Abstract
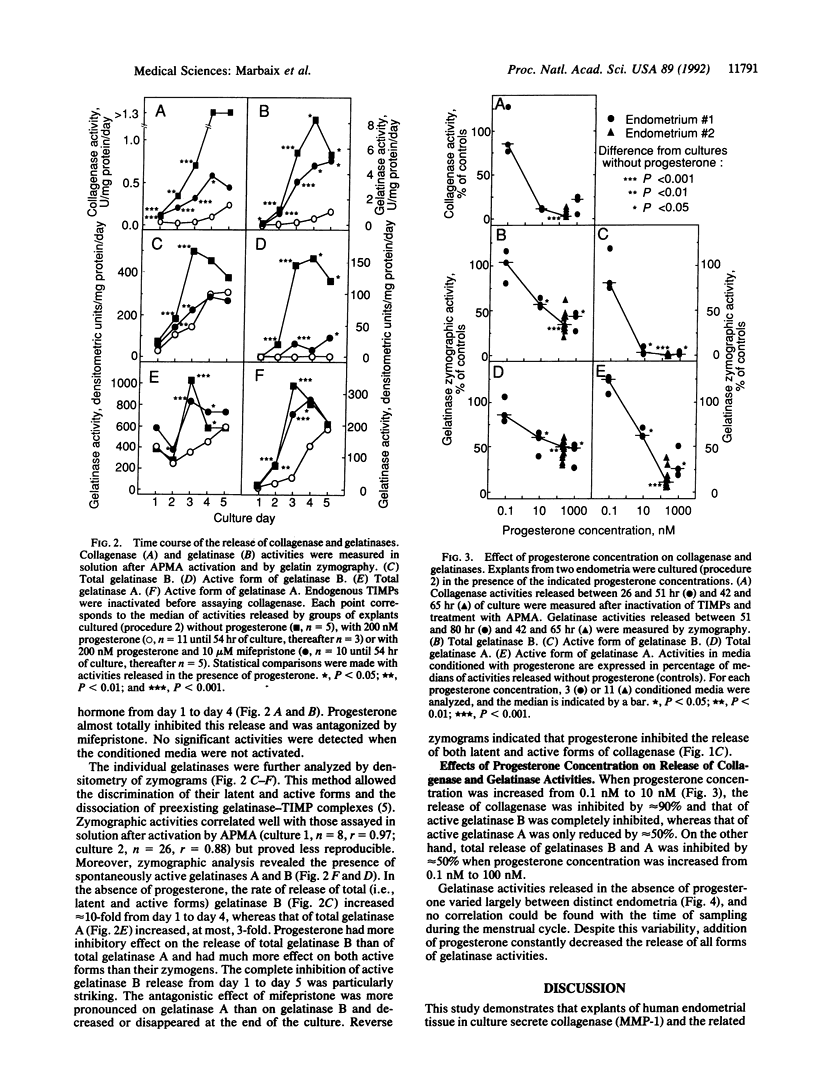
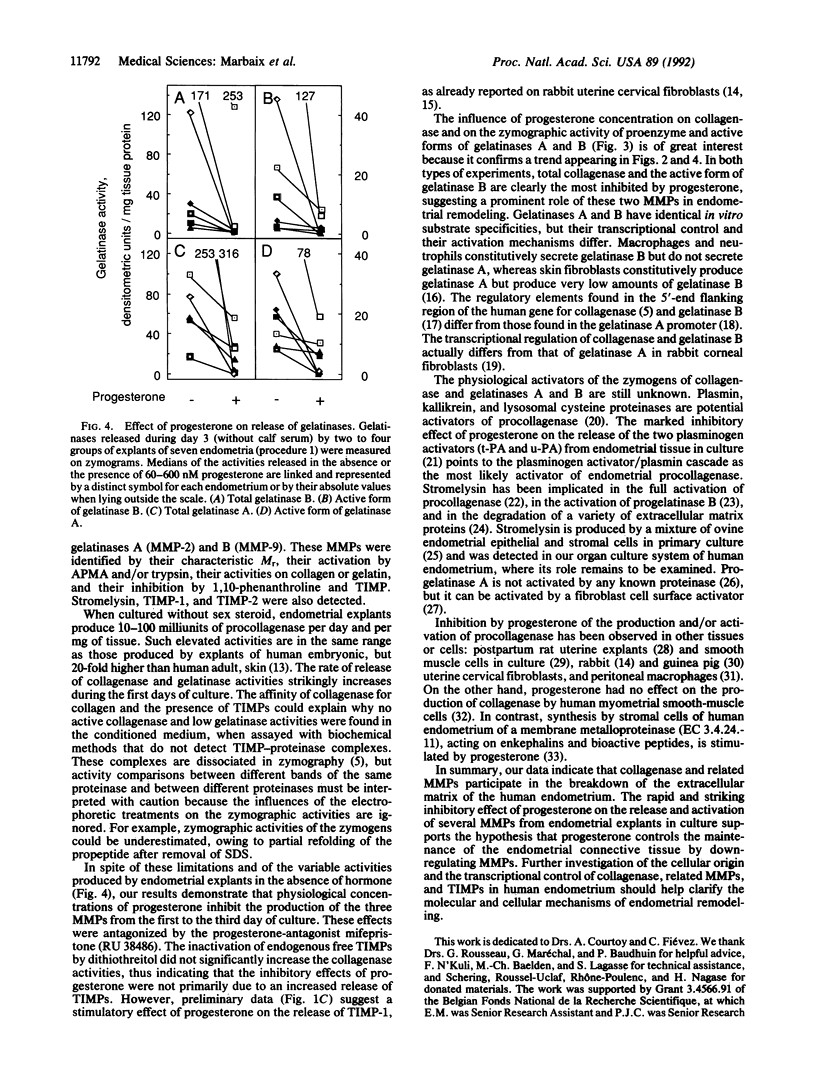
Explants of human endometrium were cultured to study the release of matrix metalloproteinases (MMPs). Analysis of conditioned media by zymography revealed latent and active forms of collagenase (MMP-1, EC 3.4.24.7), 72-kDa gelatinase A (MMP-2, EC 3.4.24.24), and 92-kDa gelatinase B (MMP-9, EC 3.4.24.35). These proteinases were identified by their M(r), their inhibition by tissue inhibitor of metalloproteinases, and the activation of their zymogens by trypsin or aminophenylmercuric acetate. In the absence of sex hormone, explants released large amounts of enzyme activities, as measured by densitometry of zymograms or in soluble assays. Physiological concentrations of progesterone (10-200 nM) almost totally abolished the release of collagenase, of total gelatinase activity, and of the active form of gelatinase B and largely inhibited the release of the active form of gelatinase A. These effects, which were antagonized by mifepristone (RU 38486), suggest that progesterone restrains endometrial tissue breakdown by blocking the secretion and activation of MMPs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruning P. F., Jonker K. M., Boerema-Baan A. W. Adsorption of steroid hormones by plastic tubing. J Steroid Biochem. 1981 Jun;14(6):553–555. doi: 10.1016/0022-4731(81)90029-7. [DOI] [PubMed] [Google Scholar]
- Canonico P. G., Beaufay H., Nyssens-Jadin M. Analytical fractionation of mouse peritoneal macrophages: physical and biochemical properties of subcellular organelles from resident (unstimulated) and cultivated cells. J Reticuloendothel Soc. 1978 Aug;24(2):115–138. [PubMed] [Google Scholar]
- Casey M. L., Smith J. W., Nagai K., Hersh L. B., MacDonald P. C. Progesterone-regulated cyclic modulation of membrane metalloendopeptidase (enkephalinase) in human endometrium. J Biol Chem. 1991 Dec 5;266(34):23041–23047. [PubMed] [Google Scholar]
- Casslén B., Andersson A., Nilsson I. M., Astedt B. Hormonal regulation of the release of plasminogen activators and of a specific activator inhibitor from endometrial tissue in culture. Proc Soc Exp Biol Med. 1986 Sep;182(4):419–424. doi: 10.3181/00379727-182-42360. [DOI] [PubMed] [Google Scholar]
- Cornillie F., Brosens I., Belsey E. M., Marbaix E., Baudhuin P., Courtoy P. J. Lysosomal enzymes in the human endometrium: a biochemical study in untreated and levonorgestrel-treated women. Contraception. 1991 Apr;43(4):387–400. doi: 10.1016/0010-7824(91)90076-r. [DOI] [PubMed] [Google Scholar]
