Abstract
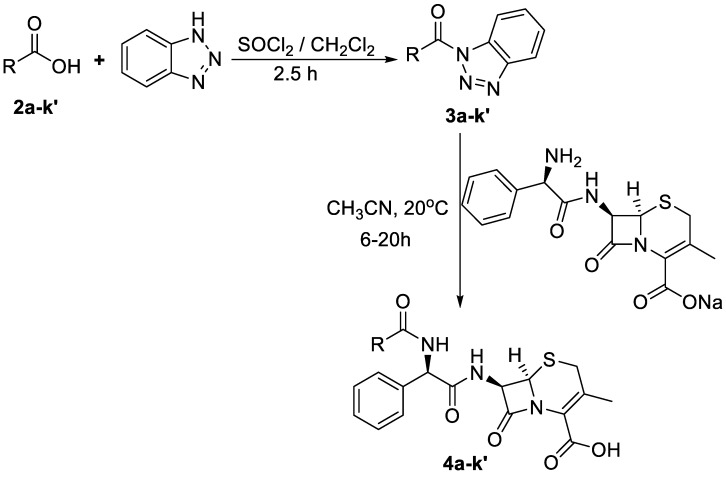
Cephalexin (1) was acylated using N-acylbenzotriazoles (3a–k′) derived from various carboxylic acids including aromatic, heterocyclic and N-Pg-α-amino acid to afford N-acylcephalexins in excellent yields (82%–96%). Antibacterial screening of the novel cephalosporins revealed that all targets (4a–j) retained the antibacterial activity of cephalexin against Staphylococcus aureus (ATCC 6538). N-Nicotinylcephalexin (4c) and N-(3,4,5-trimethoxybenzoyl)cephalexin (4g) exhibited a broader spectrum of antibacterial activity towards standard strains of Staphylococcus aureus (ATCC 6538), Paenibacillus polymyxa (ATCC 842), and Escherichia coli (ATCC 10536) as well as a resistant strain of Pseudomonas aeruginosa (ATCC 27853).
Keywords: acylation, antibiotics, benzotriazolides, drug research
1. Introduction
Effective antibiotics are needed to combat the growing bacterial resistance [1]. Cephalosporins are an important class of antimicrobials with a broad-spectrum of activity and a favourable safety profile [2]. The traditional approach to develop new antibiotics involves antimicrobial screening of natural products [3,4]. However, building new analogues of those antibiotics which are currently in use is more financially feasible because these analogues tend to have similar solubility, protein binding, and toxicity as the parent compounds [5].
Acylation reactions enabled the development of various generations of cephalosporins [6]. In addition, cephalosporins that contain nitrogen nucleophiles can be further acylated in order to modulate their antimicrobial activity, improve their solubility, and introduce fluorescent tags [7,8,9]. Such acylations have been accomplished utilizing acid chlorides, acid anhydrides, and ethyl chloroformate [7,10,11].
N-acylbenzotriazoles are advantageous acylating agents showing numerous merits over acid chlorides because: (i) they are usually isolated in high yields; (ii) they form crystals easily; (iii) they are stable in air; and (iv) chirality is preserved during the course of their preparation and reaction. These carboxylic acid surrogates are widely used when the corresponding acid chlorides are unstable or difficult to prepare [12].
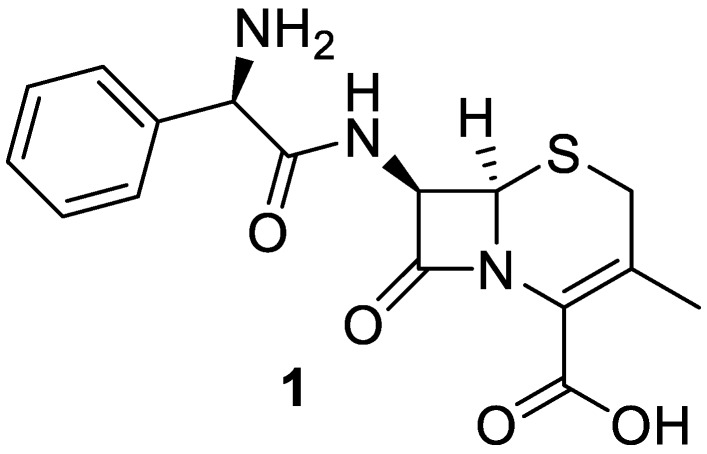
Amino acid conjugates of quinolone, metronidazole, and sulfadiazine antibiotics were recently synthesized in good yields using the benzotriazole methodology [13]. In the current work, cephalexin (Figure 1), which is a first generation cephalosporin on the World Health Organization’s list of essential medicines, was selected for further acylation with a variety of N-acylbenzotriazoles (3a–k′). N-acylcephalexins (4a–k′) were obtained in pure form in high yields by a simple work-up and their antibacterial activity was evaluated [4].
Figure 1.
Cephalexin.
2. Materials and Methods
2.1. Chemistry
Starting materials and solvents were purchased from common commercial sources and used without further purification. Melting points were determined on the Fisher Melting Apparatus, (Pittsburgh, PA, USA). 1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectra were recorded on a Bruker 400 MHz NMR Spectrometer (Bruker, Fällanden, Switzerland) in DMSO-d6. J values are given in Hz, using tetramethylsilane (TMS) as the internal standard at the Faculty of Science, Zagazig University. 1H and 13C NMR spectra of Compounds 7d–j and 8a–j can be found in Supplementary Figures S1 and S2. Elemental analyses were performed on the Carlo Erba-1106 (Thermo Fisher Scientific Inc., Waltham, MA, USA) instrument at the Regional Center for Mycology & Biotechnology, Al-Azhar University, Cairo. The reactions were followed by Thin Layer Chromatography (TLC) (silica gel, aluminum sheets 60 F254, Merck, Darmstadt, Germany). The purity of the newly synthesized compounds was assessed by TLC and elemental analysis.
Procedure for the Synthesis of 4-methyl-2-(3,4,5trimethoxybenzamido) thiazole-5-carboxylic acid (2j): Thiourea (0.152 g, 2 mmol) was refluxed in absolute ethanol with ethyl 2-chloroacetoacetate (0.28 mL, 2 mmol) for 4 h. At the end of the reaction, the solvent was evaporated and 5 mL of H2O was added, followed by neutralization with NH4OH to give compound 5 (0.32 g, off-white microcrystals). Compound (5) (0.32 g, 1.7 mmol) was subjected to hydrolysis with NaOH (0.272 g, 6.8 mmol) in (THF-H2O 3:1) under overnight reflux. At the end of the reaction, THF was evaporated and the pH was adjusted to 6–7 with HCl. After it was filtered, the solid was washed with water and then dried to give compound (6) (0.21 g). Compound (6) (0.21 g, 1.3 mmol) was refluxed with compound (3g) (0.4 g, 1.3 mmol) in dioxane in the presence of one equivalent TEA (0.18 mL). At the end of the reaction, the solvent was evaporated; ethylacetate was added and washed with diluted HCl. The organic layer was then dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to give compound (2j) (0.40 g, 90%).
Procedure for the Synthesis of N-acylbenzotriazoles (3a–k′): To 0.95 g BtH (8 mmol) dissolved in 50 mL CH2Cl2, 0.14 mL SOCl2 (2 mmol) were added. The mixture was stirred at 25 °C for 30 min, followed by the addition of the corresponding acid 2a–k′ (2 mmol) and the reaction was allowed to stir for an additional 3 h at 25 °C. The reaction was diluted with CH2Cl2 (50 mL) and the organic layer was washed with saturated Na2CO3 (20 mL, 3×), H2O (20 mL, 2×), and brine (10 mL, 1×). The organic layer was dried over anhydrous sodium sulfate. Hexane (50 mL) was added to the filtrate, then the solid obtained was dried under vacuum to give compounds 3a–k′.
Procedure for the Synthesis of N-acylcephalexines 4a–k′: Cephalexin sodium salt (0.37 g, 1 mmol) was dissolved in water (1 mL) and added to the solution of the corresponding N-acylbenzotriazoles (1 mmol) in CH3CN (7 mL). The mixture was stirred for 6–20 h (until complete consumption of N-acylbenzotriazole as monitored by TLC). The pH was adjusted to 5 using 2 N HCl, and the solvent was evaporated under reduced pressure. The residue was then extracted with ethyl acetate (20 mL, 2×) and the organic layer was washed with 4 N HCl (10 mL, 3×), water (10 mL, 1×), and brine (10 mL, 1×) then dried over anhydrous sodium sulfate. Hexane (10 mL) was added to the filtrate and the solution was left overnight in the freezer to give the desired products 4a–k′.
