Abstract
Linker histones play important roles in the genomic organization of mammalian cells. Of the linker histone variants, H1.X shows the most dynamic behavior in the nucleus. Recent research has suggested that the linker histone variants H1.X and H1.0 have different chromosomal binding site preferences. However, it remains unclear how the dynamics and binding site preferences of linker histones are determined. Here, we biochemically demonstrated that the DNA/nucleosome and histone chaperone binding activities of H1.X are significantly lower than those of other linker histones. This explains why H1.X moves more rapidly than other linker histones in vivo. Domain swapping between H1.0 and H1.X suggests that the globular domain (GD) and C-terminal domain (CTD) of H1.X independently contribute to the dynamic behavior of H1.X. Our results also suggest that the N-terminal domain (NTD), GD, and CTD cooperatively determine the preferential binding sites, and the contribution of each domain for this determination is different depending on the target genes. We also found that linker histones accumulate in the nucleoli when the nucleosome binding activities of the GDs are weak. Our results contribute to understanding the molecular mechanisms of dynamic behaviors, binding site selection, and localization of linker histones.
INTRODUCTION
Histones, including linker histones, are highly basic proteins that randomly bind to DNA. To maintain genomic integrity, DNA binding of histones should be strictly controlled. It has been established that random DNA binding of histones abrogates the sequential nucleosome assembly process (1). Histone chaperones directly bind to histones and transfer them to DNA to assemble the chromatin structure. In addition, histone chaperones have been suggested to play an important role in inhibiting the unfavorable DNA binding of histones (1). Our previous studies identified template activating factor I (TAF-I), nucleosome assembly protein 1 (NAP1), and nucleophosmin/B23 as factors involved in regulating the transcription and replication of adenovirus chromatin (2, 3). Recently, all three proteins, TAF-I, NAP1, and B23, were found to function as linker histone chaperones (4–7). A nucleolar protein, nucleolin (NCL), and prothymosin α (ProTα) were shown to regulate linker histone-chromatin binding (8–10).
Linker histones are comprised of three regions: the N-terminal domain (NTD), central globular domain (GD), and C-terminal domain (CTD). The GDs of about 80 amino acids are able to reside on the DNA entry and exit sites of a nucleosome (11). Recent structural analysis of the GD of chicken linker histone H5 demonstrated that the GD makes contact with two extended DNA strands from the nucleosome and the nucleosome dyad (12). The CTD of linker histones are generally basic and rich in lysines, alanines, and prolines. The CTD plays a critical role in the condensation of DNA through its random DNA binding activity (13, 14). The GD and CTD show distinct DNA binding activity (15), but neither is sufficient to stably associate with nucleosomes (13, 16). Fluorescent recovery after photobleaching (FRAP) assays using various combinations of mutant linker histones have demonstrated that both the GD and CTD cooperatively contribute to the stable binding of linker histones on chromatin (11).
There are seven H1 variants in somatic mammalian cells. The linker histones H1.1, H1.2, H1.3, H1.4, and H1.5 are synthesized during DNA synthesis, as are canonical core histones, whereas H1.0 and H1.X are expressed independently of DNA synthesis (17). Genetic studies have suggested that linker histones are essential for mouse growth and development and that linker histone variants play redundant roles (18–21). Depletion of single linker histone variants also has been reported to change the gene expression profiles in human cells (22), suggesting that each linker histone variant plays a distinct role in the regulation of gene expression in different tissue or cell types. Indeed, biochemical activities and stable chromatin binding in the cells of individual linker histone variants were shown to be different from each other (23–25). In particular, it was recently reported that the mobility of H1.X in the nucleus was much higher than that of other linker histone variants (26). In addition, the binding preferences of H1.0 and H1.X to the 5S rRNA genes, telomeric satellites, and actively transcribed genes were reported to be different (27). Although distinct stability and preferences of chromatin binding of individual linker histones should contribute to the organization of high-order chromatin structures and to the regulation of gene expression, the regulatory mechanism by which the chromatin binding stability and the binding site preferences of linker histones are determined is currently not understood.
In this study, we addressed the questions of why the cellular mobility of H1.X is higher than that of other linker histones and how the chromosome binding site preference of linker histones is determined. To simplify the interpretation of the results derived from in vitro experiments, we used an experimental system that used nonmodified forms of recombinant proteins to evaluate the intrinsic DNA and nucleosome binding activity of individual linker histone variants in vitro. We demonstrate that the DNA, nucleosome, and histone chaperone binding activities of H1.X are lower than those of H1.1 and H1.0 in vitro. This difference is, at least in part, because of the low DNA and histone chaperone binding ability of the H1.X CTD. Furthermore, using chimeric proteins in which the NTDs, CTDs, or GDs of H1.X and H1.0 are exchanged, we demonstrate that all three domains, NTD, GD, and CTD, contribute to stable chromatin binding. We also found that H1.0 and H1.X show distinct chromatin binding site preferences. Both the GD and CTD are suggested to be equally required for the preferential binding site selection of linker histones. Furthermore, the amino acid residues glutamic acid 73 and lysine 52 in the GDs of H1.X and H1.0, respectively, were identified to be the sites playing critical roles in the dynamic behavior, binding site determination, and nucleolar localization. Our results contribute to the understanding of the mechanisms underlying stable chromatin binding, chromatin binding site selection, and cellular localization of linker histones in vivo.
MATERIALS AND METHODS
Plasmid DNA.
To construct pEGFPC1-H1.0, pEGFPC1-H1.1, and pEGFPC1-H1.X, cDNAs were amplified from the genomic DNA extracted from HeLa cells by PCR with primer sets H1.0-F and H1.1-R, H1.1-1 and H1.1-2, and H1.X-1 and H1.1-R, respectively. Amplified DNAs were digested with BamH1 and ligated into a BamHI-digested pEGFPC1 vector (Clontech). Plasmids pET-14b-B23.1, pET14b-TAF-Iβ, pGEX2T-B23.1, pGEX6P1-TAF-Iβ, pcDNA3-H1.1-Flag, and pcDNA3-H1.X-Flag were described previously (3, 4, 28). Four human core histone cDNAs cloned in the pET22b vector were transferred to pET14b vector (Novagen) with appropriate restriction enzymes and generated pET14b-H2A, pET14b-H2B, pET14b-H3, and pET14b-H4. To construct pEGFPC1-H1.0-cX, pEGFPC1-H1.X-c0, pEGFPC1-H1.0-nX, and pEGFPC1-H1.X-n0, primer sets H1.0-cX-1 and H1.0-cX-2, H1.X-c0-1 and H1.X-c0-2, H1.0-nX-1 and H1.0-nX-2, and H1.X-n0-1 and H1.X-n0-2, respectively, were used, and pEGFPC1-H1.0 and pEGFPC1-H1.X were used as templates. To construct pEGFPC1-H1.0-gX and pEGFPC1-H1.X-g0, primer sets H1.0-gX-1 and H1.0-gX-2 as well as H1.X-g0-1 and H1.X-g0-2, respectively, were used, and pEGFPC1-H1.X-c0 and pEGFPC1-H1.0-cX were used as templates. The chimeric genes were generated by two-step PCR using primer sets described above and a primer set for the pEGFPC1 vector. The amplified DNA fragments were digested by BamHI and cloned into the same site of pEGFPC1. To construct pEGFPC1-H1.0-K52E and pEGFPC1-H1.X-E73K, primer sets H1.0-K52E-F and H1.0-K52E-R as well as H1.X-E73K-F and H1.X-E73K-R, respectively, were used. To construct pGEX6P1-H1.0-CTD, -H1.0-CTDa, -H1.0-CTDb, -H1.0-CTDc, -H1.0-CTDab, and -H1.X-CTD, cDNAs were amplified by PCR using H1.0-3 and vector primer, H1.0-3 and H1.0-4, H1.0-5 and H1.0-6, H1.0-7 and vector primer, H1.0-3 and H1.0-6, and H1.X-3 and vector primer, respectively, and appropriate pGEX6P-1 vectors as templates. Amplified cDNAs were cloned into BamHI-digested or BamHI- and EcoRI-digested pGEX6P1 vector (GE Healthcare). Primer sequences described above are shown in Table S1 in the supplemental material.
Cell culture and transfection of plasmids.
HeLa and 293T cells were maintained in Dulbecco's modified Eagle's medium (Nacalai Tesque) supplemented with 10% fetal bovine serum and 1× penicillin-streptomycin solution (Nacalai Tesque). Cell lines used were routinely tested for contamination. Transient transfection of plasmid DNA was performed using Gene Juice (Novagen) according to the manufacturer's instructions.
Purification of recombinant protein.
To express and purify the recombinant proteins, BL21 Codon Plus (DE3)-RP (Novagen) was transformed with pET14b-H1.1, pET14b-H1.0, pET14b-H1.X, pET14b-B23.1, pET14b-TAF-Iβ, pGEX6P1-H1.0-CTD, pGEX6P1-H1.X-CTD, pGEX2T-B23.1, and pGEX2T-TAF-Iβ. The transformed Escherichia coli was grown at 37°C until the optical density at 600 nm (OD600) reached 0.4. Expression of the recombinant proteins was induced by addition of 0.1 mM isopropyl β-d-thiogalactopyranoside at 30°C for 3 h. Bacterial cell pellets were sonicated in His-binding buffer (50 mM Na2HPO4, 50 mM NaH2PO4, 10 mM imidazole, 300 mM NaCl) for His-tagged proteins. His-tagged proteins were purified with HIS-Select nickel affinity gel according to the method suggested by the manufacturer (Sigma-Aldrich). Purified proteins were dialyzed against buffer H (20 mM HEPES-NaOH [pH 8.0], 50 mM NaCl, 0.1 mM EDTA, 10% [vol/vol] glycerol, 1 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride [PMSF]) or H1 buffer (20 mM Tris-HCl [pH 7.9], 200 mM NaCl, 0.1 mM PMSF) for 6 h at 4°C. Dialyzed H1 proteins were fractionated by a Mono S column (GE Healthcare) and purified with salt gradient from 0.2 M to 1 M NaCl. Glutathione S-transferase (GST)-tagged proteins were purified with glutathione-Sepharose (GE Healthcare) and further purified by a denature-renature protocol as described previously (29). Flag-NCL was expressed in 293T cells using pcDNA3.1-Flag NCL. The Flag-NCL was immunoprecipitated using anti-Flag M2 beads (Sigma), treated with micrococcal nuclease to remove RNAs, and eluted with Flag peptide (Sigma).