- Dean D. D., Woessner J. F., Jr Extracts of human articular cartilage contain an inhibitor of tissue metalloproteinases. Biochem J. 1984 Feb 15;218(1):277–280. doi: 10.1042/bj2180277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhout Y., Delaissé J. M., Vaes G. Direct extraction and assay of bone tissue collagenase and its relation to parathyroid-hormone-induced bone resorption. Biochem J. 1986 Nov 1;239(3):793–796. doi: 10.1042/bj2390793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977 Jul 15;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini M. E., Girard M. T. The pattern of metalloproteinase expression by corneal fibroblasts is altered by passage in cell culture. J Cell Sci. 1990 Oct;97(Pt 2):373–383. doi: 10.1242/jcs.97.2.373. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. An endopeptidase from rheumatoid synovial tissue culture. Biochim Biophys Acta. 1972 Feb 28;258(2):566–576. doi: 10.1016/0005-2744(72)90249-5. [DOI] [PubMed] [Google Scholar]
- Henzl M. R., Smith R. E., Boost G., Tyler E. T. Lysosomal concept of menstrual bleeding in humans. J Clin Endocrinol Metab. 1972 May;34(5):860–875. doi: 10.1210/jcem-34-5-860. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Huhtala P., Chow L. T., Tryggvason K. Structure of the human type IV collagenase gene. J Biol Chem. 1990 Jul 5;265(19):11077–11082. [PubMed] [Google Scholar]
- Huhtala P., Tuuttila A., Chow L. T., Lohi J., Keski-Oja J., Tryggvason K. Complete structure of the human gene for 92-kDa type IV collagenase. Divergent regulation of expression for the 92- and 72-kilodalton enzyme genes in HT-1080 cells. J Biol Chem. 1991 Sep 5;266(25):16485–16490. [PubMed] [Google Scholar]
- Jeffrey J. J., Coffey R. J., Eisen A. Z. Studies on uterine collagenase in tissue culture. II. Effect of steroid hormones on enzyme production. Biochim Biophys Acta. 1971 Oct;252(1):143–149. doi: 10.1016/0304-4165(71)90102-4. [DOI] [PubMed] [Google Scholar]
- Jeffrey J. J., Roswit W. T., Ehlich L. S. Regulation of collagenase production by steroids in uterine smooth muscle cells: an enzymatic and immunologic study. J Cell Physiol. 1990 May;143(2):396–403. doi: 10.1002/jcp.1041430226. [DOI] [PubMed] [Google Scholar]
- Moll U. M., Youngleib G. L., Rosinski K. B., Quigley J. P. Tumor promoter-stimulated Mr 92,000 gelatinase secreted by normal and malignant human cells: isolation and characterization of the enzyme from HT1080 tumor cells. Cancer Res. 1990 Oct 1;50(19):6162–6170. [PubMed] [Google Scholar]
- Nagase H., Enghild J. J., Suzuki K., Salvesen G. Stepwise activation mechanisms of the precursor of matrix metalloproteinase 3 (stromelysin) by proteinases and (4-aminophenyl)mercuric acetate. Biochemistry. 1990 Jun 19;29(24):5783–5789. doi: 10.1021/bi00476a020. [DOI] [PubMed] [Google Scholar]