(1H-Benzo[d][1,2,3]triazol-1-yl)(phenyl)methanone (3d): White microcrystals; yield: (0.42 g, 94%); m.p. 111–112 °C, lit. (110–112 °C) [14]. 1H-NMR (400 MHz, DMSO-d6) δ 8.33–8.28 (m, 2H, Ar-H), 8.13–8.10 (m, 2H,Ar-H), 7.85–7.76 (m, 2H, Ar-H), 7.68–7.63 (m, 3H, Ar-H). 13C-NMR (100 MHz, DMSO-d6) δ 166.5 (C=O), 145.2 (C-N=N), 133.5 (Ar-C), 131.7 (Ar-C), 131.5 (Ar-C), 131.3 (Ar-C), 130.7 (Ar-C), 128.3 (Ar-C), 126.6 (Ar-C), 120.0 (Ar-C), 114.4 (Ar-C).
(1H-Benzo[d][1,2,3]triazol-1-yl)(pyridin-3-yl)methanone (3e): White microcrystals; yield: (0.42 g, 94%); m.p. 101–102 °C, lit. (101–102 °C) [15]. 1H-NMR (400 MHz, DMSO-d6) δ 9.22 (s, 1H, N=CH-C-C=O), 8.90 (d, J = 6.5 Hz, 1H, Ar-H), 8.50–8.48 (m, 1H, Ar-H), 8.33 (dd, J = 17.6 Hz, 8.4 Hz, 2H,Ar-H), 7.86 (t, J = 7.8 Hz, 1H, Ar-H), 7.71–7.66 (m, 2H, Ar-H). 13C-NMR (100 MHz, DMSO-d6) δ 165.3 (C=O), 153.3 (CH-N), 151.3 (N=CH-CC=O), 145.2 (C-N=N), 138.8 (Ar-C), 131.4 (Ar-C), 130.9 (Ar-C), 128.0 (Ar-C), 126.8 (Ar-C), 123.3 (Ar-C), 120.1 (Ar-C), 114.3 (Ar-C).
(1H-Benzo[d][1,2,3]triazol-1-yl)(3-nitrophenyl)methanone (3f): White microcrystals; yield: (0.48 g, 90%); m.p. 159–162 °C, lit. (155.0–156 °C) [16]. 1H-NMR (400 MHz, DMSO-d6) δ 8.91 (t, J = 2.0 Hz, 1H, Ar-H), 8.60–8.57 (m, 1H, Ar-H), 8.54–8.51 (m, 1H, Ar-H), 8.34 (dd, J = 16.0, 8.0 Hz, 2H, Ar-H), 7.95 (t, J = 8.0 Hz, 1H, Ar-H), 7.89–7.85 (m, 1H, Ar-H), 7.72–7.67 (m,1H, Ar-H). 13C-NMR (100 MHz, DMSO-d6) δ 164.7 (C=O), 147.3 (C-NO2), 145.2 (C-N=N),137.2 (C=CH-CH=C-NO2), 133.2 (Ar-C), 131.5 (Ar-C), 131.0 (Ar-C), 130.1(Ar-C), 127.5 (Ar-C), 126.8 (Ar-C), 125.9 (Ar-C), 120.1 (Ar-C), 114.4 (Ar-C).
(1H-Benzo[d][1,2,3]triazol-1-yl)(3,4,5-trimethoxyphenyl)methanone (3g): White microcrystals; yield: (0.6 g, 96%); m.p. 126–128 °C, (126–128 °C) [17]. 1H-NMR (400 MHz, DMSO-d6) δ 8.28 (d, J = 10.8 Hz, 2H, Ar-H), 7.83 (t, J = 7.8 Hz, 1H, Ar-H), 7.65 (t, J = 7.8 Hz, 1H, Ar-H), 7.47 (s, 2H, Ar-H), 3.86 (s, 6H, m-(OCH3)), 3.82 (s, 3H, p-(OCH3)).
(S)-N-(1-(1H-Benzo[d][1,2,3]triazol-1-yl)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)-4-methylbenzenesulfonamide (3h): Brown microcrystals; yield: (0.80 g, 87%); m.p. 175–176 °C; 1H-NMR (400 MHz, DMSO-d6) δ 10.77 (s, 1H, NH), 8.90 (d, J = 8.4 Hz, 1H, Ar-H), 8.23 (d, J = 8.0 Hz, 1H, Ar-H), 8.03 (d, J = 8.4 Hz, 1H, Ar-H), 7.77 (t, J = 7.8 Hz, 1H, Ar-H), 7.61 (t, J = 7.6 Hz, 1H, Ar-H), 7.45 (d, J = 7.6 Hz, 1H, Ar-H), 7.27(d, J = 7.2 Hz, 2H, Ar-H), 7.24 (s, 1H, NH-SO2), 7.13 (s, 1H, CH-NH-C), 7.02 (t, J = 7.4 Hz, 1H, Ar-H), 6.92 (t, J = 7.4 Hz, 1H, Ar-H), 6.87 (d, J = 8 Hz, 2H, Ar-H), 5.55 (dd, J = 8.8, 5.6 Hz, 1H, CH-NH-SO2), 3.41 (dd, J = 14.4, 5.6 Hz, 1H, CH2-CH-NH), 3.12 (dd, J = 14.4, 8.8 Hz, 1H, CH2-CH-NH), 2.06 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 171.2 (C=O), 145.4 (C-N=N), 142.4 (=C-SO2), 136.9 (Ar-C), 136.1 (Ar-C), 131.0 (Ar-C), 130.2 (Ar-C), 128.9 (Ar-C), 126.7 (Ar-C), 126.6 (Ar-C), 126.0 (Ar-C), 124.5 (Ar-C), 120.9 (Ar-C), 120.1 (Ar-C), 118.5 (Ar-C), 117.9 (Ar-C), 113.8 (Ar-C), 111.4 (Ar-C), 108.0 (Ar-C), 55.7 (CH-NH-SO2), 28.2 (CH2-CH-NH), 20.7 (CH3). Anal. Calcd. for C24H21N5O3S: C, 62.73; H, 4.61; N, 15.24; S, 6.98; found: C, 62.85; H, 4.68; N, 15.37; S, 7.02.
N-(2-(1H-Benzo[d][1,2,3]triazole-1-carbonyl)phenyl)-4-methylbenzenesulfonamide (3i): White microcrystals; yield: (0.70 g, 89%); m.p. 148–150 °C; 1H-NMR (400 MHz, DMSO-d6) δ 10.06 (s, 1H, NHSO2), 8.29 (t, J = 8.2 Hz, 2H, Ar-H), 7.85 (t, J = 8.2 Hz, 1H,Ar-H), 7.78 (dd, J = 7.6, 1.6 Hz, 1H, Ar-H), 7.66 (t, J = 7.2 Hz, 1H,Ar-H), 7.55–7.50 (m, 1H,Ar-H), 7.45 (d, J = 8.0 Hz, 2H, Ar-H), 7.38 (t, J = 7.6 Hz, 1H, Ar-H), 7.24 (d, J = 8.0 Hz, 2H, Ar-H), 7.04 (d, J = 8.4 Hz, 1H, Ar-H), 2.27 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 165.7 (C=O), 145.4 (C-N=N), 143.2 (C-NH), 136.2 (Ar-C), 135.3 (Ar-C), 132.6 (Ar-C), 131.3 (Ar-C), 131.3 (Ar-C), 130.5 (Ar-C), 129.4 (Ar-C), 128.6 (Ar-C), 126.6 (Ar-C), 126.4 (Ar-C), 125.5 (Ar-C), 125.2 (Ar-C), 119.9 (Ar-C), 114.3 (Ar-C), 20.8 (CH3). Anal. Calcd. for C20H16N4O3S: C, 61.21; H, 4.11; N, 14.28; S, 8.17; found: C, 61.39; H, 4.17; N, 14.45; S, 8.29.