For expression and purification of recombinant core histones, E. coli transformed with pET14b-H2A, pET14b-H2B, pET14b-H3, or pET14b-H4 was grown at 37°C until the OD600 reached 0.4 and the protein expression was induced as described above. The cells were disrupted with sonication, and after centrifugation the pellet was resuspended in His-binding buffer containing 8 M urea. The pellets in the buffer were sonicated and centrifuged at 4°C. The supernatant was recovered and His-histone proteins were purified as described above in the presence of 8 M urea. The His-tagged histone proteins were further purified by a HiTrap SP XL column (GE Healthcare) with linear salt gradient from 0.2 M to 1 M NaCl. The peak fractions were collected and the same amounts of His-H2A, His-H2B, His-H3, and His-H4 were mixed and dialyzed in buffer (20 mM Tris [pH 7.9], 2 mM EDTA, 5% glycerol, 1 mM DTT, and 0.5 mM PMSF) containing 2 M NaCl for 4 h. The NaCl concentration was sequentially diluted to 1 M, 0.5 M, and 0.1 M, and dialysis was performed for 3 h at each step. The dialyzed His-H2A-His-H2B and His-H3-His-H4 complexes were treated with thrombin to remove the His tag at 25°C for 12 h. The samples were denatured by 6 M urea, loaded on a HiTrap SP XL column, and eluted with the buffer containing 1 M NaCl. The eluted samples were dialyzed and refolded as described above.
Immunoprecipitation.
Nuclear protein-rich extracts from control HeLa cells or HeLa cell lines expressing Flag-tagged H1.0, H1.1, and H1.X were prepared as described previously (4). The extracts were dialyzed in dialysis buffer (50 mM Tris [pH 7.9], 100 mM NaCl, 0.5 mM PMSF, and 10% [vol/vol] glycerol, supplemented with 0.1% Triton X-100) and mixed with Flag M2 affinity gel (Sigma-Aldrich). Precipitated proteins were eluted with the same buffer containing 0.1 mg/ml of Flag peptide (Sigma-Aldrich) and analyzed by SDS-PAGE followed by Western blotting.
Antibodies.
The following antibodies were used in this study: anti-TAF-I (KM1725; 1:100) (30), anti-B23 (32-5200; 1:1,000; Thermo Fisher Scientific), antinucleolin (D6; SC-17826; 1:1,000; Santa Cruz Biotechnology), anti-green fluorescent protein (anti-GFP) (GF200; 04363-24; 1:1,000; Nacalai Tesque), and anti-H1.X (ab116576; 1:1,000; Abcam) for Western blotting and anti-GFP (1A5; BAM-60-001; Cosmo Bio) and anti-H2A-H2B antibodies (31) for chromatin immunoprecipitation (ChIP) assay. The indicated dilution was used for Western blotting.
Reconstitution and purification of NCPs.
Nucleosome core particles (NCPs) were assembled with the 196-bp 5S rRNA gene fragment and core histones by the salt dilution method (28). Reconstituted NCPs (200 μl) incubated at 42°C for 1 h were loaded on 15% to 35% glycerol gradient in 10 mM Tris-HCl (pH 7.9), 1 mM DTT (2.2 ml). The samples were centrifuged at 54,000 rpm for 8 h at 4°C in an S55S rotor (SC100GXII; Hitachi Koki), and fractions (100 μl) were collected from the top.
FRAP assay.
HeLa cells were transiently transfected with enhanced GFP (EGFP)-tagged linker histone H1 proteins for FRAP analyses and grown on 35-mm glass-base dishes (AGC Techno Glass). The dish was set on an inverted microscope (LSM Exciter; Carl Zeiss Microimaging) in an air chamber containing 5% CO2 at 37°C and analyzed as previously described (29). The data were represented as mean values ± standard deviations (SD) from at least 10 experiments. To estimate the amounts of three distinct pools and the exchange rate constants for fast and slow mobile fractions (K1 and K2, respectively) of the EGFP-H1 proteins, the double exponential equation previously described (4) was used, and curve fitting was performed using the FRAP analysis module of the ZEN software (Carl Zeiss Microimaging).
DNA/NCP and histone chaperone binding assays.
DNA or purified NCP (10 nM DNA) was mixed and incubated with increasing amounts of His-tagged H1 proteins in buffer (15 mM Tris-HCl, pH 7.9, 150 mM NaCl, 12% glycerol, and 200 μg/ml bovine serum albumin [BSA]) at 30°C for 30 min. Samples were separated on 6% native-PAGE gel in 0.5× Tris-borate-EDTA (TBE) at 18.75 V/cm for 80 min. DNA was visualized by GelRed (Biotium) staining. For the experiments shown in Fig. 4, His-tagged TAF-I or B23 mixed and incubated with His-tagged H1 was incubated with DNA or NCP for 30 min at 30°C. For histone chaperone binding assays, His-tagged histone chaperone proteins were mixed with increasing amounts of His-tagged H1 and incubated for 30 min at room temperature in a buffer containing 15 mM Tris-HCl, pH 7.9, 150 mM NaCl, and 12% glycerol. Samples were separated on native-PAGE gel in 0.5× TBE at 18.75 V/cm for 90 min. Proteins were visualized by Coomassie brilliant blue (CBB) staining.
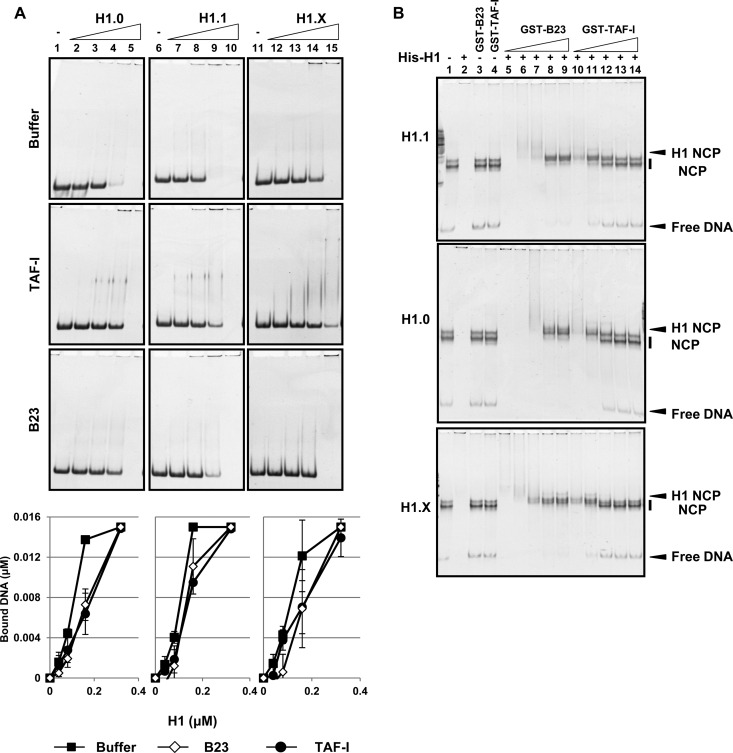
FIG 4.
Effects of histone chaperones on the DNA/nucleosome binding activity of linker histones. (A) DNA binding activity. Linker histones His-H1.0, His-H1.1, and His-H1.X (lanes 1 to 5, 6 to 10, and 11 to 15, respectively) (0, 0.04, 0.08, 0.16, and 0.32 μM) were preincubated with or without His-TAF-I or His-B23 (0.4 μM), the 196-bp DNA fragments (0.01 μM) were added, and the mixture was further incubated. The mixtures were loaded on 6% native PAGE gel, and DNA was visualized by GelRed staining. The amounts of free DNA in each fraction relative to those of the input were calculated and are shown in graphs at the bottom of each panel. Three independent experiments were performed, and error bars indicate ±SD. (B) Nucleosome binding activity. NCPs (0.01 μM DNA) were mixed without (lane 1) or with (lanes 2 to 4) His-tagged H1, GST-B23, or GST-TAF-I (0.06 μM for H1s and 0.045 μM for GST proteins). His-tagged H1 variants (0.06 μM) were preincubated with GST-B23 (lanes 5 to 9) or GST-TAF-I (lanes 10 to 14) (0.03, 0.06, 0.12, 0.24, 0.3, and 0.45 μM GST proteins), and then NCP (0.01 μM DNA) was added and further incubated. His-H1.1, His-H1.0, and His-H1.X were used for top, middle, and bottom panels, respectively. The mixtures were loaded on 6% native PAGE gel, and DNA was visualized by GelRed staining. Positions of free DNA, NCP, and H1-bound NCP are indicated at the right of the gels.
Chromatin immunoprecipitation assay.
HEK293T cells (1 × 106 cells) were transiently transfected with 1 μg of plasmids (pEGFPC1-H1 variants) and were fixed with 1% formaldehyde for 10 min 24 h after transfection. ChIP assays were performed according to the manual of the ChIP assay kit (Millipore). The protein extracts were prepared and subjected to immunoprecipitation with control Ig or anti-GFP antibody, and the precipitated DNA was used for quantitative PCR with the primer sets for the human rRNA gene, 5S rRNA gene, RNU2 gene, and CES1 gene. The regions A to D of the rRNA gene correspond to positions 42960 to 42999 and 1 to11, 6626 to 6676, 18370 to 18420, and 30857 to 30907, respectively, relative to the rRNA transcription start site (+1). Primer sequences used were previously described (32). Primer sequences for the 5S rRNA gene, RNU2 gene, and CES1 gene are shown in Table S2 in the supplemental material.
RESULTS
Dynamics of linker histones in the cell.
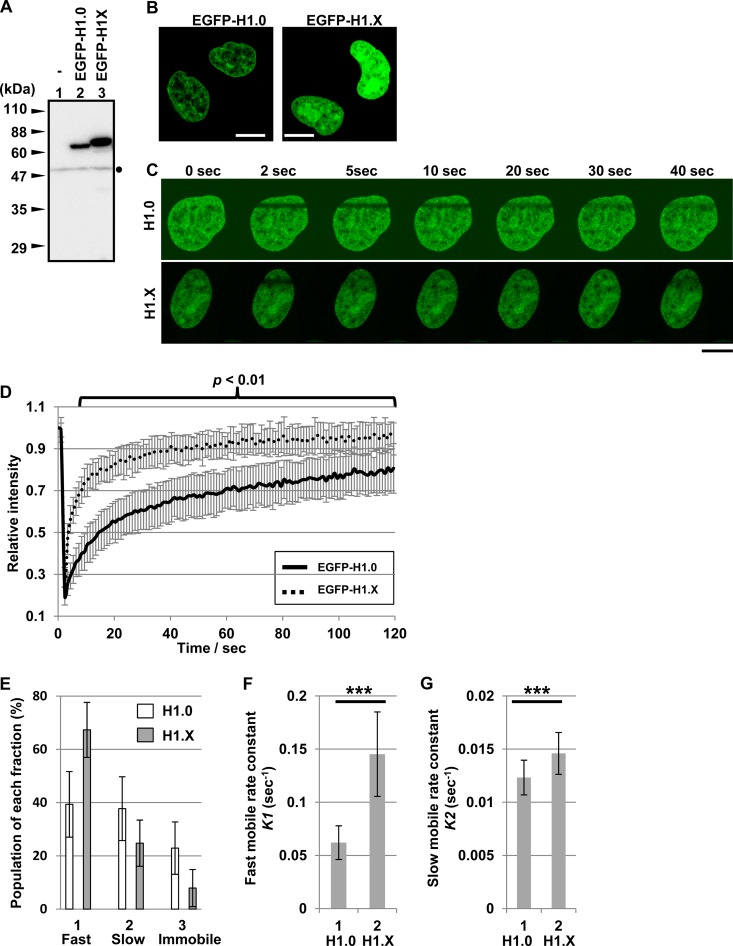
To examine the dynamic behavior of linker histone H1 variants in HeLa cells, FRAP assays were performed using N-terminally EGFP-tagged H1.0 and H1.X. The integrities of EGFP-tagged proteins transiently expressed in HeLa cells were confirmed by Western blotting (Fig. 1A). EGFP-tagged H1 proteins were localized to the nucleus, and H1.X was also detected in the nucleoli of some cells (Fig. 1B). H1.X was previously reported to be localized to the nucleoli during the G1 phase (33), although we did not examine the cell cycle stages of individual cells expressing the EGFP-H1 proteins. Consistent with a previous report (26), FRAP assay demonstrated that the recovery rate of H1.X was significantly higher than that of H1.0 (Fig. 1C and D). Based on FRAP recovery curves, it has been proposed that linker histones in the nuclei are classified into at least three distinct populations, low chromatin-bound (fast mobile), high chromatin-bound (slow mobile), and stable chromatin-bound (immobile) pools (17). To examine these distinct pools of H1.0 and H1.X, the recovery curves were analyzed by a curve-fitting method using a double exponential equation as described previously (4) (Fig. 1E). The fast mobile pool of H1.X was estimated to be about 70% and the immobile pool was less than 10%. In contrast, the fast mobile pool of H1.0 was much smaller than that of H1.X, whereas the immobile pool of H1.0 was significantly larger than that of H1.X. In addition, the exchange rate constants (K1 and K2) of the EGFP proteins for the fast and slow mobile fractions indicated that H1.X in both fractions exchanged more rapidly than H1.0 (Fig. 1F and G). K1 of H1.0 and H1.X were 0.0621 ± 0.0159 s−1 and 0.145 ± 0.0398 s−1, respectively, whereas K2 of H1.0 and H1.X were 0.0123 ± 0.0016 s−1 and 0.0146 ± 0.0020 s−1, respectively.
