Abstract
Urachal carcinoma is an uncommon cancer whose rarity has precluded its study and evidence-based management strategies are lacking. This study assessed all urachal carcinomas in Ireland and clinical parameters in order to improve understanding. Urachal carcinomas diagnosed from 1994 to 2011 were identified from the National Cancer Registry in Ireland. Data obtained included patient age, gender, diagnostic year, pathology, tumor stage, patient treatment strategies and survival. Twenty-six urachal carcinomas were identified, the majority being adenocarcinoma. This comprised 0.3% of all invasive bladder tumors. Patients were predominantly male (62%) and over 50 years of age (58%). Twenty-two patients (85%) underwent surgery, with only six (23%) undergoing chemotherapy. On average, median overall survival was 2.6 years (range 0-15.2 yrs). Survival was longer in women (5 vs. 1.9 yrs), patients under 50 years of age (3.6 vs. 1.9 yrs), those without confirmed metastasis (4.1 vs. 0.7 yrs) and those who received chemotherapy (3.6 vs. 2.6 yrs). The overall survival of urachal carcinoma in Ireland is less than expected from published literature. This study highlights the need for centralization of rare tumors with international collaboration to identify the optimal treatment strategy and improve outcome.
Key words: Urachal carcinoma, epidemiology, management, survival
Introduction
Urachal carcinoma comprises less than 1% of all bladder carcinomas and reportedly affects 0.01-0.02% of total adult cancers.1-3 It was first described in 1863 by Hue and Jacquin4 and since then, single and joint institutional reviews have increased the understanding of this rare disease.5-10 Urachal carcinomas are predominantly adenocarcinoma (80-90%) and are thought to develop from malignant transformation of columnar metaplastic epithelium of the embryological remnant of the allantois that connects the umbilicus with the fetal bladder, the urachus.2,6 Tumors can be difficult to stage as they arise from the bladder wall. Sheldon and colleagues created the most widely used international staging system for urachal carcinoma (Table 1).2
Table 1.
Sheldon staging system of urachal carcinoma.
| Sheldon staging system | |
|---|---|
| pT1 | No invasion beyond the urachal mucosa |
| pT2 | Invasion confined to the urachus |
| pT3 | a) Local extension to the bladder |
| b) Local extension to the abdominal wall | |
| c) Local extension to viscera other than the bladder | |
| pT4 | a) Metastasis to regional lymph nodes |
| b) Metastasis to distant sites | |
Adapted from Sheldon et al.2
The cornerstone of treatment remains surgical excision involving partial or radical cystectomy with en bloc resection including the umbilicus.1,11,12 Urachal tumors have a reported local recurrence rate of 15 to 41% after primary resection within the first two years of follow-up.3,13,14 Clinicopathological risk factors for early relapse and poor outcome include positive surgical margins following resection, advanced tumor stage and grade as well as lymph node involvement and presence of distant metastasis at initial diagnosis.8,13-16 Recurrence is best treated with resection and if complete, a salvage cure rate of up to 50% has been reported.14,17 Distant metastases are most commonly seen in the liver, lymph nodes, lungs and bones and overall signify a very poor survival.8,13,14 Five-year survival depending on pathological stage is up to 49% but this is center-specific.8,14,17 In general, urachal cancers tend to be relatively resistant to radiotherapy.5 The benefit of adjuvant chemotherapy for urachal carcinoma remains unclear. The rarity of this cancer complicates, if not precludes, the conduct of randomized clinical trials. Chemotherapy is generally reserved for relapsed and recurrent disease that cannot be surgically removed.5 There are numerous reports in the literature of benefit from chemotherapeutic agents, with response rates around 15%,5,18-21 however the optimal regimens in a first or second line setting have not been definitively established.5,15,22 Treatment strategies have been led by isolated case reports, institutional case series and multicenter reviews that have assessed the outcome of various chemotherapeutic and radiation therapy regimens, including platinum-containing regimens,18,23 5-fluorouracil-based treatment22,24 and the newer biological therapies targeting the oncogene, KRAS and the epidermal growth factor receptor,25 as well as antiangiogenic agents.26-28 In an attempt to increase international knowledge of this rare malignancy, this paper sets out to identify all urachal carcinomas in Ireland utilizing the Irish National Cancer Registry and correlates this incidence with demographic data, treatment received and overall patient outcome.
Materials and Methods
Patient data was obtained from the prospective National Cancer Registry in Ireland database. This was developed in 1994 for the purpose of collecting accurate cancer information to allow the determination of incidence, prevalence and mortality in Ireland permitting international comparisons. Data available from this resource included patient age, gender, year of diagnosis of urachal cancer, pathologic subtype, confirmed metastasis, patient management including surgery, radiotherapy and chemotherapy and overall survival. Information was obtainable from 1994 to the end of 2011.