N-(5-(1H-Benzo[d][1,2,3]triazole-1-carbonyl)-4-methylthiazol-2-yl)-3,4,5-trimethoxybenzamide (3j): White microcrystals; yield: (0.74 g, 82%); m.p. 107–110 °C; 1H-NMR (400 MHz, DMSO-d6) δ 8.29 (d, J = 8.4 Hz, 2H, Ar-H), 7.83 (t, J = 6.8 Hz, 1H, Ar-H), 7.66 (t, J = 7.0 Hz, 1H, Ar-H), 7.47 (s, 2H, Ar-H), 7.14 (s, 1H, NH), 3.86 (s, 6H, m-(OCH3)), 3.82 (s, 3H, p-(OCH3)) 1.17 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 165.80 (C=O), 152.42 (C=O), 145.2 (N-C-CH3), 142.3 (C-OCH3), 131.9 (Ar-C), 130.7 (Ar-C), 128.2 (Ar-C), 127.4 (Ar-C), 126.6 (Ar-C), 126.2 (Ar-C), 125.7 (Ar-C), 120.0 (Ar-C), 114.3 (Ar-C), 109.4 (Ar-C), 60.3 (p-OCH3), 56.2 (m-OCH3), 14.1 (CH3). Anal. Calcd. for C21H19N5O5S: C, 55.62; H, 4.22; N, 15.44; S, 7.07.; found: C, 55.78; H, 4.30; N, 15.61; S, 7.15.
(S)-N-(1-(1H-Benzo[d][1,2,3]triazole-1-yl)-1-oxopropan-2-yl)-4-methylbenzenesulfonamide (3k): White microcrystals; yield: (0.62 g, 90%); m.p. 142–144 °C; 1H-NMR (400 MHz, DMSO-d6) δ 8.78 (d, J = 7.8 Hz, 1H, NH-SO2), 8.26 (d, J = 8.2 Hz, 1H, Ar-H), 8.03 (d, J = 8.2 Hz, 1H, Ar-H), 7.78 (t, J = 7.6 Hz, 1H, Ar-H), 7.63 (t, J = 7.6 Hz, 1H, Ar-H), 7.57 (d, J = 8.0 Hz, 2H, Ar-H), 7.16 (d, J = 8.0 Hz, 2H, Ar-H), 5.35–5.28 (m, 1H, NH-CH-CH3), 2.18 (s, 3H, p-CH3), 1.45(d, J = 6.8 Hz, 3H, NH-CH-CH3). Anal. Calcd. for C16H16N4O3S: C, 55.80; H, 4.68; N, 16.27; S, 9.31; found: C, 55.96; H, 4.76; N, 16.43; S, 9.41.
(RS)-N-(1-(1H-Benzo[d][1,2,3]triazole-1-yl)-1-oxopropan-2-yl)-4-methylbenzenesulfonamide (3k, 3k′): White microcrystals; yield: (0.62 g, 90%); m.p. 142–144 °C; 1H-NMR (400 MHz, DMSO-d6) δ 8.78 (d, J = 8.4 Hz, 1H, NH-SO2), 8.27(d, J = 8.4 Hz, 1H, Ar-H), 8.03 (d, J = 8.4 Hz, 1H, Ar-H), 7.79 (t, J = 8.0 Hz, 1H, Ar-H), 7.63 (t, J = 8.0 Hz, 1H, Ar-H), 7.57 (d, J = 8.0 Hz, 2H, Ar-H), 7.16 (d, J = 8.0 Hz, 2H, Ar-H), 5.35–5.27 (m, 1H, NH-CH-CH3), 2.18 (s, 3H, p-CH3), 1.44 (d, J = 6.8 Hz, 3H NH-CH-CH3). Anal. Calcd. for C16H16N4O3S: C, 55.80; H, 4.68; N, 16.27; S, 9.31; found: C, 55.94; H, 4.71; N, 16.60; S, 9.44.
(6R,7R)-7-((R)-2-(2-(((Benzyloxy)carbonyl)amino)acetamido)-2-phenylacetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (4a): White microcrystals; yield: (0.47 g, 87%); m.p. 210–212 °C; 1H-NMR (400 MHz, DMSO-d6) δ 13.21 (s, 1H, COOH), 9.35 (d, J = 8.4 Hz, 1H, NH-CH(CO)-CH-), 8.53 (d, J = 8.4 Hz, 1H, NH-CH(CO)-Ph), 7.49–7.43 (m, 3H, NH-CO-O-, 2Ar-H), 7.35–7.24 (m, 8H, Ar-H), 5.70 (d, J = 8.4 Hz, 1H, NH-CH(CO)-Ph), 5.62 (dd, J = 8.4, 4.8 Hz, 1H, NH-CH(CO)-CH-), 5.03 (s, 2H, Ph-CH2-O-), 4.96 (d, J = 4.8 Hz, 1H, -CH-S-), 3.73 (d, J = 6.0 Hz, 2H, NH-CH2-CO-), 3.46 (d, J = 18.0 Hz, 1H, -S-CH2-), 3.28 (d, J = 18.0 Hz, 1H, -S-CH2-), 1.98 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 170.6 (CO-NH-CH), 168.7 (CO-NH-CH-Ph), 163.9 (CO-N-), 163.5 (COOH), 156.5(CO-O-CH2), 138.1 (Ar-C), 137.1 (Ar-C), 128.3 (Ar-C), 128.2 (Ar-C), 127.8 (Ar-C), 127.6 (Ar-C), 127.0 (CH3-C=C), 122.8 (-C=C-COOH), 65.4 (O-CH2-Ph), 58.4 (-CH-S-), 57.1 (CH-CH-S-), 55.4 (CH(CO)Ph), 43.3 (CH2-NH-CO-O-), 28.9 (-S-CH2-), 19.4 (CH3). Anal. Calcd. for C26H26N4O7S: C, 57.98; H, 4.87; N, 10.4; S, 5.95; found: C, 58.13; H, 4.91; N, 10.62; S, 6.04.
(6R,7R)-7-((R)-2-((S)-2-(((benzyloxy)carbonyl)amino)-3-phenylpropanamido)-2-phenylacetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (4b): White microcrystals; yield: (0.54 g, 86%); m.p. 216–218 °C; 1H-NMR (400 MHz, DMSO-d6) δ 9.37 (d, J = 8.4 Hz, 1H, NH-CH(CO)-CH-), 8.83 (d, J = 8.4 Hz, 1H, NH-CH(CO)-Ph), 7.56 (d, J = 8.4 Hz, 1H, NH-CH(CO)CH2-Ph), 7.40–7.19 (m, 15H, Ar-H), 5.74 (d, J = 8.4 Hz, 1H, NH-CH(CO)-Ph), 5.63 (dd, J = 8.4, 4.4 Hz, 1H, NH-CH(CO)-CH-), 4.96 (d, J = 4.4 Hz, 1H, -CH-S-), 4.94 (s, 2H, Ph-CH2-O-), 4.50–4.45 (m, 1H, NH-CH(CO)-CH2-Ph), 3.46 (d, J = 18.4 Hz,1H, -S-CH2-), 3.25 (d, J = 18.4 Hz, 1H, -S-CH2-), 2.96 (dd, J = 13.6, 4.0 Hz, 1H, NH-CH(CO)-CH2-Ph), 2.71 (dd, J = 13.6, 10.4 Hz, 1H, NH-CH(CO)-CH2Ph ), 1.97 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 171.2 (CO-NH-CH(CO)-Ph), 170.6 (CO-NH-CH(CO)-Ph), 163.9 (CO-N-), 163.5 (COOH), 155.8 (CO-O-CH2), 138.3 (Ar-C), 137.9 (Ar-C), 137.0 (Ar-C), 129.8 (Ar-C), 129.3(Ar-C), 128.3 (Ar-C), 128.2 (Ar-C), 128.0 (Ar-C), 127.7 (Ar-C), 127.4 (Ar-C), 126.8 (Ar-C), 126.2 (Ar-C), 125.8 (CH3-C=C), 122.7 (-C=C-COOH), 65.2 (O-CH2-Ph), 58.4 (-CH-S-), 57.2 (CH(CO)-CH-S-), 56.0 (CH(CO)Ph), 55.3 (CH(CO)-NH-CO-O-), 28.9 (-S-CH2-), 19.4 (CH-CH2-Ph), 13.9 (CH3). Anal. Calcd. for C33H32N4O7S: C, 63.04; H, 5.13; N, 8.91; S, 5.1; found: C, 63.21; H, 5.19; N, 9.04; S, 5.18.