- Ogata Y., Enghild J. J., Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992 Feb 25;267(6):3581–3584. [PubMed] [Google Scholar]
- Okada Y., Morodomi T., Enghild J. J., Suzuki K., Yasui A., Nakanishi I., Salvesen G., Nagase H. Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts. Purification and activation of the precursor and enzymic properties. Eur J Biochem. 1990 Dec 27;194(3):721–730. doi: 10.1111/j.1432-1033.1990.tb19462.x. [DOI] [PubMed] [Google Scholar]
- Rajabi M., Solomon S., Poole A. R. Hormonal regulation of interstitial collagenase in the uterine cervix of the pregnant guinea pig. Endocrinology. 1991 Feb;128(2):863–871. doi: 10.1210/endo-128-2-863. [DOI] [PubMed] [Google Scholar]
- Roswit W. T., Rifas L., Gast M. J., Welgus H. G., Jeffrey J. J. Purification and characterization of human myometrial smooth muscle collagenase. Arch Biochem Biophys. 1988 Apr;262(1):67–75. doi: 10.1016/0003-9861(88)90169-5. [DOI] [PubMed] [Google Scholar]
- Sakyo K., Ito A., Ogawa C., Mori Y. Hormonal control of collagenase inhibitor production in rabbit uterine cervical fibroblast-like cells. Biochim Biophys Acta. 1986 Oct 1;883(3):517–522. doi: 10.1016/0304-4165(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Salamonsen L. A., Nagase H., Woolley D. E. Production of matrix metalloproteinase 3 (stromelysin) by cultured ovine endometrial cells. J Cell Sci. 1991 Oct;100(Pt 2):381–385. doi: 10.1242/jcs.100.2.381. [DOI] [PubMed] [Google Scholar]
- Sato T., Ito A., Mori Y., Yamashita K., Hayakawa T., Nagase H. Hormonal regulation of collagenolysis in uterine cervical fibroblasts. Modulation of synthesis of procollagenase, prostromelysin and tissue inhibitor of metalloproteinases (TIMP) by progesterone and oestradiol-17 beta. Biochem J. 1991 May 1;275(Pt 3):645–650. doi: 10.1042/bj2750645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai H., Nagai Y. A latent collagenase from embryonic human skin explants. J Biochem. 1977 May;81(5):1261–1268. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Enghild J. J., Morodomi T., Salvesen G., Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin). Biochemistry. 1990 Nov 6;29(44):10261–10270. doi: 10.1021/bi00496a016. [DOI] [PubMed] [Google Scholar]
- Wahl L. M. Hormonal regulation of macrophage collagenase activity. Biochem Biophys Res Commun. 1977 Jan 24;74(2):838–845. doi: 10.1016/0006-291x(77)90379-5. [DOI] [PubMed] [Google Scholar]
- Ward R. V., Atkinson S. J., Slocombe P. M., Docherty A. J., Reynolds J. J., Murphy G. Tissue inhibitor of metalloproteinases-2 inhibits the activation of 72 kDa progelatinase by fibroblast membranes. Biochim Biophys Acta. 1991 Aug 30;1079(2):242–246. doi: 10.1016/0167-4838(91)90132-j. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]