FIG 1.
FRAP analyses of linker histones. (A) Western blotting of EGFP-tagged linker histones. Cell extracts were prepared from HeLa cells transfected without (lane 1) or with vectors for the expression of EGFP-H1.0 and EGFP-H1.X (lanes 2 and 3) and then were subjected to Western blotting with anti-GFP antibody. Positions of molecular size markers are shown at the left of the panel. A dot at the right side of the panel indicates a nonspecific band. (B) Localization of EGFP-tagged linker histones. HeLa cells grown on glass-base dishes were transiently transfected with vectors for the expression of EGFP-H1.0 and -H1.X as described for panel A, and localization of EGFP proteins in living cells was examined by confocal microscopy. Bars at the bottom of each panel indicate 10 μm. (C and D) FRAP analyses of linker histones. HeLa cells prepared as described for panel B were subjected to FRAP assays as described in Materials and Methods. Cells showing similar EGFP intensity were picked up (10 cells of each sample) and examined. Rectangular regions in the nuclei as shown in panel C were bleached with a 488-nm laser line, and the EGFP intensity before and after photobleach was measured. The recovery curves for EGFP-H1.0 and -H1.X were plotted as a function of time. Error bars of recovery curves indicate ±SD. Typical images for EGFP-H1.0 and -H1.X before and after photobleach are shown in panel C. Statistical analysis was performed by two-tailed Student's t test, and the P values for the data 5 s after photobleach are less than 0.01. (E) The fast mobile, slow mobile, and immobile pools were estimated by curve fitting using the ZEN software. (F and G) The exchange rate constants of slow (F) and fast (G) mobile pools were estimated by the ZEN software. Error bars indicate ±SD. Statistical analyses were performed with two-tailed Student's t test. ***, P < 0.01.
DNA and nucleosome binding of linker histones in vitro.
For chromatin binding proteins, including linker histones, mobility in living cells reflects their intrinsic chromatin/DNA binding (34). Therefore, we next examined the DNA/nucleosome binding activity of linker histones using purified recombinant proteins. Nucleosome core particle (NCP) reconstituted with four recombinant core histones and a 196-bp long DNA fragment was purified using a glycerol gradient, and the peak NCP fractions were used for binding assays (Fig. 2A and B). The reconstituted NCPs were detected as two bands on native PAGE gel because of the different positioning of NCP along the 196-bp DNA fragment (28). Fixed amounts of DNA and NCPs (Fig. 2C, top and bottom) were mixed with increasing amounts of H1.0, H1.1, and H1.X, and the complexes were resolved using native PAGE. All H1 variants tested, H1.0, H1.1, and H1.X, bound to both naked DNA and NCP in a dose-dependent manner, and their affinities toward NCP were higher than those toward naked DNA (Fig. 2C, right). In addition, both the DNA- and NCP-binding activities of H1.X were lower than those of H1.1 and H1.0, suggesting that the high mobility of H1.X in living cells is at least in part because of its low affinity for DNA and NCP.
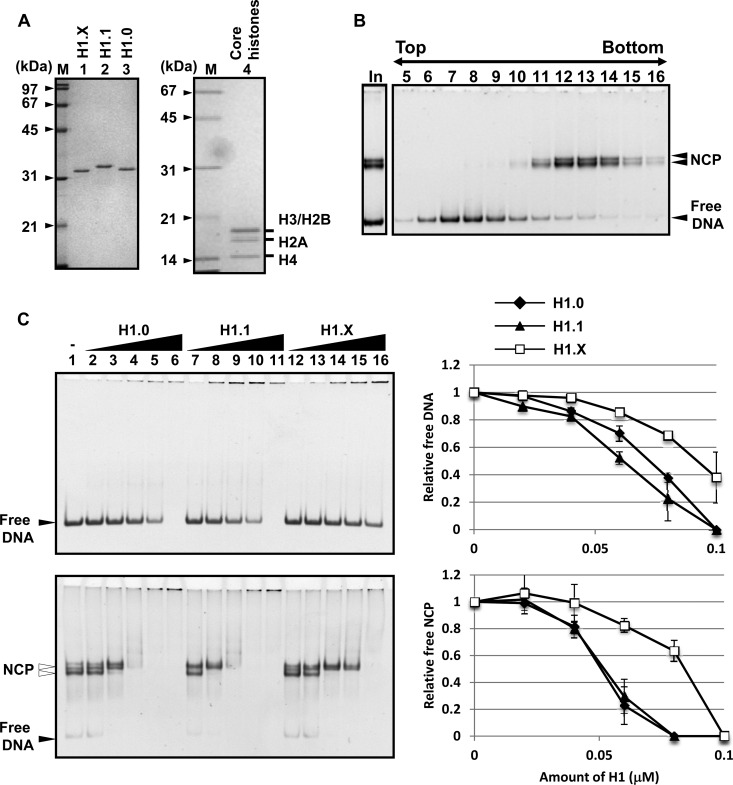
FIG 2.
DNA and nucleosome binding activity of linker histones. (A) Purified recombinant proteins. Recombinant His-H1.X, His-H1.1, and His-H1.0 (lanes 1 to 3) and recombinant histone octamers (lane 4) were separated by 12.5% SDS-PAGE and visualized with Coomassie brilliant blue (CBB) staining. Lanes M indicate molecular size markers. (B) Purification of nucleosome core particles (NCP). Reconstituted NCP assembled on the 196-bp 5S rRNA gene fragment with recombinant core histone octamers was purified by a 15% to 35% glycerol gradient. Fractions 5 to 16 (5 μl) were analyzed by 6% native PAGE. DNA was visualized by GelRed staining. Fractions 12 to 14 were pooled and used for the assays described for panel C. Positions of free DNA and NCPs are indicated. Lane In indicates the sample before glycerol gradient fractionation. (C) DNA and NCP binding activity of H1 variants. Naked DNA (196-bp 5S rRNA gene fragment) (upper) or NCP assembled on the same DNA fragment (lower) (0.01 μM each) were mixed and incubated without or with 0.02, 0.04, 0.06, 0.08, and 0.1 μM H1.0 (lanes 2 to 6), H1.1 (lanes 7 to 11), and H1.X (lanes 12 to 16), followed by native PAGE analysis. The intensities of free DNA (upper) and NCP (lower) were measured, and the amounts of free DNA and NCP are graphically shown at the right. Two independent experiments were performed, and error bars indicate ±SD.
Histone chaperone binding activity of H1.X is lower than that of other linker histone variants.
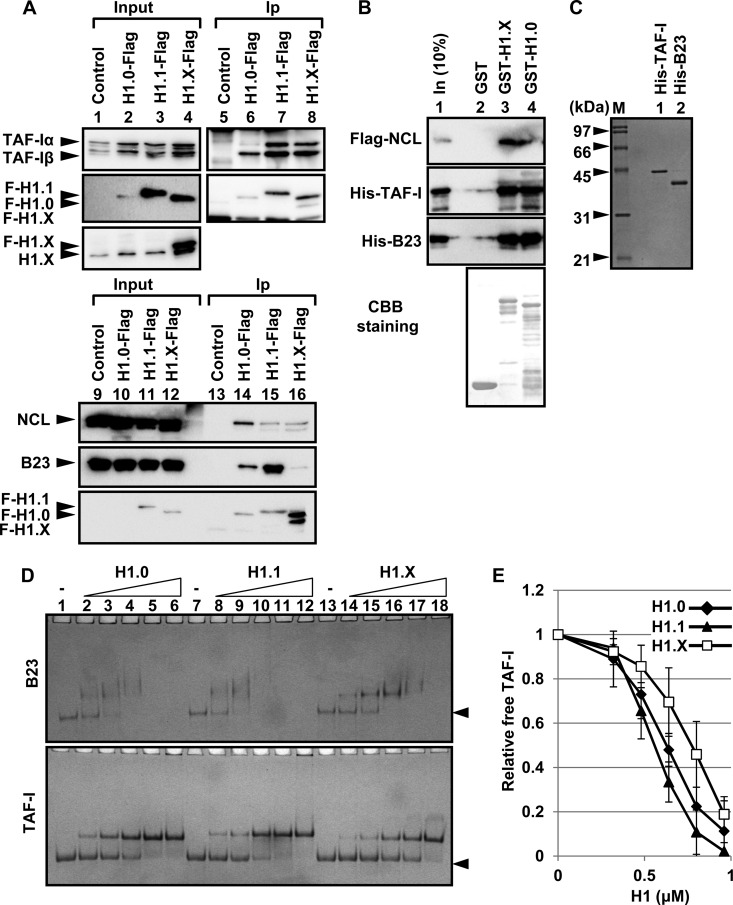
It was previously demonstrated that the mobility of H1 was regulated by histone chaperones (4, 10, 35). Therefore, we tested whether H1.X, like other H1 variants, associates with previously known linker histone chaperones. For this purpose, we used HeLa cells stably expressing Flag-tagged H1.0, H1.1, and H1.X and prepared nuclear extracts for immunoprecipitation assay (Fig. 3A). The expression level of H1.0-Flag was lower than those of H1.1-Flag and H1.X-Flag (lanes 2 and 10), and the expression level of Flag-tagged H1.X was similar to that of endogenous H1.X (lane 4, bottom). Flag-tagged linker histones were successfully purified from the extracts (Fig. 3A, lanes 5 to 8 and 13 to 16). The histone chaperones nucleophosmin/B23, nucleolin (NCL), and TAF-I were copurified with Flag-tagged H1.0, H1.1, and H1.X from the extracts. These results suggested that the histone chaperones regulate the dynamic behavior of linker histones in the nucleus. The interactions of NCL and B23 with Flag-H1.1 was not affected by DNase I treatment (see Fig. S1 in the supplemental material), suggesting that the interaction was not mediated by fragmented chromatin.
