of mapping biopsy of the anal canal enabled us to determine the appropriate resection margin in more personalized way. With 1 cm margin from positive biopsy sites within the anal canal, we accomplished negative resection margins and no local recurrence in the anal canal was observed suggesting the effectiveness of mapping biopsy of the anal canal in determining adequate resection margins.
On the other hand, owing to the small sample size, we could not sufficiently analyze the indication of the covering colostomy and the postoperative anorectal function. We will further analyze by accumulating more EMPD patients.
In summary, although the sample size was small, this report may add an additional perspective to the treatment strategy for perianal and vulvar EMPD. Mapping biopsy of the anal canal may be a useful method for the evaluation of disease extent within the canal.
References
- 1.Kanitakis J. Mammary and extramammary Paget's disease. J Eur Acad Dermatol Venereol. 2007;21:581–590. doi: 10.1111/j.1468-3083.2007.02154.x. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd J, Flanagan AM. Mammary and extramammary Paget's disease. J Clin Pathol. 2000;53:742–749. doi: 10.1136/jcp.53.10.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minicozzi A, Borzellino G, Momo R, Steccanella F, Pitoni F, de Manzoni G. Perianal Paget's disease: presentation of six cases and literature review. Int J Colorectal Dis. 2010;25:1–7. doi: 10.1007/s00384-009-0797-9. [DOI] [PubMed] [Google Scholar]
- 4.Kyriazanos ID, Stamos NP, Miliadis L, Noussis G, Stoidis CN. Extra-mammary Paget's disease of the perianal region: a review of the literature emphasizing the operative management technique. Surg Oncol. 2011;20:e61–e71. doi: 10.1016/j.suronc.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Chan JY, Li GK, Chung JH, Chow VL. Extramammary Paget's disease: 20 years of experience in chinese population. Int J Surg Oncol. 2012 doi: 10.1155/2012/416418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunn RA, Gallager HS. Vulvar Paget's disease: a topographic study. Cancer. 1980;46:590–594. doi: 10.1002/1097-0142(19800801)46:3<590::aid-cncr2820460327>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Coldiron BM, Goldsmith BA, Robinson JK. Surgical treatment of extramammary Paget's disease. A report of six cases and a reexamination of Mohs micrographic surgery compared with conventional surgical excision. Cancer. 1991;67:933–938. doi: 10.1002/1097-0142(19910215)67:4<933::aid-cncr2820670413>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Zollo JD, Zeitouni NC. The roswell park cancer institute experience with extramammary Paget's disease. Br J Dermatol. 2000;142:59–65. doi: 10.1046/j.1365-2133.2000.03242.x. [DOI] [PubMed] [Google Scholar]
- 9.Fanning J, Lambert HC, Hale TM, Morris PC, Schuerch C. Paget's disease of the vulva: prevalence of associated vulvar adenocarcinoma, invasive Paget's disease, and recurrence after surgical excision. Am J Obstet Gynecol. 1999;180:24–27. doi: 10.1016/s0002-9378(99)70143-2. [DOI] [PubMed] [Google Scholar]
- 10.Hendi A, Brodland DG, Zitelli JA. Extramammary Paget's disease: surgical treatment with Mohs micrographic surgery. J Am Acad Dermatol. 2004;51:767–773. doi: 10.1016/j.jaad.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Mohs FE, Blanchard L. Microscopically controlled surgery for extramammary Paget's disease. Arch Dermatol. 1979;115:706–708. [PubMed] [Google Scholar]
- 12.Fishman DA, Chambers SK, Schwartz PE, Kohorn EI, Chambers JT. Extramammary Paget's disease of the vulva. Gynecol Oncol. 1995;56:266–270. doi: 10.1006/gyno.1995.1044. [DOI] [PubMed] [Google Scholar]
- 13.Kim BJ, Park SK, Chang H. The effectiveness of mapping biopsy in patients with extramammary Paget's disease. Arch Plast Surg. 2014;41:753–758. doi: 10.5999/aps.2014.41.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato T, Fujimoto N, Fujii N, Tanaka T. Mapping biopsy with punch biopsies to determine surgical margin in extramammary Paget's disease. J Dermatol. 2013;40:968–972. doi: 10.1111/1346-8138.12347. [DOI] [PubMed] [Google Scholar]
- 15.Rajendran S, Koh CE, Solomon MJ. Extramammary Paget's disease of the perianal region: a 20-year experience. ANZ J Surg. 2014 doi: 10.1111/ans.12814. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]