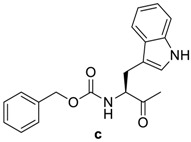
(6R,7R)-7-((R)-2-((S)-2-(((benzyloxy)carbonyl)amino)-3-(1H-indol-3-yl)propanamido)-2-phenylacetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (4c): White microcrystals; yield: (0.56 g, 84%); m.p. 168–170 °C; 1H-NMR (400 MHz, DMSO-d6) δ 13.16 (s, 1H, COOH), 10.80 (s, 1H, NH), 9.34 (d, J = 8.0 Hz, 1H, NH-CH(CO)-CH-), 8.75 (d, J = 8.0 Hz, 1H, NH-CH(CO)-Ph), 7.65 (d, J = 8.0 Hz, 1H, NH-CO-O), 7.46 (d, J = 8.4 Hz, 1H, Ar-H), 7.37–7.22 (m, 11H, Ar-H), 7.14 (s, 1H, =CH-NH), 7.05 (t, J = 7.4 Hz, 1H, Ar-H), 6.95 (t, J = 7.4 Hz, 1H, Ar-H), 5.71 (d, J = 8.0 Hz, 1H, NH-CH(CO)-Ph), 5.65 (dd, J = 8.0, 4.4 Hz, 1H, NH-CH(CO)-CH-), 4.99 (d, J = 4.4 Hz, 1H, -CH-S-), 4.95 (s, 2H, Ph-CH2-O-), 4.53–4.47 (m, 1H, CH-NH-CO-O-), 3.48 (d, J = 18.4 Hz, 1H, -S-CH2-), 3.28 (d, J = 18.4 Hz, 1H, -S-CH2-), 3.09 (dd, J = 14.0, 4.4 Hz, 1H, CH2-CH-NH-CO-O-), 2.91 (dd, J = 14.0, 9.6 Hz, 1H, CH2-CH-NH-CO-O-), 1.99 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 171.6 (CO-NH-CH(CO)-Ph), 170.6 (CO-NH-CH(CO)-Ph), 163.9 (CO-N-), 163.5 (COOH), 155.8 (CO-O-CH2), 138.2 (Ar-C), 137.0 (Ar-C), 136.1 (Ar-C), 129.8(Ar-C), 128.3 (Ar-C), 128.2 (Ar-C), 127.7 (Ar-C), 127.6 (Ar-C), 127.4 (Ar-C), 127.3 (Ar-C), 126.9(Ar-C), 124.0 (CH3-C=C), 122.8 (-C=C-COOH), 120.8 (Ar-C), 118.7 (Ar-C), 118.2 (Ar-C), 111.2 (Ar-C), 109.9 (Ar-C), 65.3 (O-CH2-Ph), 58.5 (-CH-S-), 57.2 (CH(CO)-CH-S-), 55.5 (CH(CO)Ph), 55.4 (CH(CO)-NH-CO-O-), 28.9 (-S-CH2-), 27.9 (CH2-CH-NH-(CO)-O-), 19.4 (CH3). Anal. Calcd. for C35H33N5O7S: C, 62.96; H, 4.98; N, 10.49; S, 4.8; found: C, 63.14; H, 5.06; N, 10.67; S, 4.91.
(6R,7R)-7-((R)-2-benzamido-2-phenylacetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (4d): Yellow microcrystals; yield: (0.38 g, 84%); m.p. 132–134 °C; 1H-NMR (400 MHz, DMSO-d6) δ 13.11 (s, 1H, COOH), 9.27 (d, J = 8.0 Hz, 1H, NH-CH(CO)-CH-), 8.86 (d, J = 8.0 Hz, 1H, NH-CH(CO)-Ph), 7.92 (d, J = 7.2 Hz, 2H, Ar-H), 7.59–7.44 (m, 5H, Ar-H), 7.37–7.28 (m, 3H, Ar-H), 5.89 (d, J = 8.0 Hz, 1H, NH-CH(CO)-Ph), 5.66 (dd, J = 8.0, 4.4 Hz, 1H, NH-CH(CO)-CH-), 5.00 (d, J = 4.4 Hz, 1H, -CH-S-), 3.48 (d, J = 18.4 Hz, 1H, -S-CH2-), 3.28 (d, J = 18.4 Hz, 1H, -S-CH2-), 1.99 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 170.7 (CO-NH-CH(CO)-Ph), 166.2 ((CO)-Ph), 163.9 (CO-N-), 163.4 (COOH), 137.7 (Ar-C), 133.8 (Ar-C), 132.8 (Ar-C), 131.4 (Ar-C), 129.7 (Ar-C), 129.2 (Ar-C), 128.5 (Ar-C), 128.2 (Ar-C), 128.1 (Ar-C), 127.7 (Ar-C), 127.5 (CH3-C=C), 122.7 (-C=C-COOH), 58.5 (-CH-S-), 57.1 (CH(CO)Ph), 56.5 (CH(CO)-CH-S-), 28.9 (-S-CH2-), 19.3 (CH3). Anal. Calcd. for C23H21N3O5S: C, 61.18; H, 4.69; N, 9.31; S, 7.1; found: C, 61.42; H, 4.76; N, 9.43; S, 7.19.
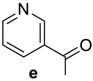
(6R,7R)-3-methyl-7-((R)-2-(nicotinamido)-2-phenylacetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (4e): White microcrystals; yield: (0.37 g, 82%); m.p. 200–202 °C; 1H-NMR (400 MHz, DMSO-d6) δ 9.29 (d, J = 8.4 Hz, 1H, NH-CH(CO)-CH-), 9.21 (d, J = 8.0 Hz, 1H, NH-CH(CO)-Ph), 9.04 (s, 1H, Ar-H), 8.70 (dd, J = 4.8, 1.6 Hz, 1H, Ar-H), 8.27–8.24 (m, 1H, Ar-H), 7.54 (d, J = 6.8 Hz, 2H, Ar-H), 7.51–7.48 (m, 1H, Ar-H), 7.38–7.29 (m, 3H, Ar-H ), 5.90 (d, J = 8.0 Hz, 1H, NH-CH(CO)-Ph), 5.59 (dd, J = 8.4, 4.8 Hz, 1H, NH-CH(CO)-CH-), 4.94 (d, J = 4.4 Hz, 1H, -CH-S-), 3.42 (d, J = 18.4 Hz, 1H, -S-CH2-), 3.18 (d, J = 18.4Hz, 1H, -S-CH2-), 1.95 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 171.1 (CO-NH-CH(CO)-Ph), 165.5 (CO-NH-CH(CO)-Ph), 164.9 (CO-N-), 163.8 (COOH), 152.5 (C=N-C=C-CO), 149.4 (C=N-C=C-CO), 138.0(Ar-C), 136.0 (Ar-C), 130.0 (Ar-C), 128.8 (Ar-C), 128.7(Ar-C), 128.3 (Ar-C), 128.1 (Ar-C), 127.9 (CH3-C=C), 123.8 (-C=C-COOH), 58.8 (-CH-S-), 57.6 (CH(CO)-CH-S-), 57.1 (NH-CH(CO)-Ph), 29.2 (-S-CH2-), 19.8 (CH3). Anal. Calcd. for C22H20N4O5S: C, 58.40; H, 4.46; N, 12.38; S, 7.09; found: C, 58.63; H, 4.53; N, 12.61; S, 7.21.
(6R,7R)-3-methyl-7-((R)-2-(3-nitrobenzamido)-2-phenylacetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (4f): Yellow microcrystals; yield: (0.43 g, 87%); m.p. 226–228 °C; 1H-NMR (400 MHz, DMSO-d6) δ 13.44 (s, 1H, COOH), 9.40 (d, J = 7.6 Hz, 1H, NH-CH(CO)-Ph), 9.33 (d, J = 8.0 Hz, 1H, NH-CH(CO)-CH- ), 8.76–8.62 (m, 1H, Ar-H), 8.48–8.46 (m, 1H, Ar-H), 8.40–8.34 (m, 2H, Ar-H), 7.83–7.75 (m, 2H, Ar-H), 7.54 (d, J = 7.2 Hz, 1H, Ar-H), 7.39–7.30 (m, 2H, Ar-H), 5.91 (d, J = 7.6 Hz, 1H, NH-CH(CO)-Ph), 5.67 (dd, J = 8.0, 4.8 Hz, 1H, NH-CH(CO)-CH-), 5.00 (d, J = 4.8 Hz, 1H, -CH-S-), 3.48 (d, J = 18.0 Hz, 1H, -S-CH2-), 3.28 (d, J = 18.0 Hz, 1H, -S-CH2-), 1.99 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 170.6 (CO-NH-CH(CO)-Ph), 165.5 (CO-NH-CH(CO)-Ph), 164.5 (CO-N-), 163.6 (COOH), 147.60 (C-NO2), 137.4(Ar-C), 135.4 (Ar-C), 134.4 (Ar-C), 132.5 (Ar-C), 130.5 (Ar-C), 129.9 (Ar-C), 128.3 (Ar-C), 127.3 (CH3-C=C), 126.1 (Ar-C), 123.7(Ar-C), 122.7(-C=C-COOH), 58.6 (-CH-S-), 57.2 (NH-CH(CO)-Ph), 56.9 (CH(CO)-CH-S-), 28.9 (-S-CH2-), 19.4 (CH3). Anal. Calcd. for C23H20N4O7S: C, 55.64; H, 4.06; N, 11.28; S, 6.46; found: C, 55.78; H, 4.03; N, 11.52; S, 6.52.