FIG 3.
Histone chaperone binding of linker histones. (A) Interaction of linker histones with linker histone chaperones in the extracts. Nuclear extracts were prepared from control HeLa cells and HeLa cells expressing H1.0-Flag, H1.1-Flag, or H1.X-Flag, and immunoprecipitation analyses were performed with anti-Flag M2 beads. Input (lanes 1 to 4 and 9 to 12) and immunoprecipitated (lanes 5 to 8 and 13 to 16) proteins were separated by SDS-PAGE and subjected to Western blotting with anti-TAF-I, -NCL, -B23, -Flag, and -H1.X antibodies. (B) GST pulldown assay. GST, GST-H1.X, or GST-H1.X (1 μg each) was expressed and immobilized on glutathione-Sepharose beads and mixed with 1 μg of Flag-NCL, His-TAF-I, or His-B23. After extensive washing, the proteins on the beads were analyzed by SDS-PAGE and Western blotting with anti-NCL, -TAF-I, and -B23 antibodies. (Bottom) The membrane was stained with CBB. (C) Purified proteins. His-tagged TAF-I and B23 (200 ng each) were separated by 10% SDS-PAGE and visualized by CBB staining. Lane M indicates molecular size markers. (D and E) Native PAGE analyses of linker histone-histone chaperone complex formation. B23 and TAF-I (1.6 μM each) were incubated with increasing amounts (0, 0.32, 0.48, 0.64, 0.8, and 0.96 μM) of His-H1.0, His-H1.1, and His-H1.X at room temperature for 30 min. The mixture was separated by native PAGE and visualized by CBB staining. Positions of free chaperones are shown by arrowheads. The relative amounts of free TAF-I are plotted in panel E. Three independent experiments were performed, and error bars indicate ±SD.
To determine if NCL, B23, and TAF-I directly associated with linker histones, GST pulldown assays were performed using GST-tagged linker histones (Fig. 3B). NCL, TAF-I, and B23 were efficiently pulled down with GST-H1.X and GST-H1.0 but not with GST alone, indicating that the chaperones directly associate with both H1.X and H1.0. The interaction between the chaperones and linker histones next were quantitatively analyzed (Fig. 3C to E). Because both B23 and TAF-I are highly acidic proteins, they move to the cathode on native PAGE gel. Consistent with the GST pulldown assay, free B23 and TAF-I (indicated by arrowheads) decreased upon addition of increasing amounts of linker histones. The amount of free TAF-I and B23 was quantitatively analyzed (Fig. 3E; see also Fig. S2 in the supplemental material). The result demonstrated that the affinity of TAF-I and B23 to H1.X was clearly lower than that to H1.0 and H1.1. We could not purify NCL at high concentration and did not test the interaction between NCL and linker histones quantitatively.
The histone chaperones inhibit random DNA binding of histones and mediate proper histone-DNA complex formation. We next examined whether NCL, TAF-I, and B23 function as chaperones for linker histones. Although NCL was identified as a binding protein for linker histones (Fig. 3A and B), we observed that NCL bound to DNA and NCPs, and we could not evaluate the histone chaperone function of NCL in our assay system (see Fig. S3 in the supplemental material). Consistent with the data shown in Fig. 2, linker histones bound randomly to DNA, and the activity of H1.X was lower than those of H1.0 and H1.1 (Fig. 4A, bottom graphs). However, the DNA binding of linker histones was inhibited by the addition of both B23 and TAF-I. We next examined the effect of B23 and TAF-I on the H1-nucleosome complex formation. Under the assay conditions in which the concentration of linker histones was six times higher than that of NCP, NCPs mixed with linker histones formed large aggregates and could not enter native PAGE gel (Fig. 4B, lane 2). However, the NCP-H1 complex appeared upon addition of increasing amounts of TAF-I and B23 (lanes 5 to 9 and 10 to 14). This result indicated that both B23 and TAF-I were able to mediate deposition of linker histones, including H1.X, on NCPs. The deposition of all linker histones on NCPs was impaired, and free NCPs were clearly detected upon addition of excess TAF-I. From these results, we concluded that the linker histone chaperones TAF-I and B23 inhibited the random DNA binding of linker histones, including H1.X, and mediated proper H1-NCP complex formation. In addition, the biochemical activities of B23 and TAF-I as linker histone chaperones were different from each other.
Functions of the NTD, GD, and CTD in the mobility of linker histones.
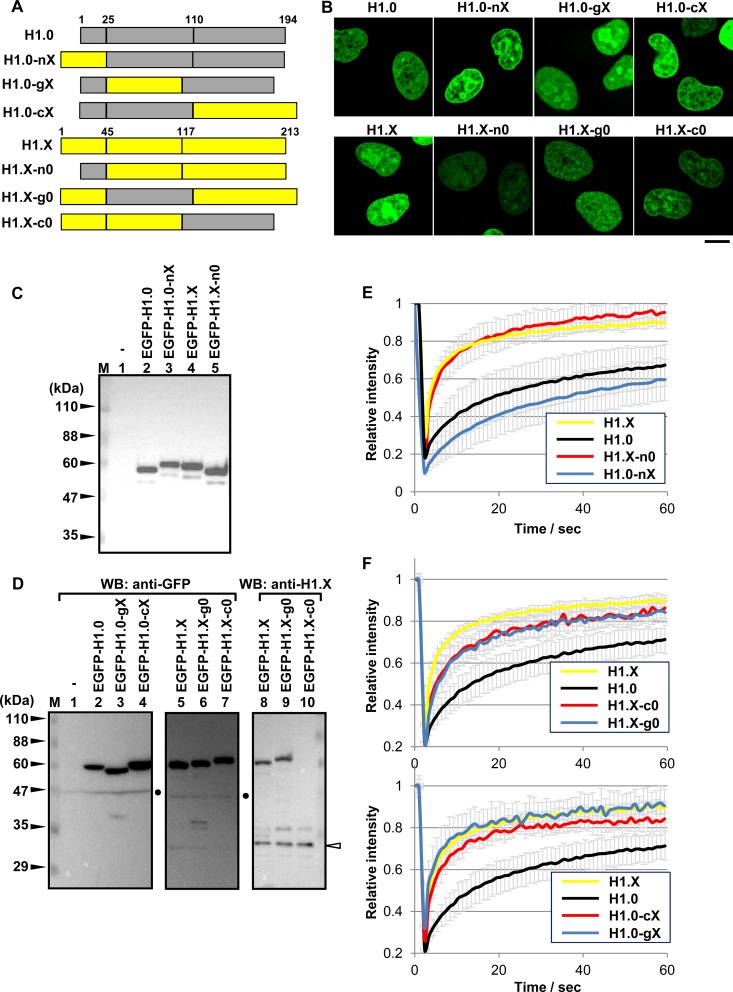
Previous studies have demonstrated that the dynamics of linker histones are cooperatively regulated by both the GD and CTD (11), and the deletion of the NTD also decreased the stable chromatin binding of linker histones (36). To determine the individual contributions of the NTD, GD, and CTD to the dynamic behavior of H1.X, we performed domain swapping analyses. We constructed chimeric H1 proteins that comprised H1.0 containing the NTD, GD, and CTD of H1.X (H1.0-nX, H1.0-gX, and H1.0-cX, respectively) and H1.X containing NTD, GD, and CTD of H1.0 (H1.X-n0, H1.X-g0, and H1.X-c0, respectively) (Fig. 5A). EGFP-tagged versions of these chimeric proteins were expressed in HeLa cells to analyze the mobility using FRAP assay. All of the chimeric proteins were localized in the nuclei as wild-type proteins (Fig. 5B). The integrities of the proteins were confirmed by Western blotting with anti-GFP antibody (Fig. 5C and D). The expression level of the exogenous proteins relative to the endogenous protein was also examined by anti-H1.X antibody that recognizes the CTD of H1.X (Fig. 5D, lanes 8 to 10). We demonstrated that the mobility of H1.X was slightly increased by replacement of its NTD with that of H1.0, whereas the mobility of H1.0 was decreased by replacement of its NTD with that of H1.X (Fig. 5E). FRAP assays clearly demonstrated that the mobility of H1.X slightly but significantly decreased by the replacement of either its GD or CTD with that of H1.0 (Fig. 5F, top). In contrast, the mobility of H1.0 markedly increased upon the replacement of either its GD or CTD with that of H1.X (Fig. 5F, bottom). Thus, we concluded that both GD and CTD of H1.X similarly but independently contribute to the dynamic behavior of H1.X in the cell nucleus. These results also imply that the cooperative functions of both GD and CTD of H1.0 are required for the stable chromatin binding of H1.0 in vivo, because either GD or CTD alone was not sufficient for stable binding to chromatin. To further confirm the effect of NTD, the FRAP results for H1.0-gX and H1.X-c0 and those for H1.0-cX and H1.X-g0 were shown in one graph (see Fig. S5 in the supplemental material). H1.0-cX and H1.X-g0 as well as H1.0-cX and H1.X-g0 have the same GDs and CTDs. These results suggest that the NTD of H1.0 increases and that of H1.X decreases the mobility of linker histones.
FIG 5.
FRAP assays for the chimeric H1.0 and H1.X mutant proteins. (A) Schematic representation of the mutant chimeric proteins. H1.0 and H1.X are represented by gray and yellow, respectively. Amino acid numbers of the border between the NTD, GD, and CTD are indicated at the top of each protein. (B) Localization of the chimeric H1 proteins. HeLa cells transiently expressing the EGFP-H1 mutant proteins as indicated at the top of each panel were observed under a confocal microscope. The scale bar indicates 10 μm. (C and D) Detection of EGFP-H1 proteins by Western blotting (WB). EGFP-H1.0, EGFP-H1.X, or chimeric proteins were transiently expressed in HeLa cells and detected by Western blotting with anti-GFP and anti-H1.X (lanes 8 to 10 in panel D) antibodies. The extracts derived from untransfected HeLa cells (lanes 1) were also examined as a control. Lane M indicates molecular size markers, and the positions of marker proteins are indicated on the left. Dots on the right indicate a nonspecific band. The position of the endogenous H1.X protein is indicated by an arrowhead. (E and F) FRAP assays of the chimeric H1 proteins. (E) EGFP-H1.0, -H1.X, -H1.0-nX, and -H1.X-n0 are shown. (F, top) FRAP data for EGFP-H1.0, -H1.X, -H1.X-g0, and -H1.X-c0 are shown. (Bottom) FRAP data for EGFP-H1.X, -H1.0, -H1.0-gX, and -H1.0-cX are shown. The data for EGFP-H1.0 and EGFP-H1.X for both graphs are from the same experiment sets. FRAP data were obtained from at least 10 cells, and error bars are ±SD.