Statistical analysis was performed using two tailed students t-test.
Results
Between 1994 and the end of 2011, the National Cancer Registry database identified 26 histopathological confirmed urachal carcinomas in the Republic of Ireland. Figure 1 depicts the numbers of cancers diagnosed per year along with the annual incidence of bladder carcinomas allowing comparison.
Figure 1.
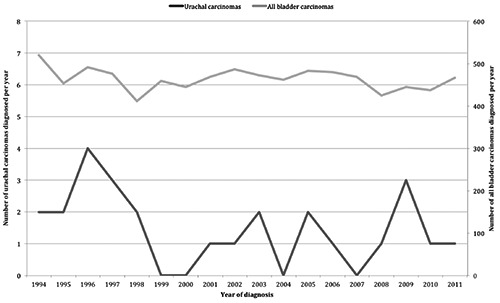
Annual incidence of urachal cancer (values on left) and invasive bladder cancer (values on right) diagnosed from 1994-2011.
The overall incidence was 0.3% of all invasive bladder cancers (n=8351). The total number of all invasive cancers diagnosed in Ireland between 1994 and 2011 obtained from the National Cancer Registry was 274,357 cases; therefore urachal carcinomas comprised 0.0095% of all invasive cancers diagnosed in Ireland during this 18-year period. The demographic breakdown is depicted in Table 2.
Table 2.
Demographic data of urachal carcinoma patients, 1994-2011.
| Demographic data | N (%) |
|---|---|
| Age | |
| <30 years | 0 (0) |
| 30-39 years | 4F+1M (19.2) |
| 40-49 years | 3F (11.5) |
| 50-59 years | 3M (11.5) |
| 60-69 years | 3F+3M (23.1) |
| 70-79 years | 5M (19.2) |
| 80-89 years | 4M (15.4) |
| Gender | |
| Male | 16 (61.5) |
| Female | 10 (38.5) |
| Histopathology | |
| Adenocarcinoma | 15 (57.7) |
| Transitional carcinoma | 7 (26.9) |
| Squamous cell carcinoma | 1 (3.8) |
| Unspecified | 3 (11.5) |
| Metastasis | |
| Metastatic at diagnosis | 3 (11.5) |
| Non-metastatic at diagnosis | 23 (88.5) |
| Treatment | |
| No treatment | 3 (11.5) |
| Surgery alone | 15 (57.7) |
| Surgery + RT | 2 (7.7) |
| Surgery + RT + chemo | 2 (7.7) |
| Chemo alone | 1 (3.8) |
| Surgery + chemo | 3 (11.5) |
| Surgery details | |
| Excision of lesion | 4 (15.4) |
| Partial cystectomy | 8 (30.8) |
| Radical cystectomy | 9 (34.6) |
| Radical cystectomy + hysterectomy | 1 (3.8) |
| Biopsy only (no surgery) | 4 (15.4) |
F, female; M, male; RT, radiotherapy; chemo, chemotherapy.
Patients were predominantly male (n=16, 62%), with a ratio of male to female patients of 1.6:1. Urachal carcinoma was more common in those over 50 years of age (n=18, 69%). Table 2 also charts the proportional age groups of patients and gender. Females presented at a younger age than men. Overall 7 women (70%) presented before the age of 50 years and 15 men (94%) presented over the age of 50 years, with 9 over 70 years old (56%). Most patients presented with no confirmed identifiable metastatic disease (n=23, 88%). Of the 3 patients with metastatic disease at diagnosis, 2 had pulmonary metastasis and one had hepatic metastasis. Two further patients were known to have developed metastatic disease during the course of the follow-up; one developed cutaneous disease and the other, pulmonary disease.
Histopathologic subtype
Of the 26 urachal cancers identified, 15 were adenocarcinoma, 7 were transitional cell carcinoma and one squamous cell carcinoma (Table 2). Three tumors were unspecified by the reporting institution. All seven transitional cell carcinomas occurred in patients over the age of 70 years and all were found in men. In comparison, 14 adenocarcinomas were diagnosed in patients aged between 30 and 69 years. Females were more commonly diagnosed with adenocarcinoma (70%).
Treatment strategies
Twenty-two of the 26 patients (85%) underwent surgical resection (Table 2). The remaining 4 patients only had a biopsy for pathological diagnosis. Most patients underwent a radical cystectomy (n=9) with one additional patient also documented as having a concurrent hysterectomy. Eight patients underwent a partial cystectomy and the four remaining patients had local excision of the bladder lesion alone.