(6R,7R)-3-methyl-8-oxo-7-((R)-2-phenyl-2-(3,4,5-trimethoxybenzamido)acetamido)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (4g): Yellow microcrystals; yield: (0.49 g, 91%); m.p. 197–199 °C; 1H-NMR (400 MHz, DMSO-d6) δ 9.26 (d, J = 8.4 Hz, 1H, NH-CH(CO)-CH-), 8.92 (d, J = 8.0 Hz, 1H, NH-CH(CO)-Ph), 7.52 (d, J = 7.2 Hz, 2H, Ar-H ), 7.37–7.29 (m, 3H, Ar-H), 7.26 (s, 2H, Ar-H), 5.92 (d, J = 8.0 Hz, 1H, NH-CH(CO)-Ph), 5.57 (dd, J = 8.4, 4.4 Hz, 1H, NH-CH(CO)-CH-), 4.92 (d, J = 4.4 Hz, 1H, -CH-S-), 3.83 (s, 6H, M-OCH3), 3.70 (s, 3H, P-OCH3), 3.39 (d, J = 20.0 Hz, 1H, -S-CH2-), 3.14 (d, J = 20.0 Hz, 1H, -S-CH2-), 1.93 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 170.8 (CO-NH-CH(CO)-Ph), 165.8 (CO-NH-CH(CO)-Ph), 164.0 (CO-N-), 163.5 (COOH), 152.5 (m-OCH3-C=), 140.2 (p-OCH3-C=), 137.8 (Ar-C), 129.7 (Ar-C), 129.0 (Ar-C), 128.2 (Ar-C), 127.7 (Ar-C), 127.6 (CH3-C=C), 122.7 (-C=C-COOH), 105.4 (Ar-C), 60.1 (-CH-S-), 58.6 (P-OCH3), 57.2 (NH-CH(CO)-Ph), 56.7 (CH(CO)-CH-S-), 56.1 (M-OCH3), 28.9 (-S-CH2-), 19.4 (CH3). Anal. Calcd. for C26H27N3O8S: C, 57.66; H, 5.03; N, 7.76; S, 5.92; found: C, 57.94; H, 5.11; N, 7.88; S, 6.11.
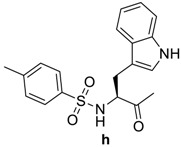
(6R,7R)-7-((R)-2-((S)-3-(1H-indol-3-yl)-2-(4-methylphenylsulfonamido)propanamido)-2-phenylacetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (4h): Brown microcrystals; yield: (0.59 g, 86%); m.p. 125–127 °C; 1H-NMR (400 MHz, DMSO-d6) δ 12.56 (s, 1H, COOH), 10.76 (s, 1H, NH), 9.32 (d, J = 8.0 Hz, 1H, NH-CH(CO)-CH-), 8.70 (d, J = 8.0 Hz, 1H, NH-CH(CO)-Ph), 8.09 (d, J = 9.2 Hz, 1H, Ar-H), 7.91 (d, J = 8.4 Hz, 1H, Ar-H), 7.46 (d, J = 8.0 Hz, 2H, Ar-H), 7.40 (d, J = 8.0 Hz, 1H, Ar-H), 7.30–7.24 (m, 4H, 3 Ar-H, 1H, NH-SO2 ), 7.17 (d, J = 8.0 Hz, 2H, Ar-H), 7.08–7.02 (m, 3H, Ar-H), 6.92 (t, J = 7.6, 1H, Ar-H), 5.68 (dd, J = 8.0, 4.0 Hz, 1H, NH-CH(CO)-CH-), 5.58 (d, J = 8.0 Hz, 1H, NH-CH(CO)-Ph), 4.98 (d, J = 4.0 Hz, 1H, -CH-S-), 3.88 (dd, J = 8.4, 6.8 Hz, 1H, CH-NH-SO2), 3.40 (d, J = 8.0 Hz, 1H, -S-CH2-), 3.37 (d, J = 8.0 Hz, 1H, -S-CH2-), 3.04 (dd, J = 14.4, 6.8 Hz, 1H, CH2-CH-NH-SO2), 2.84 (dd, J = 14.4, 8.4 Hz, 1H, CH-NH-SO2), 2.31 (s, 3H, CH3), 2.00 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 172.6 (CO-NH-CH(CO)-Ph), 170.3 (CO-NH-CH(CO)-Ph), 163.9 (CO-N-), 163.4 (COOH), 142.1 (SO2-C=), 137.9 (Ar-C), 136.0 (Ar-C), 129.0 (Ar-C), 128.9 (Ar-C), 128.0 (Ar-C), 126.9 (Ar-C), 126.6 (Ar-C), 126.2 (Ar-C), 126.0 (CH3-C=C), 123.9 (-C=C-COOH), 120.7 (Ar-C), 120.6 (Ar-C), 118.3 (Ar-C), 117.7 (Ar-C), 111.3 (Ar-C), 108.8 (Ar-C), 64.83 (-CH-S-), 57.18 (CH(CO)-CH-S-), 56.76 (NH-CH(CO)-Ph), 56.48 (CH-NH-SO2), 28.61 (CH2-CH-NH-SO2), 28.22 (-S-CH2-), 20.88 (CH3), 15.07 (CH3). Anal. Calcd. for C34H33N5O7S2: C, 59.37; H, 4.84; N, 10.18; S, 9.32; found: C, 59.51; H, 4.89; N, 10.26; S, 9.46.
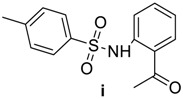
(6R,7R)-3-methyl-7-((R)-2-(2-(4-methylphenylsulfonamido)benzamido)-2-phenylacetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid(4i): White microcrystals; yield: (0.51 g, 82%); m.p. 137–139 °C; 1H-NMR (400 MHz, DMSO-d6) δ 13.16 (s, 1H, COOH), 11.07 (s, 1H, NHSO2), 9.32 (d, J = 8.0 Hz, 1H, NH-CH(CO)-CH-), 9.11 (d, J = 8.0 Hz, 1H, NH-CH(CO)-Ph), 7.84 (d, J = 8.4 Hz, 1H, Ar-H), 7.67 (d, J = 8.4 Hz, 1H, Ar-H ), 7.59–7.26 (m, 10H, Ar-H), 7.15–7.10 (m, 1H, Ar-H), 5.81 (d, J = 8.0 Hz, 1H, NH-CH(CO)-Ph), 5.68 (dd, J = 8.0, 4.0 Hz, 1H, NH-CH(CO)-CH-), 5.01 (d, J = 4.0 Hz, 1H, -CH-S-), 3.49 (d, J = 18.4 Hz, 1H, -S-CH2-), 3.28 (d, J = 18.4 Hz, 1H, -S-CH2-), 2.30 (s, 3H, CH3), 1.98 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 170.1 (CO-NH-CH(CO)-Ph), 167.7 (CO-NH-CH(CO)-Ph), 163.8 (CO-N-), 163.4 (COOH), 137.0 (SO2-C=), 129.7 (Ar-C), 129.7 (Ar-C), 129.5 (Ar-C), 129.4 (Ar-C), 128.2 (Ar-C), 127.8 (Ar-C), 127.7 (Ar-C), 126.7(Ar-C), 126.7 (CH3-C=C), 122.7 (-C=C-COOH), 121.2 (Ar-C), 120.0 (Ar-C), 58.5 (-CH-S-), 57.1 (NH-CH(CO)-Ph), 56.6 (CH(CO)-CH-S-), 28.8 (-S-CH2-), 20.9 (CH3), 19.3 (CH3). Anal. Calcd. for C30H28N4O7S2: C, 58.05; H, 4.55; N, 9.03; S, 10.33; found: C, 58.31; H, 4.62; N, 9.14; S, 10.52.