Functions of the NTD, GD, and CTD of linker histones on their chromosomal binding site preference.
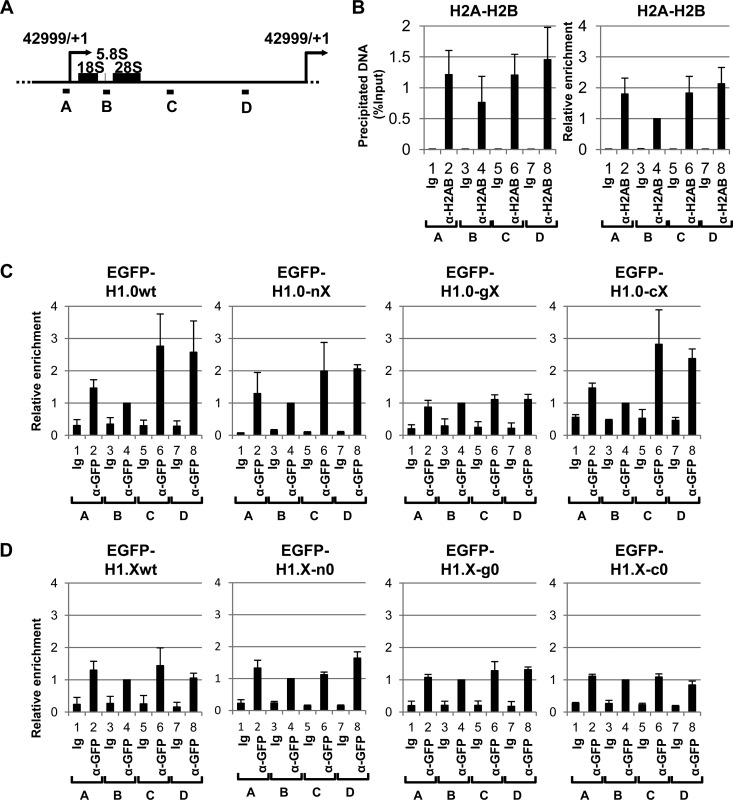
It was recently reported that H1.0 and H1.X show distinct localization patterns along the rRNA gene repeats (27). We examined the effect of NTD, GD, and CTD substitutions on the localization of these linker histones along the rRNA genes. EGFP-tagged H1 proteins were transiently expressed in 293T cells, and the binding along the rRNA gene repeats (Fig. 6A) was examined using chromatin immunoprecipitation (ChIP) assays. Because the amounts of linker histones were expected to depend on core histone density, we first examined core histone density along the rRNA gene using anti-H2A-H2B antibody (Fig. 6B). Core histone H2A-H2B density around the coding region (region B) was lower than that of the rRNA gene promoter or intergenic regions (regions A, C, and D). H1.0 enrichment on the rRNA gene promoter and rRNA coding regions (regions A and B, respectively) was lower than that on the intergenic regions (regions C and D). Conversely, the enrichment of H1.X on the rRNA gene promoter and coding regions was similar to that on the intergenic regions (Fig. 6C and D). These results indicate that H1.0 and H1.X have distinct chromosomal binding site preferences and that the binding site preferences of H1.0 and H1.X are not explained simply by core histone density. H1.0-nX and H1.X-n0 as well as H1.0-cX and H1.X-c0 showed binding patterns similar to those of the wild-type H1.0 and H1.X, respectively, suggesting that the NTD and CTD of linker histones contribute less efficiently to their binding site preference to the rRNA gene. In sharp contrast, replacement of the GD of H1.0 with that of H1.X clearly changed the pattern of binding, and H1.0-gX distributed evenly throughout the rRNA gene in a manner similar to that of H1.X. However, H1.X-g0, in which the GD of H1.X was replaced with that of H1.0, also showed a binding pattern similar to that of H1.X. From these results, we concluded the GD of H1.0 was required for the enrichment of H1.0 at the intergenic region of the rRNA genes. However, the GD of H1.0 alone was not sufficient for establishing the binding site preference, and the cooperative function of either NTD or CTD is required.
FIG 6.
Binding preference of linker histones to the rRNA gene. (A) Schematic representation of the human rRNA gene repeats. A unit of the human rRNA gene (42,999 bp) is schematically represented. Transcription start sites (position +1) are indicated by arrows, and the positions of 18S, 5.8S, and 28S rRNAs are shown by boxes. Positions A to D show the sites of primer sets for ChIP-quantitative PCR (qPCR) analyses. (B) Histone density along the rRNA gene repeats. Chromatin immunoprecipitation assays were performed with anti-H2A-H2B antibody, and the precipitated DNA was analyzed by qPCR using primer sets A to D. The graph on the left represents the amount of precipitated DNA with percent input, and the graph on the right represents the amount of precipitated DNA as relative values by setting the amount of precipitated DNA of the B region as 1.0. (C and D) Association of linker histone mutants with the rRNA genes. EGFP-tagged H1.0, H1.0-nX, H1.0-gX, and H1.0-cX (C) and EGFP-H1.X, H1.X-n0, H1.X-g0, and H1.X-c0 (D) were transiently expressed in 293T cells, and chromatin immunoprecipitation assays were performed. Precipitated DNA was quantitatively analyzed by qPCR with primer sets A to D. The DNA amounts at primer set B (coding region) precipitated with GFP antibody were set as 1.0, and the relative amounts for each sample were estimated. The experiments were independently repeated three times, the graphs are averages, and error bars indicate ±SD.
To confirm the observations seen in the rRNA genes, we further examined the effects of three domains on the binding site selection of linker histones using two RNA polymerase (Pol) II-driven genes, the U2 snRNA (RNU2) and CES1 genes, and one RNA polymerase III-driven gene, the 5S rRNA gene (Fig. 7). The RNU2 and 5S rRNA genes encode splicing snRNA and large ribosome subunit RNA, respectively, and should be actively transcribed in growing cells. It is also known that both the RNU2 and 5S rRNA genes are tandemly repeated as rRNA genes (Fig. 7A) (37, 38). On the other hand, the CES1 gene was reported to be silenced in 293T cells by DNA methylation of CpG sites around the promoter region (39). Core histone H2A-H2B densities of the rRNA gene B region and the RNU2 gene were lower than those of the 5S rRNA and CES1 genes (Fig. 7B). The binding of EGFP-H1 proteins to the genes were represented by both percent input and relative enrichment by setting the DNA amount of the rRNA gene B region to 1.0 (Fig. 7C). ChIP assays demonstrated that H1.0 showed clear preference for 5S rRNA, RNU2, and CES1 genes relative to the rRNA gene B region (Fig. 7C). On the other hand, H1.X showed similar binding preferences for all genes with slight enrichment for the 5S rRNA gene. These results also supported the notion that H1.0 and H1.X have distinct binding site preferences. When the CTD of H1.0 was replaced by that of H1.X (H1.0-cX), the binding site preference for all three genes was significantly decreased. In addition, the replacement of the GD of H1.0 with that of H1.X also decreased its binding to the RNU2 gene, whereas it did not show a significant effect on the binding to the 5S rRNA and CES1 genes. These results suggest that both the CTD and GD are required for the binding site selection of linker histones, and the contributions of the CTD and GD of H1.0 to determine the preferred binding sites are distinct depending on the target genes.
FIG 7.
Effects of GD and CTD on the binding preference of linker histones to the Pol II- and Pol III-transcribed genes. (A) Schematic representation of the 5S rRNA (Pol III-transcribed gene) and RNU2 and CES1 genes (Pol II-transcribed genes). The 5S rRNA and RNU2 (U2 snRNA) genes are tandemly repeated, and the transcription start sites (+1) are shown by arrows. Coding regions of the genes are represented by boxes. Arrowheads show the primer sites used for ChIP assays. Scales of nucleotide length are shown at the bottom of each gene. (B) Histone density on the rRNA, 5S rRNA, RNU2, and CES1 genes. ChIP assays were performed with anti-H2A-H2B antibody, and precipitated DNA was analyzed using the primer sets shown in panel A. (C) ChIP assays with the extracts derived from cells expressing either EGFP-H1.0wt, EGFP-H1.0-cX, EGFP-H1.0-gX, or H1.X-wt were performed as described for Fig. 6. (Top) Precipitated DNA was analyzed by qPCR and represented by percent input. Three independent experiments were performed, and error bars indicate ±SD. (Bottom) Because the expression of EGFP proteins was not constant in each experiment, the results are also represented by normalized DNA amounts using the precipitated DNA of the B region of the rRNA gene.
Biochemical properties of the CTDs of H1.0 and H1.X.
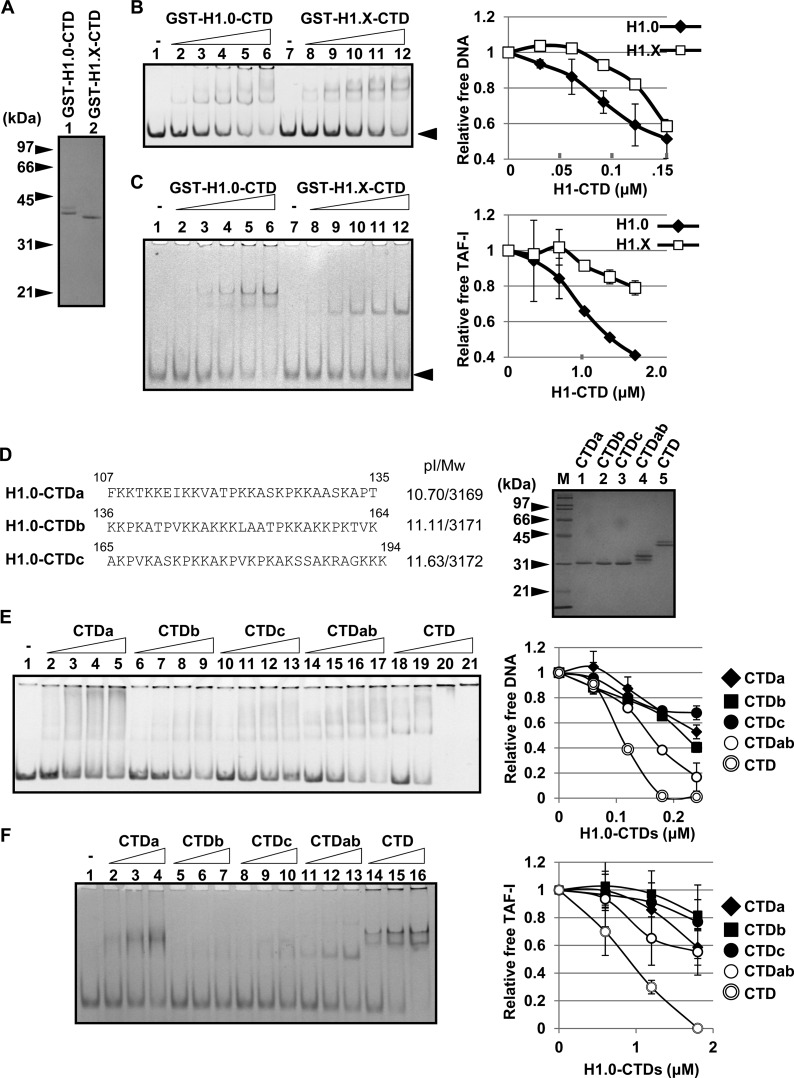
As previously reported (13, 15, 16), we demonstrated that the CTD plays a critical role in the cellular dynamics of linker histones (Fig. 5). In addition, the association of linker histones with both TAF-I and B23 was mediated by the CTD (4, 5). Therefore, it was assumed that the CTD activity of H1.X was lower than that of other linker histones. To test this idea, the GST-tagged CTD of H1.0 and H1.X were purified (Fig. 8A) and used for DNA and histone chaperone binding assays (Fig. 8B and C). The affinity of the H1.0 CTD to DNA was higher than that of the H1.X CTD. Similarly, the affinity of the H1.X CTD to TAF-I was significantly lower than that of the H1.0 CTD. These results support the idea that low DNA and histone chaperone binding of H1.X was because of its poor CTD activity.