Multidisciplinary treatment combinations are outlined in Table 2. Radiotherapy was administered to four patients only. Chemotherapy was utilized in six patients (23%). One of these six patients is known to have had chemotherapy outside of Ireland. Patients who received chemotherapy were generally younger; on average, patients who received chemotherapy were less than 59 years of age, whereas those who did not receive chemotherapy were older. However there was a wide variation with patients receiving chemotherapy aged between 30 and 74 years of age. Three of the five patients with known metastatic disease received chemotherapy. Three patients received adjuvant chemotherapy in combination with radiotherapy and surgery. The majority of patients who received chemotherapy were alive by the end of 2011 (n=4, 67%) including two patients who had known pulmonary metastasis. The details of the six patients who received chemotherapy are depicted in Table 3.
Table 3.
Details of patients receiving chemotherapy.
| Year of diagnosis | Histopathology | Gender | Age (years) | Survival (years) | Alive at end 2011 | Management | Details |
|---|---|---|---|---|---|---|---|
| 1997 | Adeno | M | 35-39 | 4.1 | No | Sx (partial cystectomy), RT, Chemo | Adjuvant chemotherapy |
| 2001 | Adeno | M | 55-59 | 10.8 | Yes | Sx (partial cystectomy) + chemo | Adjuvant chemotherapy |
| 2003 | TCC | M | 70-74 | 0.6 | No | Chemo alone | Pulmonary metastasis at diagnosis |
| 2005 | Adeno | F | 45-49 | 6.9 | Yes | Sx (excision of lesion), RT, Chemo | Adjuvant chemotherapy |
| 2008 | Adeno | F | 30-34 | 3.1 | Yes | Sx (total cystectomy) + chemo | Pulmonary metastasis 3 years post diagnosis |
| 2010 | Adeno | F | 60-64 | 0.7 | Yes | Sx (partial cystectomy) + chemo | Pulmonary metastasis at diagnosis |
Adeno, adenocarcinoma; M, male; Sx, surgery; RT, radiotherapy; chemo, chemotherapy; TCC, transitional cell carcinoma; F, female.
Survival
Full survival data until the end of 2011 revealed that 17 of the 26 patients diagnosed with urachal carcinoma were known to have died (65%). Of these deceased patients, 3 (18%) were known to have confirmed metastatic disease. Overall 13 patients who died underwent a surgical procedure (76%), 3 received radiotherapy (18%) and 3 received chemotherapy (18%). Of the latter, 2 had metastatic disease at diagnosis and the remaining patient received chemotherapy on the development of metastasis. Three patients (18%) received no form of active treatment.
Of the 9 patients who were alive at the end of 2011, the majority was female (n=6, 67%) and diagnosed at less than 50 years of age (n=6, 67%). None were over the age of 65 years. All patients underwent surgical excision of the primary tumor. Two patients received adjuvant chemotherapy and one patient received chemotherapy 3.5 years later upon the diagnosis of lung metastases. One patient also received adjuvant radiotherapy in conjunction with surgery and chemotherapy.
On average, patients with urachal carcinoma survived a median of 2.7 years following diagnosis (range 0-15.2 years). Those that received chemotherapy had a median survival of 3.6 years (range 0.6-10.8 years) as compared with median overall survival of 2.6 years (range 0-15.2 years) in patients who did not receive any chemotherapy. There were only 3 patients who received chemotherapy as adjuvant treatment. Their survival ranged from 4.1 to 10.8 years, with a median of 6.9 years (P=0.92). Table 4 depicts comparative median survival in patients based upon age, gender, year of diagnosis, confirmed metastasis, extent of surgical excision and chemotherapy.
Table 4.
Median survival in years grouping patients according to age, gender, year of diagnosis, presence of metastasis and receipt of chemotherapy.