(6R,7R)-3-methyl-7-((R)-2-(4-methyl-2-(3,4,5-trimethoxybenzamido)thiazole-5-carboxamido)-2-phenylacetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (4j): Yellow microcrystals; yield: (0.61 g, 89%); m.p. 179–181 °C; 1H-NMR (400 MHz, DMSO-d6) δ 9.28 (d, J = 8.0 Hz, 1H, NH-CH(CO)-CH-), 8.88 (d, J = 8.0 Hz, 1H, NH-CH(CO)-Ph), 7.56 (s, 1H, NH), 7.52 (d, J = 7.2 Hz, 2H, Ar-H), 7.38–7.30 (m, 3H, Ar-H), 7.26 (s, 2H, Ar-H), 5.91 (d, J = 8.0 Hz, 1H, NH-CH(CO)-Ph), 5.67 (dd, J = 8.0, 4.0 Hz, 1H, NH-CH(CO)-CH-), 5.01 (d, J = 4.0Hz, 1H, -CH-S-), 3.83 (s, 6H, m-OCH3), 3.71 (s, 3H, p-OCH3), 3.48 (d, J = 18.4 Hz, 1H, -S-CH2-), 3.28 (d, J = 18.4 Hz, 1H, -S-CH2-), 1.99 (s, 3H, CH3), 1.91 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 170.8 (CO-NH-CH(CO)-Ph), 165.7 (CO), 164.0 (CO), 163.4 (COOH), 152.8 (Ar-C), 152.4 (Ar-C), 140.2 (Ar-C), 137.7 (Ar-C), 129.8 (Ar-C), 128.9 (Ar-C), 128.2 (Ar-C), 127.6 (Ar-C), 127.6 (CH3-C=C), 122.7 (-C=C-COOH), 122.7 (Ar-C), 105.4 (Ar-C), 60.0 (-CH-S-), 58.5 (p-OCH3), 57.2 (CH(CO)-CH-S-), 56.7 (NH-CH(CO)-Ph), 56.0 (m-OCH3), 28.9 (-S-CH2-), 21.0 (CH3), 19.3 (CH3). Anal. Calcd. for C31H31N5O9S2: C, 54.62; H, 4.58; N, 10.27; S, 9.41; found: C, 54.89; H, 4.61; N, 10.38; S, 9.5.
(6R,7R)-3-methyl-7-((R)-2-(2-((S)-4-methylphenylsulfonamido)propanamido)-2-phenylacetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (4k): White microcrystals; yield: (0.53 g, 93%); m.p. 234–236 °C; 1H-NMR (400 MHz, DMSO-d6) δ 9.33 (d, J = 8.0 Hz, 1H, NH-CH(CO)-CH-), 8.57 (d, J = 8.0 Hz, 1H, NH-CH(CO)-Ph), 7.99 (s, 1H, NH-SO2), 7.65 (d, J = 7.6 Hz, 2H, Ar-H), 7.39 (d, J = 7.6 Hz, 2H, Ar-H), 7.35–7.23 (m, 5H, Ar-H), 5.65 (dd, J = 8.0, 4.4 Hz, 1H, NH-CH(CO)-CH-), 5.57 (d, J = 8.0 Hz, 1H NH-CH(CO)-Ph), 4.95 (d, J = 4.4 Hz, 1H, -CH-S-), 4.07–3.95 (m, 1H, NH-CH-CH3), 3.44 (d, J = 18.0 Hz, 1H, -S-CH2-), 3.23 (d, J = 18.0 Hz, 1H, -S-CH2-), 2.39 (s, 3H, p-CH3), 1.97 (s, 3H, CH3), 1.03 (d, J = 6.8 Hz, 3H, NH-CH-CH3).13C-NMR (100 MHz, DMSO-d6) δ 170.8 (CO-NH-CH(CO)-Ph), 170.4 (CO), 163.9 (CO), 163.4 (CO), 142.5 (Ar-C), 138.1(Ar-C), 137.8(Ar-C), 129.4 (Ar-C), 128.2 (Ar-C), 127.6 (Ar-C), 126.9 (Ar-C), 126.6 (Ar-C), 126.5 (CH3-C=C), 122.7 (-C=C-COOH), 58.3 (-CH-S-), 57.1 (CH(CO)-CH-S-), 55.3 (NH-CH(CO)-Ph), 51.7 (NH-CH-CH3), 28.9 (-S-CH2-), 21.0 (p-CH3), 19.4 (CH3), 18.9 (NH-CH-CH3). Anal. Calcd. for C26H28N4O7S2: C, 54.53; H, 4.93; N, 9.78; S, 11.20; found: C, 54.80; H, 4.96; N, 9.88; S, 11.30.
(6R,7R)-3-methyl-7-((R)-2-(2-((RS)-4-methylphenylsulfonamido)propanamido)-2-phenylacetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (4k, 4k′): White microcrystals; yield: (0.51 g, 89%); m.p. 224–227 °C; 1H-NMR (400 MHz, DMSO-d6) δ 9.35 (d, J = 7.6 Hz, 1H, NH-CH(CO)-CH-), 8.57 (d, J = 7.6 Hz, 1H, NH-CH(CO)-Ph), 8.01 (d, J = 8 Hz, 1H, NH-SO2), 7.72–7.58 (m, 2H, Ar-H), 7.45–7.22 (m, 7H, Ar-H), 5.73–5.62 (m, 1H, NH-CH(CO)-CH-), 5.57 (d, J = 8.0 Hz, 1H NH-CH(CO)-Ph), 5.46 (d, J = 7.6 Hz, 1H, -CH-S-), 4.07–3.92 (m, 1H, NH-CH-CH3), 3.45 (d, J = 17.2 Hz, 1H, -S-CH2-), 3.27 (d, J = 17.2 Hz, 1H, -S-CH2-), 2.36 (d, J = 15.6, 3H, p-CH3), 1.98 (s, 3H, CH3), 1.07–1.03 (m, 3H, NH-CH-CH3). 13C-NMR (100 MHz, DMSO-d6) δ 170.8 (CO-NH-CH(CO)-Ph (k)), 170.7 (CO-NH-CH(CO)-Ph (k′)), 170.4 (CO), 163.9 (CO), 163.4 (CO), 142.6 (Ar-C (k′)), 142.5 (Ar-C (k)), 138.2(Ar-C (k′)), 138.1(Ar-C (k)), 137.8 (Ar-C), 129.9 (Ar-C (k)), 129.8 (Ar-C (k′)), 129.4 (Ar-C (k)), 129.3 (Ar-C (k′)), 128.2 (Ar-C), 126.9 (Ar-C), 126.6 (Ar-C), 126.5 (CH3-C=C), 122.7 (-C=C-COOH), 58.4 (-CH-S-), 57.2 (CH(CO)-CH-S-), 55.3 (NH-CH(CO)-Ph), 51.7 (NH-CH-CH3 (k)), 51.5 (NH-CH-CH3 (k′)), 28.9 (-S-CH2-), 21.0 (p-CH3 (k)), 20.9 (p-CH3 (k′)), 19.4 (CH3), 19.1 (NH-CH-CH3 (k)), 18.9 (NH-CH-CH3(k)). Anal. Calcd. for C26H28N4O7S2: C, 54.53; H, 4.93; N, 9.78; S, 11.20; found: C, 54.77; H, 4.98; N, 9.86; S, 11.32.
2.2. Antimicrobial Activity
2.2.1. Sensitivity Testing (Agar Diffusion Method)
Compounds (4a–j) and Cephalexin (Ceporex®) were dissolved in DMSO in the concentration 1 mg/mL. The microorganisms were cultivated overnight, and optical densities were adjusted 0.2–0.8 and diluted 1:100. Constructed compounds were tested against standard microbial strains in Mueller Hinton agar; Ceporex® and DMSO were tested in parallel as a reference and negative control, respectively. Diameters of inhibition zones were measured in mm.
2.2.2. Minimum Inhibitory Concentration MIC (Broth Dilution Method)
Compounds (4a-j) and Cephalexin (Ceporex®) were dissolved in DMSO in the concentration 2 mg/mL as a stock solution and then diluted two-fold serially to 1 µg/mL in a Mueller Hinton broth (Sigma-Aldrich, Darmstadt, Germany). The microorganisms were cultivated overnight, and optical densities were adjusted to 0.2–0.8 and diluted 1:100. The constructed compounds were tested against the standard microbial strains; Ceporex® and DMSO were tested in parallel as a reference and negative control, respectively.