FIG 8.
DNA and histone chaperone binding activities of the CTD. (A) Purified proteins. GST-tagged H1.0 CTD and H1.X CTD were purified, separated by SDS-PAGE, and visualized with CBB staining. (B) DNA binding activity of GST-tagged CTDs. The 196-bp DNA fragment (0.01 μM) was incubated without (lanes 1 and 7) or with increasing amounts of GST-H1.0 CTD (lanes 2 to 6) or GST-H1.X CTD (lanes 8 to 12) (0.03, 0.06, 0.09, 0.12, and 0.15 μM GST-CTD proteins), separated by 6% native PAGE, and visualized with GelRed staining. The position of free DNA is shown by an arrowhead. The relative amount of free DNA was plotted as shown on the right. (C) TAF-I binding activity of H1 CTDs. His–TAF-I (1.6 μM) was incubated without (lanes 1 and 7) or with increasing amounts of GST-H1.0 CTD (lanes 2 to 6) or GST-H1.X CTD (lanes 8 to 12) (0.3, 0.6, 0.9, 1.2, and 1.5 μM the GST-CTD proteins), separated by 6% native PAGE, and visualized with CBB staining. The position of free TAF-I is shown by an arrowhead. The amounts of free TAF-I are plotted on the right. (D) Expression and purification of the three regions of H1.0 CTD. H1.0 CTD is separated into three regions, CTDa, CTDb, and CTDc, and amino acid sequences, theoretical isoelectric points (pI), and molecular weights (Mw) are shown. GST-tagged CTDa, CTDb, CTDc, CTDab (amino acids 107 to 164), and CTD (amino acids 107 to 194) were expressed, purified, and separated by SDS-PAGE followed by CBB staining. Lane M indicates molecular mass markers. (E) DNA binding activity of the CTD peptides. The purified proteins (0.06, 0.12, 0.18, and 0.24 μM), prepared as described for panel D, were mixed with 196-bp-long DNA and separated by native PAGE. The relative amount of free DNA was plotted as shown on the right. (F) TAF-I binding of the CTD peptides. His–TAF-I (1.6 μM) was incubated without (lane 1) or with increasing amounts of GST-H1.0 CTD peptides (0.6, 1.2, and 1.8 μM), separated by 6% native PAGE, and visualized with CBB staining. The amounts of free TAF-I are plotted on the right. For panels B, C, E, and F, the data in the graphs are averages from two independent experiments and error bars indicate ±SD.
It was previously reported that the CTD of H1.0 is divided into functional subdomains to bind linker DNA, stabilize the folding of nucleosome arrays, and facilitate the self-association of nucleosomes (40). To determine the subdomain involved in the DNA and histone chaperone binding of the H1.0 CTD, the CTD region was divided into three regions, CTDa, CTDb, and CTDc (amino acids 107 to 135, 136 to 164, and 165 to 194, respectively) (Fig. 8D), and their DNA and histone chaperone binding activities were examined. Positively charged lysines (K) are almost evenly distributed, and the theoretical isoelectric points (pI) of three peptides are similar. The three peptides similarly bound to naked DNA with lower affinity than full-length CTD (Fig. 8E). The CTDab peptide (amino acids 107 to 164) showed higher affinity than the individual short peptides but lower affinity than the full-length peptide, suggesting that the random DNA binding activity of the CTD is proportional to its length. B23 bound to all three CTD peptides with similar efficiency and showed stronger activity with CTDab and the full CTD than short peptides (see Fig. S4 in the supplemental material). Interestingly, we found that the binding of CTDa to TAF-I was stronger than those of CTDb and CTDc and enhanced with the increase of CTD length (Fig. 8F). The binding of the H1.0 CTD with TAF-I is not simply explained by charge, because the CTDa region was less basic than CTDb and CTDc. Considered together, we conclude that the entire CTD region contributes to determine the affinity to DNA and B23, whereas the contribution of the CTDa region of H1.0 CTD to TAF-I binding is the most prominent.
Identification of the amino acid position in the GD causing different dynamics between H1.0 and H1.X.
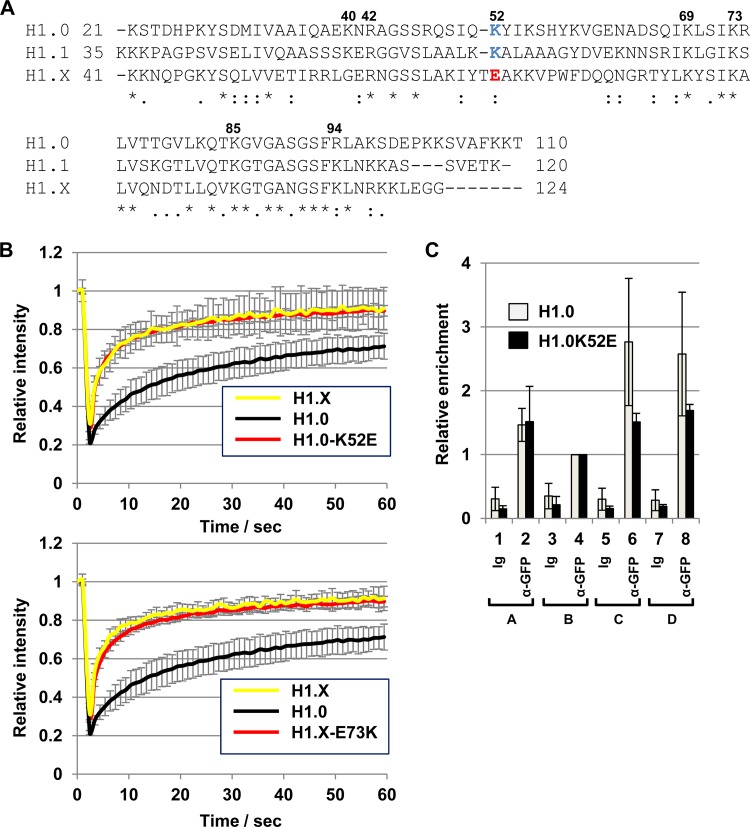
We next sought to determine the amino acid(s) in the globular domains affecting the stable chromatin binding of H1.0 and H1.X. Previous mutation analyses have suggested that two DNA binding sites in the GD play roles in stable nucleosome binding: one includes Lys69, Arg73, and Lys85, and the other includes Lys40, Arg42, Lys52, and Arg94 (12, 41). Among these amino acids, lysines at positions 40 and 52 in H1.0 were not conserved in H1.X (Gly60 and Glu73, respectively) (Fig. 9A). In addition, the amino acid in H1.X corresponding to H1.0 Lys52 (K52) is a negatively charged amino acid (Glu at position 73 [E73]). Therefore, we focused on Lys52 in H1.0 and prepared a mutant H1.0 in which Lys52 was replaced by Glu (H1.0-K52E) and a mutant H1.X in which Glu73 was replaced by Lys (H1.X-E73K). FRAP assays clearly demonstrated that the substitution at Lys52 to Glu dramatically enhanced the mobility of H1.0, whereas the substitution in H1.X at Glu73 to Lys did not strongly affect the mobility of H1.X (Fig. 9B). We also found that preferential binding of H1.0 to the rRNA gene intergenic regions (C and D regions) was lost by the alteration at Lys52 to Glu (Fig. 9C). These results indicate that Glu73 in H1.X is one of the critical residues that confers dynamic behavior on H1.X, although this amino acid alone is not sufficient to explain its level of dynamic behavior being higher than that of H1.0.
FIG 9.
Identification of the amino acid in GD responsible for the dynamic behavior of H1.X. (A) Alignment of the globular domains of H1.0, H1.1, and H1.X. Amino acid sequences of the globular domains of H1.0, H1.1, and H1.X are aligned. Amino acid positions suggested to be responsible for the nucleosomal DNA binding are indicated at the top of the sequences. The amino acid numbers shown are for human H1.0. K52 in H1.0 and the corresponding amino acid in H1.1 K67 are shown in blue, whereas the corresponding position of H1.X (E73) is shown in red. (B) FRAP analyses. EGFP–H1.0-K52E (top) and EGFP–H1.X-E73K (bottom) were transiently expressed and FRAP assays were performed. The data of EGFP-H1.0 and H1.X in both graphs were from the same experiment sets shown in Fig. 1D. FRAP data were obtained from 10 cells, and error bars are ±SD. (C) ChIP assay for the H1.0-K52E mutant. EGFP–H1.0-K52E was transiently expressed and a ChIP assay was performed using control Ig and anti-GFP antibody. The association of the proteins at the rRNA gene was quantitatively analyzed. The amount of region B was set to 1.0, and relative amounts of regions A to D were analyzed. Three independent experiments were performed, and error bars indicate ±SD. The data set for wild-type H1.0 was the same as the data set shown in Fig. 6C.
The activity of GDs affects the localization of linker histones.