3. Results and Discussion
3.1. Chemistry
N-tosyl-l-tryptophan (2h), N-tosylanthranilic acid (2i), N-tosyl-l-alanine (2k), and N-tosyl-dl-alanine (2k, 2k′) were prepared according to the reported procedure by the reaction of the corresponding amino acid with p-toluenesulfonyl chloride in the presence of triethylamine TEA [18]. The presence of the thiazole moiety in many cephalosporin antibiotics motivated us to synthesize 4-methyl-2-(3,4,5-trimethoxy benzamido)thiazole-5-carboxylic acid (2j) (Scheme 1) [19]. Condensation of thiourea and ethyl-2-chloroacetoacetate gave ethyl 2-amino-4-methylthiazole-5-carboxylate (5) in 95% yield [20]. Compound (5) was then hydrolyzed using sodium hydroxide to give the free carboxylic acid (6) [21,22]. Coupling of (6) with N-(3,4,5-trimethoxybenzoyl)benzotriazole (3g) afforded 4-methyl-2-(3,4,5-trimethoxybenzamido)thiazole-5-carboxylic acid (2j) in 90% yield.
Scheme 1.
Synthesis of 4-methyl-2-(3,4,5-trimethoxybenzamido)thiazole-5-carboxylic acid (2j).
N-acylbenzotriazoles 3a–k′ (Scheme 2, Table 1) were prepared in 82%–96% yields via the reaction of carboxylic acids 2a–k′ with four equivalents of 1H-benzotriazole and one equivalent of SOCl2 in CH2Cl2 at 25 °C for 3 h [23]. Subsequently, N-acylbenzotriazoles 3a–j and the racemic mixture 3k, 3k′ were stirred with cephalexin sodium in acetonitrile for 6–20 h at 25 °C to afford N-acylcephalexins 4a–j and the diastereomeric mixture 4k, 4k′ in 82%–92% yield (Scheme 2, Table 1). Novel N-acylbenzotriazoles 3h–k′ and N-acylcephalexins 4a–k′ were characterized by 1H-NMR, 13C-NMR, and elemental analysis. The diastereomeric mixture 4k, 4k′ was prepared to confirm that the original chirality was maintained during the reaction of cephalexin with chiral N-acylbenzotriazoles under the current reaction conditions. The presence of strong absorption at 1764–1770 cm−1 in the IR spectra of 4a,b,d and g confirmed that the β-lactam ring was not cleaved during coupling with N-acylbenzotriazoles.
Scheme 2.
Synthesis of target compounds N-acylcephalexins (4a–k′).
Table 1.
RCO-, melting points (m.p.), and yields of N-acylbenzotriazoles 3a-k′ and N-acylcephalexines 4a-k′.
| RCO-a–k′ | 3a–k′ Yield % | 3a–k′m.p. (°C) m.p. (°C) [Lit.] | Time/h 4a–k′ | 4a–k′ Yield % | 4a–k′ m.p. (°C) |
|---|---|---|---|---|---|
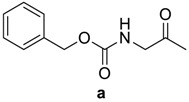 |
94 | 107–109 (106–108) [24] | 10 | 87 | 210–212 |
 |
95 | 150–153 (151–152) [24] | 12 | 86 | 216–218 |
 |
95 | 99–101 (100–101) [25] | 12 | 84 | 168–170 |
 |
94 | 111–112 (112–113) [14] | 9 | 84 | 132–134 |
 |
94 | 101–102 (101–102) [15] | 8 | 82 | 200–202 |
 |
90 | 156–157 (155–156) [16] | 10 | 87 | 226–228 |
 |
96 | 125–127 (126–128) [17] | 6 | 91 | 197–199 |
 |
87 | 175–176 | 12 | 86 | 125–127 |
 |
89 | 148–150 | 12 | 82 | 137–139 |
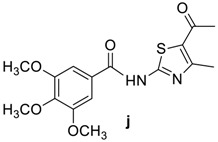 |
82 | 107–110 | 20 | 89 | 179–181 |
 |
90 | 142–144 | 14 | 93 | 234–236 |
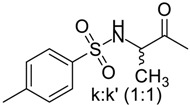 |
90 | 142–144 | 14 | 89 | 224–227 |
3.2. Antimicrobial Activity
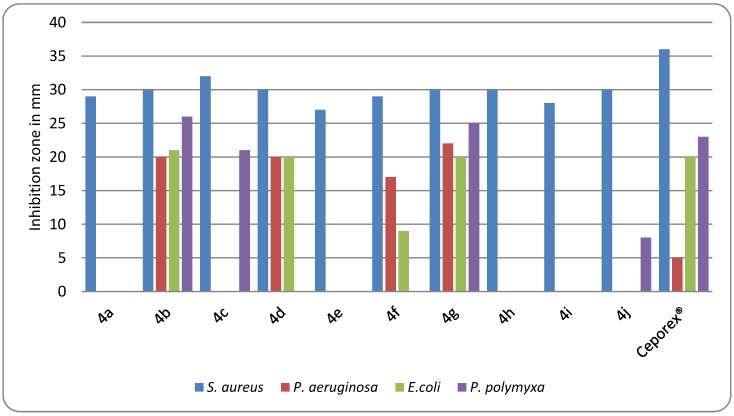
Synthesized compounds 4a–j were tested in vitro for their antimicrobial activity against Staphylococcus aureus (ATCC 6538), Pseudomonas aeruginosa (ATCC 27853), Escherichia coli (ATCC 10536), Paenibacillus polymyxa (ATCC 842), and Candida albicans (ATCC 10231). Cephalexin (Ceporex®) manufactured by: SmithKline Beecham, Harm-Giza, Egypt was used as a reference compound. Sensitivity testing (agar diffusion method) and MIC (broth microdilution method) were performed according to the reported method [26]. The antimicrobial activity of the test compounds as well as cephalexin sodium is depicted in Table 2 and Table 3 and (Figure 2).
Table 2.
Inhibition zones measured in mm for N-acylcephalexins 4a–j and cephalexin (Ceporex®).
| Target | Staphylococcus Aureus | Pseudomonas Aeruginosa | E. coli | Paenibacillus Polymyxa |
|---|---|---|---|---|
| 4a | 27 | - | - | - |
| 4b | 29 | 17 | 9 | - |
| 4c | 30 | 22 | 20 | 25 |
| 4d | 29 | - | - | - |
| 4e | 30 | 20 | 20 | - |
| 4f | 32 | - | - | 21 |
| 4g | 30 | 20 | 21 | 26 |
| 4h | 30 | - | - | - |
| 4i | 28 | - | - | - |
| 4j | 30 | - | - | 8 |
| Ceporex | 36 (S) | 5 (R) | 20 (S) | 23 (S) |
S: sensitive; R: resistance
Table 3.
Broth dilution method measured in µg/mL for N-acylcephalexins 4a–j and cephalexin (Ceporex®).
| Target | Staphylococcus Aureus | Pseudomonas Aeruginosa | E. coli | Paenibacillus Polymyxa |
|---|---|---|---|---|
| 4a | 4 | - | - | - |
| 4b | 2 | 16 | - | - |
| 4c | ≤1 | 8 | 8 | 4 |
| 4d | 2 | - | - | - |
| 4e | ≤1 | 8 | 8 | - |
| 4f | ≤1 | - | - | 4 |
| 4g | ≤1 | 8 | 4 | 4 |
| 4h | ≤1 | - | - | - |
| 4i | 2 | - | - | - |
| 4j | ≤1 | - | - | - |
| Ceporex | ≤1 (S) | - | 8 (S) | 4 (S) |
Figure 2.
Inhibition measured in mm.
All the synthesized compounds 4a–j showed antimicrobial activity against S.aureus comparable to Ceporex®. Furthermore, compounds 4c,e,g exhibited bactericidal activity against E. coli (G-ve bacteria) and P. polymyxa (Gm+ve anaerobic bacteria) close to that of Ceporex®. The most interesting result was the strong antimicrobial activity of 4b,c,e,g against the resistant strain of P.aeruginosa which was significantly higher than that of Ceporex®. N-nicotinylcephalexin (4c) and N-(3,4,5-trimethoxybenzoyl)cephalexin (4g) enjoyed a broader spectrum of antibacterial activity than cephalexin.
4. Conclusions
In conclusion, we have utilized a mild and efficient protocol for acylating cephalexin as an example of cephalosporins containing amino nucleophiles using N-acylbenzotriazole methodology. The protocol described herein enabled the modulation of the antibacterial activity of cephalosporins. The preliminary antimicrobial susceptibility testing of the novel synthesized targets led to two promising cephalosporin antimicrobials with a broader spectrum of activity.
Acknowledgements
The authors express no acknowledgement.
Supplementary Materials
Detailed NMR spectra are available online at http://www.mdpi.com/2218-0532/84/3/484/s1.
Author Contributions
N.E.A.-D., K.A.A. and T.S.I. conceived and designed the experiments; E.H.A.-A. and N.E.A.-D. analyzed the data for the synthesis section; K.A.A. performed the synthesis experiments; N.E.A.-D., T.S.I. and E.H.A.-A. wrote the paper, W.A.H. performed the antibacterial screening experiments.
Conflicts of Interest
The authors have no conflicts of interest.
References
- 1.Zucca M., Savoia D. The Post-Antibiotic Era: Promising Developments in the Therapy of Infectious Diseases. Int. J. Biomed. Sci. 2010;6:77–86. [PMC free article] [PubMed] [Google Scholar]
- 2.Biek M., Critchley I.A., Riccobene T.A., Thye D.A. Ceftaroline fosamil: A novel broad-spectrum cephalosporin with expanded anti-Gram-positive activity. J. Antimicrob. Chemother. 2010;65:iv9–iv16. doi: 10.1093/jac/dkq251. [DOI] [PubMed] [Google Scholar]
- 3.Singh S.B., Barrett J.F. Empirical antibacterial drug discovery—Foundation in natural products. Biochem. Pharmacol. 2006;71:1006–1015. doi: 10.1016/j.bcp.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Tareq F.S., Lee M.A., Lee H.S., Lee Y.J., Lee J.S., Hasan C.M., Islam M.T., Shin H.J. Gageotetrins A–C., Noncytotoxic Antimicrobial Linear Lipopeptides from a Marine Bacterium Bacillus subtilis. Org. Lett. 2014;16:928–931. doi: 10.1021/ol403657r. [DOI] [PubMed] [Google Scholar]
- 5.Coates A.R., Halls G., Hu Y. Novel classes of antibiotics or more of the same? Br. J. Pharmacol. 2011;163:184–194. doi: 10.1111/j.1476-5381.2011.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heyningen E.V. The Chemistry of Cephalosporin Antibiotics. III. Acylation of Cephalosporadesates. J. Med. Chem. 1965;8:22–25. doi: 10.1021/jm00325a005. [DOI] [PubMed] [Google Scholar]
- 7.Alwan S.M. Synthesis and Preliminary Antimicrobial Activities of New Arylideneamino-1,3,4-thiadiazole-(thio/dithio)-acetamido Cephalosporanic Acids. Molecules. 2012;17:1025–1038. doi: 10.3390/molecules17011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hecker S.J., Calkins T., Price M.E., Huie K., Chen S., Glinka T.W., Dudley M.N. Prodrugs of Cephalosporin RWJ-333441 (MC-04,546) with Improved Aqueous Solubility. Antimicrob. Agents Chemother. 2003;47:2043–2046. doi: 10.1128/AAC.47.6.2043-2046.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe S., Mizukami S., Akimoto Y., Hori Y., Kikuchi K. Intracellular Protein Labeling with Prodrug-Like Probes Using a Mutant β-Lactamase Tag. Chem. A Eur. J. 2011;17:8342–8349. doi: 10.1002/chem.201100973. [DOI] [PubMed] [Google Scholar]
- 10.Daloia E., Lim G., Melton J., Roubie J.A. Convenient Method for the Preparation of (Z)-(2-Aminothiazol-4-yl)-2-methoxyimino-acetylchloride Hydrochloride. Synth. Commun. 1993;23:2617–2622. doi: 10.1080/00397919308012597. [DOI] [Google Scholar]
- 11.Saud M.D., Muna I.K., Huda A.H., Ikbal R.H. Synthesis of New Derivatves of β-Lactam Antibiotics. Int. J. PharmTech Res. 2014;6:1018–1027. [Google Scholar]
- 12.Katritzky A.R., Suzuki K., Wang Z. Acylbenzotriazoles as Advantageous N-, C-, S-, and O-Acylating Agents. Synlett. 2005:1656–1665. doi: 10.1055/s-2005-871551. [DOI] [Google Scholar]
- 13.Ibrahim M.A., Panda S.S., Birs A.S., Serrano J.C., Gonzalez C.F., Alamry K.A., Katritzky A.R. Synthesis and antibacterial evaluation of amino acid–antibiotic conjugates. Bioorg. Med. Chem. Lett. 2014;24:1856–1861. doi: 10.1016/j.bmcl.2014.01.065. [DOI] [PubMed] [Google Scholar]
- 14.Katritzky A.R., Vakulenko A., Jain R. The preparation of N-acylbenzotriazoles from aldehydes. Arkivoc. 2003;14:131–139. [Google Scholar]
- 15.El-Nachef C., Bajaj K., Koblick J., Katritzky A.R. Microwave-Assisted Formation of Peptide–Vitamin Conjugates. Eur. J. Org. Chem. 2012;23:4412–4419. doi: 10.1002/ejoc.201200323. [DOI] [Google Scholar]
- 16.Wet-osot S., Duangkamol C., Pattarawarapan M., Phakhodee W. Facile synthesis of N-acylbenzotriazoles from carboxylic acids. Monatshefte Chem. 2015;146:959–963. doi: 10.1007/s00706-014-1408-1. [DOI] [Google Scholar]
- 17.Agarwal P.K., Dathi M.D., Saifuddin M., Kundu B. Engineering of indole-based tethered biheterocyclic alkaloid meridianin into β-carboline-derived tetracyclic polyheterocycles via amino functionalization/6-endo cationic π-cyclization. Beilstein J. Org. Chem. 2012;8:1901–1908. doi: 10.3762/bjoc.8.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simsek S., Horzella M., Kalesse M. Oxazaborolidinone-Promoted Vinylogous Mukaiyama Aldol Reactions. Org. Lett. 2007;9:5637–5639. doi: 10.1021/ol702640w. [DOI] [PubMed] [Google Scholar]
- 19.Chantot J.F., Bryskier A. HR 810 (cefpirome). Experimental evaluation of the in vitro and in vivo antibiotic activity of a new amino-2-thiazole methoxy- imino cephalosporin. Pathol. Biol. 1985;33:482–486. [PubMed] [Google Scholar]
- 20.Oniga S., Oniga O., Chirtoc I., Ionescu M., Tiperciuc B., Ghiran D. Activitatea antimicrobiană a unor 3-N-acetil-5[2′-(acetil-amino)-4′metil- 5′tiazolil]-2-aril-Δ4-1,3,4-oxadiazoline. Farmacia. 2005;53:28–35. [Google Scholar]
- 21.Gharat L.A., Narayana L., Thomas A., Khairatkar N., Shah D.M. Thiazole Derivatives as Stearoyl CoA Desaturase Inhibitors. WO2010007482 A3. 2010 Dec 2;
- 22.Fu J., Hou D., Kamboj R. Aminothiazole Derivatives as Human Stearoyl-Coa Desaturase Inhibitors. WO2007130075A1. 2008 Jan 24;
- 23.Katritzky A.R., Abo-Dya N.E., Tala S.R., Ghazvini-Zadeh E.H., Bajaj K., El-Feky S.A. Efficient and selective syntheses of S-Acyl and N-Acyl glutathiones. Synlett. 2010;9:1337–1340. doi: 10.1055/s-0029-1219837. [DOI] [Google Scholar]
- 24.Katritzky A.R., Abo-Dya N.E., Tala S.R., Gyanda K., Abdel-Samii Z.K. An efficient method for the preparation of peptide alcohols. Org. Biomol. Chem. 2009;7:4444–4447. doi: 10.1039/B905730G. [DOI] [PubMed] [Google Scholar]
- 25.Katritzky A.R., Tala S.R., Abo-Dya N.E., Ibrahim T.S., El-Feky S.A., Gyanda K., Pandya K.M. Chemical Ligation of S-Scylated Cysteine Peptides to Form Native Peptides via 5-, 11-, and 14-Membered Cyclic Transition States. J. Org. Chem. 2011;76:85–96. doi: 10.1021/jo1015757. [DOI] [PubMed] [Google Scholar]
- 26.Luber P., Bartelt E., Genschow E., Wagner J., Hahn H. Comparison of Broth Microdilution, E Test, and Agar Dilution Methods for Antibiotic Susceptibility Testing of Campylobacter jejuni and Campylobacter coli. J. Clin. Microbiol. 2003;41:1062–1068. doi: 10.1128/JCM.41.3.1062-1068.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.