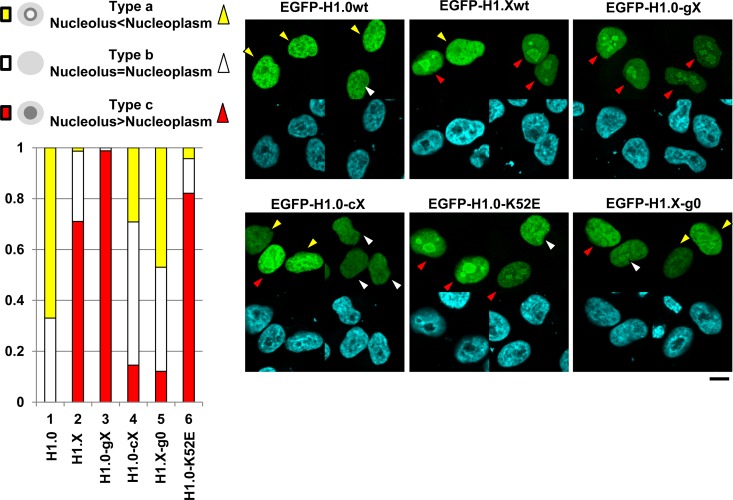
We observed that not only EGFP-H1.X but also the EGFP-H1.0 chimeric proteins showed nucleolar accumulation in some, but not all, cells. To address the conditions under which linker histones localize to the nucleoli, the localizations of EGFP-H1.0, -H1.X, -H1.0-gX, -H1.0-cX, H1.0-K52E, and H1.X-g0 were examined (Fig. 10). Their localization patterns were categorized into the following three groups: type a (nucleolus localization is weaker than nucleoplasm localization), type b (nucleolus and nucleoplasm localizations are the same), and type c (nucleolus localization is stronger than nucleoplasm localization), as shown by yellow, white, and red arrowheads, respectively. Wild-type H1.0 was localized mainly to the nucleoplasm (type a or b), whereas wild-type H1.X showed nucleolar accumulation (type c) in about 70% of expressing cells. Although the FRAP recovery curves of H1.0-gX and H1.0-cX were very similar, as shown in Fig. 5F, their localization patterns were similar to those of H1.X and H1.0, respectively. When the GD of H1.X was replaced by that of H1.0 (H1.X-g0), the number of cells showing nucleolar accumulation (type c) was clearly decreased. In addition, the K52E mutation of H1.0 increased the type c localization pattern. These results suggest that linker histones accumulate in the nucleolus when their GD activity is low. We also examined the recovery rates of EGFP-H1.X in the nucleoplasm and the nucleolus by FRAP assay as shown in Fig. S6 in the supplemental material. We found that the recovery rate of EGFP-H1.X was not different between nucleolar H1.X and nucleoplasmic H1.X. This indicates that EGFP-H1.X accumulates at the nucleolus by associating with nucleolar components with affinity similar to that for chromatin.
FIG 10.
Functions of the GD on the nucleolar localization of linker histones. HeLa cells were transiently transfected with vectors for the expression of EGFP-H1.0, -H1.X, H1.0-gX, -H1.0-cX, H1.X-g0, and H1.0-K52E. The cells were fixed 24 h posttransfection and stained with DAPI, and localization of the proteins and DNA was observed under a confocal microscope. The protein localization patterns are categorized into types a, b, and c as shown at the top left. Cells with type a, b, and c are indicated by yellow, white, and red arrowheads. Two typical fields for each sample are shown at the right. (Bottom left) The percentages of cells with type a, b, and c were calculated and graphically represented. For all EGFP proteins, 10 different fields were observed (more than 25 cells were examined).
DISCUSSION
In this study, we analyzed the molecular mechanisms by which linker histone variants show different dynamic behaviors and chromosomal binding site preferences. Biochemical analyses showed that intrinsic DNA and nucleosome binding activity correlates with the dynamic behavior of linker histones. As summarized in Table 1, we demonstrated that the slow FRAP mobility and binding site preferences of H1.0 are contributed by H1.0 GD and CTD, whereas fast FRAP mobility of H1.X is contributed by both the GDs and CTDs and nucleolar accumulation of H1.X is contributed by its GD. Contrary to the functions of GDs and CTDs, the NTD of H1.0 slightly increases the mobility of linker histones, and the NTD of H1.X slightly decreases the mobility of linker histones. In addition, the NTDs of H1.0 play distinct roles in binding site selection, because H1.0-cX and H1.X-g0 have the same amino acid sequences, except the NTDs showed a distinct binding pattern (Fig. 6). In addition, linker histones tend to accumulate at the nucleoli when their DNA binding activities of GD are low. Our results contribute to the understanding of the molecular mechanisms by which linker histone variants show distinct dynamic behaviors in the nucleus and select their preferential binding sites.
TABLE 1.
Summary of the resultsa
| H1 variant and property | NTD | GD | CTD |
|---|---|---|---|
| H1.0 | |||
| FRAP mobility | Fast | Slow | Slow |
| Binding site preference | Yes | Yes | Yes |
| Nucleolar localization | – | – | – |
| H1.X | |||
| FRAP mobility | Slow | Fast | Fast |
| Binding site preference | – | – | – |
| Nucleolar localization | – | Yes | – |
When the contribution of the domains is clear, the functions (fast, slow, or yes) are in boldface, whereas when the contribution of the domains is not very high, the functions are in italics. When the contributions of the domains are not detected or not determined, the functions are represented by a dash.
The higher mobility of H1.X than H1.0 can be explained by the weak DNA and nucleosome binding activities of H1.X (Fig. 2 and 8). Our biochemical analyses indicated that the CTD of H1.X is a reason for weak DNA and nucleosome binding of H1.X among various linker histone variants. The CTDs of linker histones are highly basic, which is probably important for association with the linker DNA region by stabilizing GD binding to the DNA entry and exit sites of the nucleosome (11). The basic amino acid content of the H1.X CTD (32 lysine and arginine residues in amino acids 117 to 213 [33.0%]) is lower than that of the H1.0 CTD (43 lysine and arginine residues in amino acids 97 to 194 [43.9%]). This difference between H1.X and H1.0 leads to the different affinities of the CTDs to DNA and acidic histone chaperone proteins. Consistent with this idea, we demonstrated that the mobility of H1.0 was increased by the replacement of its CTD with that of H1.X. Furthermore, it was previously reported that the mobility of linker histones in the nucleus was increased when the positive charge of the CTD was neutralized by phosphorylation (42). The other potential reason for higher mobility of H1.X than H1.0 is their different GD affinities to nucleosomes because of a positive charge at position 73 in the GD of H1.X.
Among the somatic linker histone variants other than H1.X, H1.1 moves most quickly (24, 43). It was recently reported that a positive charge located around the border between the GD and CTD plays a critical role in the quick mobility of mouse H1.1 (43). The corresponding amino acid residue in human H1.1 is not conserved, and this position is a serine (Ser115). Glutamic acid (E; a negatively charged amino acid) is located around a similar position in H1.X (position 122) (Fig. 9A), and two positively charged amino acids around the border between the GD and CTD (Asp99 and Glu100) (Fig. 9A) are located in H1.0. These observations suggest that the mobility of linker histones is not explained simply by the positively charged amino acids at the border between the GD and CTD. It should also be noted that the intrinsic DNA binding activity of H1.1 was similar to or slightly higher than that of H1.0 (Fig. 2C), although the mobility of H1.1 in the nucleus is higher than that of H1.0 (24). Consistent with our result, it was previously reported that H1.1 purified from mouse liver showed higher DNA binding activity in vitro than H1.0 (44), and H1.1 purified from yeast cells showed lower NCP binding activity than H1.0 (23). These results suggest that posttranslational modifications of H1.1 regulate its DNA and nucleosome binding.
The expression level of linker histone variants is divergent depending on cell types (45). The expression of H1.0 is high in differentiated cells, whereas that of H1.1 is very limited to some specific tissues and cell types. On the other hand, the expression of H1.2 and H1.4 is detected in a variety of cell types. It has been shown that H1.4 shows lower mobility than H1.2, and the localization patterns of these variants are different (24). Based on our findings, it is reasonably speculated that the GDs and CTDs of these variants cooperatively determine their properties in vivo. The biochemical assay system established in this study will contribute to the understanding of the different behaviors of the most abundant linker histones, H1.2 and H1.4.
Consistent with a previous report (27), we also found that the binding preference was different between H1.0 and H1.X. Both the GDs and CTDs contribute to determining the binding site (Fig. 6 and 7). When linker histones reside on a nucleosome core particle, the GD makes direct contact with nucleosomal DNA. If any of these DNA sites contain methylated CpG, the binding of linker histones may be affected. Because previous studies suggested that the DNA methylation affects the nucleosome binding of linker histones (46–48), it will be interesting to test the effect of DNA methylation on the DNA binding activity of the GD and CTD in the future.
As shown in Fig. 3, we found that H1.X and H1.0 associated with the previously known linker histone chaperones TAF-I, B23, and NCL. We showed that TAF-I and B23 mediated the deposition of linker histones on NCPs, whereas TAF-I, but not B23, removed H1s from H1-bound NCPs in vitro. Consistent with these findings, H1 mobility was decreased by TAF-I knockdown (4). In addition, both B23 and TAF-I bind to linker histones through the CTD, although TAF-I and B23 showed different binding preferences toward the CTD regions (Fig. 8F; also see Fig. S4 in the supplemental material). The differences in CTD binding of TAF-I and B23 may cause differences in biochemical activity in linker histone assembly and disassembly.
Supplementary Material
ACKNOWLEDGMENTS
We thank Shinichi Tate (Hiroshima University, Japan) for the original human core histone expression plasmids.
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to M.O. (26440021), M.H. (26830123), and K.N. (24115002 and 25291001).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00200-16.
REFERENCES
- 1.Andrews AJ, Chen X, Zevin A, Stargell LA, Luger K. 2010. The histone chaperone Nap1 promotes nucleosome assembly by eliminating nonnucleosomal histone DNA interactions. Mol Cell 37:834–842. doi: 10.1016/j.molcel.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagata K, Kawase H, Handa H, Yano K, Yamasaki M, Ishimi Y, Okuda A, Kikuchi A, Matsumoto K. 1995. Replication factor encoded by a putative oncogene, set, associated with myeloid leukemogenesis. Proc Natl Acad Sci U S A 92:4279–4283. doi: 10.1073/pnas.92.10.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okuwaki M, Iwamatsu A, Tsujimoto M, Nagata K. 2001. Identification of nucleophosmin/B23, an acidic nucleolar protein, as a stimulatory factor for in vitro replication of adenovirus DNA complexed with viral basic core proteins. J Mol Biol 311:41–55. doi: 10.1006/jmbi.2001.4812. [DOI] [PubMed] [Google Scholar]
- 4.Kato K, Okuwaki M, Nagata K. 2011. Role of template activating factor-I as a chaperone in linker histone dynamics. J Cell Sci 124:3254–3265. doi: 10.1242/jcs.083139. [DOI] [PubMed] [Google Scholar]
- 5.Gadad SS, Senapati P, Syed SH, Rajan RE, Shandilya J, Swaminathan V, Chatterjee S, Colombo E, Dimitrov S, Pelicci PG, Ranga U, Kundu TK. 2011. The multifunctional protein nucleophosmin (NPM1) is a human linker histone H1 chaperone. Biochemistry 50:2780–2789. doi: 10.1021/bi101835j. [DOI] [PubMed] [Google Scholar]
- 6.Shintomi K, Iwabuchi M, Saeki H, Ura K, Kishimoto T, Ohsumi K. 2005. Nucleosome assembly protein-1 is a linker histone chaperone in Xenopus eggs. Proc Natl Acad Sci U S A 102:8210–8215. doi: 10.1073/pnas.0500822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kepert JF, Mazurkiewicz J, Heuvelman GL, Toth KF, Rippe K. 2005. NAP1 modulates binding of linker histone H1 to chromatin and induces an extended chromatin fiber conformation. J Biol Chem 280:34063–34072. doi: 10.1074/jbc.M507322200. [DOI] [PubMed] [Google Scholar]
- 8.Kondili K, Tsolas O, Papamarcaki T. 1996. Selective interaction between parathymosin and histone H1. Eur J Biochem 242:67–74. doi: 10.1111/j.1432-1033.1996.0067r.x. [DOI] [PubMed] [Google Scholar]
- 9.Karetsou Z, Sandaltzopoulos R, Frangou-Lazaridis M, Lai CY, Tsolas O, Becker PB, Papamarcaki T. 1998. Prothymosin alpha modulates the interaction of histone H1 with chromatin. Nucleic Acids Res 26:3111–3118. doi: 10.1093/nar/26.13.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erard MS, Belenguer P, Caizergues-Ferrer M, Pantaloni A, Amalric F. 1988. A major nucleolar protein, nucleolin, induces chromatin decondensation by binding to histone H1. Eur J Biochem 175:525–530. doi: 10.1111/j.1432-1033.1988.tb14224.x. [DOI] [PubMed] [Google Scholar]
- 11.Stasevich TJ, Mueller F, Brown DT, McNally JG. 2010. Dissecting the binding mechanism of the linker histone in live cells: an integrated FRAP analysis. EMBO J 29:1225–1234. doi: 10.1038/emboj.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou BR, Jiang J, Feng H, Ghirlando R, Xiao TS, Bai Y. 2015. Structural mechanisms of nucleosome recognition by linker histones. Mol Cell 59:628–638. doi: 10.1016/j.molcel.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu X, Hamkalo B, Parseghian MH, Hansen JC. 2009. Chromatin condensing functions of the linker histone C-terminal domain are mediated by specific amino acid composition and intrinsic protein disorder. Biochemistry 48:164–172. doi: 10.1021/bi801636y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roque A, Iloro I, Ponte I, Arrondo JLR, Suau P. 2005. DNA-induced secondary structure of the carboxyl-terminal domain of histone H1. J Biol Chem 280:32141–32147. doi: 10.1074/jbc.M505636200. [DOI] [PubMed] [Google Scholar]
- 15.Caterino TL, Fang H, Hayes JJ. 2011. Nucleosome linker DNA contacts and induces specific folding of the intrinsically disordered H1 carboxyl-terminal domain. Mol Cell Biol 31:2341–2348. doi: 10.1128/MCB.05145-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allan J, Hartman PG, Crane-Robinson C, Aviles FX. 1980. The structure of histone H1 and its location in chromatin. Nature 288:675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- 17.Raghuram N, Carrero G, Th'ng J, Hendzel MJ. 2009. Molecular dynamics of histone H1. Biochem Cell Biol 87:189–206. doi: 10.1139/O08-127. [DOI] [PubMed] [Google Scholar]
- 18.Sirotkin AM, Edelmann W, Cheng G, Klein-Szanto A, Kucherlapati R, Skoultchi AI. 1995. Mice develop normally without the H1(0) linker histone. Proc Natl Acad Sci U S A 92:6434–6438. doi: 10.1073/pnas.92.14.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Q, Sirotkin A, Skoultchi AI. 2000. Normal spermatogenesis in mice lacking the testis-specific linker histone H1t. Mol Cell Biol 20:2122–2128. doi: 10.1128/MCB.20.6.2122-2128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan Y, Nikitina T, Morin-Kensicki EM, Zhao J, Magnuson TR, Woodcock CL, Skoultchi AI. 2003. H1 linker histones are essential for mouse development and affect nucleosome spacing in vivo. Mol Cell Biol 23:4559–4572. doi: 10.1128/MCB.23.13.4559-4572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Q, Inselman A, Han X, Xu H, Zhang W, Handel MA, Skoultchi AI. 2004. Reductions in linker histone levels are tolerated in developing spermatocytes but cause changes in specific gene expression. J Biol Chem 279:23525–23535. doi: 10.1074/jbc.M400925200. [DOI] [PubMed] [Google Scholar]
- 22.Sancho M, Diani E, Beato M, Jordan A. 2008. Depletion of human histone H1 variants uncovers specific roles in gene expression and cell growth. PLoS Genet 4:e1000227. doi: 10.1371/journal.pgen.1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clausell J, Happel N, Hale TK, Doenecke D, Beato M. 2009. Histone H1 subtypes differentially modulate chromatin condensation without preventing ATP-dependent remodeling by SWI/SNF or NURF. PLoS One 4:e0007243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Th'ng JP, Sung R, Ye M, Hendzel MJ. 2005. H1 family histones in the nucleus. Control of binding and localization by the C-terminal domain. J Biol Chem 280:27809–27814. [DOI] [PubMed] [Google Scholar]
- 25.Orrego M, Ponte I, Roque A, Buschati N, Mora X, Suau P. 2007. Differential affinity of mammalian histone H1 somatic subtypes for DNA and chromatin. BMC Biol 5:22. doi: 10.1186/1741-7007-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takata H, Matsunaga S, Morimoto A, Ono-Maniwa R, Uchiyama S, Fukui K. 2007. H1.X with different properties from other linker histones is required for mitotic progression. FEBS Lett 581:3783–3788. doi: 10.1016/j.febslet.2007.06.076. [DOI] [PubMed] [Google Scholar]
- 27.Mayor R, Izquierdo-Bouldstridge A, Millan-Arino L, Bustillos A, Sampaio C, Luque N, Jordan A. 2015. Genome distribution of replication-independent histone H1 variants shows H1.0 associated with nucleolar domains and H1X associated with RNA polymerase II-enriched regions. J Biol Chem 290:7474–7491. doi: 10.1074/jbc.M114.617324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okuwaki M, Kato K, Shimahara H, Tate S, Nagata K. 2005. Assembly and disassembly of nucleosome core particles containing histone variants by human nucleosome assembly protein I. Mol Cell Biol 25:10639–10651. doi: 10.1128/MCB.25.23.10639-10651.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okuwaki M, Sumi A, Hisaoka M, Saotome-Nakamura A, Akashi S, Nishimura Y, Nagata K. 2012. Function of homo- and hetero-oligomers of human nucleoplasmin/nucleophosmin family proteins NPM1, NPM2 and NPM3 during sperm chromatin remodeling. Nucleic Acids Res 40:4861–4878. doi: 10.1093/nar/gks162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagata K, Saito S, Okuwaki M, Kawase H, Furuya A, Kusano A, Hanai N, Okuda A, Kikuchi A. 1998. Cellular localization and expression of template-activating factor I in different cell types. Exp Cell Res 240:274–281. doi: 10.1006/excr.1997.3930. [DOI] [PubMed] [Google Scholar]
- 31.Hisaoka M, Ueshima S, Murano K, Nagata K, Okuwaki M. 2010. Regulation of nucleolar chromatin by B23/nucleophosmin jointly depends upon its RNA binding activity and transcription factor UBF. Mol Cell Biol 30:4952–4964. doi: 10.1128/MCB.00299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murano K, Okuwaki M, Hisaoka M, Nagata K. 2008. Transcription regulation of the rRNA gene by a multifunctional nucleolar protein, B23/nucleophosmin, through its histone chaperone activity. Mol Cell Biol 28:3114–3126. doi: 10.1128/MCB.02078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoldt S, Wenzel D, Schulze E, Doenecke D, Happel N. 2007. G1 phase-dependent nucleolar accumulation of human histone H1x. Biol Cell 99:541–552. doi: 10.1042/BC20060117. [DOI] [PubMed] [Google Scholar]
- 34.Catez F, Ueda T, Bustin M. 2006. Determinants of histone H1 mobility and chromatin binding in living cells. Nat Struct Mol Biol 13:305–310. doi: 10.1038/nsmb1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George EM, Brown DT. 2010. Prothymosin alpha is a component of a linker histone chaperone. FEBS Lett 584:2833–2836. doi: 10.1016/j.febslet.2010.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oberg C, Belikov S. 2012. The N-terminal domain determines the affinity and specificity of H1 binding to chromatin. Biochem Biophys Res Commun 420:321–324. doi: 10.1016/j.bbrc.2012.02.157. [DOI] [PubMed] [Google Scholar]
- 37.Little RD, Braaten DC. 1989. Genomic organization of human 5 S rDNA and sequence of one tandem repeat. Genomics 4:376–383. doi: 10.1016/0888-7543(89)90345-5. [DOI] [PubMed] [Google Scholar]
- 38.Pavelitz T, Rusche L, Matera AG, Scharf JM, Weiner AM. 1995. Concerted evolution of the tandem array encoding primate U2 snRNA occurs in situ, without changing the cytological context of the RNU2 locus. EMBO J 14:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hori T, Hosokawa M. 2010. DNA methylation and its involvement in carboxylesterase 1A1 (CES1A1) gene expression. Xenobiotica 40:119–128. doi: 10.3109/00498250903431794. [DOI] [PubMed] [Google Scholar]
- 40.Lu X, Hansen JC. 2004. Identification of specific functional subdomains within the linker histone H10 C-terminal domain. J Biol Chem 279:8701–8707. doi: 10.1074/jbc.M311348200. [DOI] [PubMed] [Google Scholar]
- 41.Brown DT, Izard T, Misteli T. 2006. Mapping the interaction surface of linker histone H1(0) with the nucleosome of native chromatin in vivo. Nat Struct Mol Biol 13:250–255. doi: 10.1038/nsmb1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Contreras A, Hale TK, Stenoien DL, Rosen JM, Mancini MA, Herrera RE. 2003. The dynamic mobility of histone H1 is regulated by cyclin/CDK phosphorylation. Mol Cell Biol 23:8626–8636. doi: 10.1128/MCB.23.23.8626-8636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flanagan TW, Files JK, Casano KR, George EM, Brown DT. 2016. Photobleaching studies reveal that a single amino acid polymorphism is responsible for the differential binding affinities of linker histone subtypes H1.1 and H1.5. Biol Open 5:372–380. doi: 10.1242/bio.016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talasz H, Sapojnikova N, Helliger W, Lindner H, Puschendorf B. 1998. In vitro binding of H1 histone subtypes to nucleosomal organized mouse mammary tumor virus long terminal repeat promotor. J Biol Chem 273:32236–32243. doi: 10.1074/jbc.273.48.32236. [DOI] [PubMed] [Google Scholar]
- 45.Meergans T, Albig W, Doenecke D. 1997. Varied expression patterns of human H1 histone genes in different cell lines. DNA Cell Biol 16:1041–1049. doi: 10.1089/dna.1997.16.1041. [DOI] [PubMed] [Google Scholar]
- 46.McArthur M, Thomas JO. 1996. A preference of histone H1 for methylated DNA. EMBO J 15:1705–1714. [PMC free article] [PubMed] [Google Scholar]
- 47.Nightingale K, Wolffe AP. 1995. Methylation at CpG sequences does not influence histone H1 binding to a nucleosome including a Xenopus borealis 5 S rRNA gene. J Biol Chem 270:4197–4200. doi: 10.1074/jbc.270.9.4197. [DOI] [PubMed] [Google Scholar]
- 48.Gilbert N, Thomson I, Boyle S, Allan J, Ramsahoye B, Bickmore WA. 2007. DNA methylation affects nuclear organization, histone modifications, and linker histone binding but not chromatin compaction. J Cell Biol 177:401–411. doi: 10.1083/jcb.200607133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.