| Survival | Median survival (years) | Range (years) | P-value | |
|---|---|---|---|---|
| Age | Less than 50 years of age | 3.6 | 1.3-15.2 | 0.50 |
| 50 years of age or older | 1.9 | 0-15.2 | ||
| Gender | Male | 1.9 | 0-15.2 | 0.22 |
| Female | 5.0 | 0.7-15.2 | ||
| Year of diagnosis | Earlier than 2000 | 2.7 | 0.1-15.2 | 0.42 |
| Year 2000 or later | 2.6 | 0-10.8 | ||
| Extent of surgery | Partial surgery | 5.7 | 0.7-15.2 | 0.20 |
| Radical / total surgery | 2.9 | 0.1-15.2 | ||
| Metastasis | Metastasis identified | 0.7 | 0-3.1 | 0.10 |
| No metastasis identified | 4.1 | 0.1-15.2 | ||
| Chemotherapy | Chemotherapy | 3.6 | 0.6-10.8 | 0.92 |
| No chemotherapy | 2.6 | 0-15.2 | ||
| Adjuvant chemotherapy | 6.9 | 4.1-10.8 | ||
| No adjuvant chemotherapy | 2.6 | 0-15.2 |
Discussion
The incidence of urachal carcinoma in the literature has been documented as between 0.01 and 0.02% of all invasive cancers and the Irish national experience reflects this. Over an 18-year period in Ireland, an incidence of 0.01% of all invasive cancers was recorded. As expected in this series, urachal carcinoma had a demographic preponderance of males (69%) and those above the age of 50 (62%). Females tended to present earlier, with 70% diagnosed before the age of 50 years. Furthermore, women were more inclined to live longer than their male counterparts (5 vs. 1.9 years, Table 4). The reasons underlying these marked gender differences in age at diagnosis and survival is unclear and has not been identified in prior analyses of urachal carcinoma. The P values from all results are not statistically significant on account of small patient numbers as well as a wide range of results.
Management strategies hinge upon surgical resection with recommendations encouraging en bloc excision including umbilicus. Postoperatively, there is no data to support chemotherapy in the adjuvant setting and it remains a treatment for unresectable and/or metastatic disease. International guidelines continue to recommend individualizing treatment on a case by case basis and employing radiotherapy and/or chemotherapy regimens at the responsible physician’s discretion.1 In this national cohort, the majority of patients (85%) underwent surgical resection. A total of 17 patients underwent cystectomy, 9 of whom had a radical procedure and 8 patients underwent partial excision. There was no appreciable difference in survival with greater resections, although the small numbers may not reflect this accurately. Out of the 26 urachal carcinomas, 4 received adjunctive radiotherapy and less than one quarter of patients received chemotherapy (n=6, 23%), half in the metastatic setting. Full survival data until the end of 2011 revealed that 17 of the 26 patients diagnosed with urachal carcinoma were known to have died. A total of 10 patients survived more than 5 years (38%), which is inferior to international survival data of approximately 49%.8,14,15 Table 4 reveals that patients younger than 50 years of age, females and those diagnosed at an earlier stage had a better overall survival. There has been no significant change in survival between 1994 and 2011 despite changes in management of this disease (Table 4), although small numbers make comparisons difficult. As each of these cases is spread throughout Ireland and over an 18-year period, it is possible that the caseload for each institution is too small to induce an improvement in the clinical management of this rare disease. This might suggest that the centralization of rare diseases such as urachal carcinomas should take place in order to improve overall patient survival. This would allow clinicians in the fields of surgery, medical and radiation oncology to enhance their skills and knowledge base, benefiting patient outcome. It is also noteworthy that there is little data on targeted biologic therapy in urachal cancer.
A recent retrospective review of urachal carcinomas identified seventeen patients over a thirteen-year period in a Chinese University hospital, identified similar age of presentation and male preponderance.29 Chen and colleagues reported a median overall survival of 57.6 months (4.8 years), which is much greater than this research identified at 2.7 years. In the Chinese study, there was no information on whether any patients received adjuvant or palliative chemotherapy or radiotherapy in the management of this disease.
Limitations of this study include an overall small number of urachal cancers within the whole country and analyzing subgroups. Examining the effect of treatments, such as chemotherapy and radiotherapy, was made difficult by small numbers and the variation in patient outcomes. It was not possible to unblind the data collected to identify particular chemotherapeutic regimes administered in individual cases. The National Cancer Registry is a dedicated independent research institute that collects data on every cancer diagnosed in Ireland. Potential inaccuracies or failures with data collection and/or data reporting to the Registry could result in errors. In order to identify, address and improve this problem, the Registry performs ongoing evaluation. The latest review has revealed the estimated levels of data completeness are satisfactory.30 Finally, there have been difficulties with the diverse coding and varied definitions of invasive bladder cancer between countries and indeed organizations, with an increasing proportion of bladder tumors being coded as in situ or uncertain. This can lead to potential inaccuracies in national data collection worldwide.
Conclusions
This paper reports all urachal carcinomas diagnosed in Ireland between 1994 and the end of 2011 as recorded by the national database: the National Cancer Registry. Urachal carcinoma has an incidence comparable to other international series. Epidemiological assessment revealed a younger age of presentation for females and a marked increased survival in this group as compared to men. Furthermore, the data identified a lower five-year survival than that reported in the literature (38 vs. 49%) and questions whether rare diseases and carcinomas, such as urachal cancer, should be treated exclusively in a specialist center to improve overall patient outcome. This report hopes to add to the dearth of literature on this rare disease to improve international understanding and enhance the management strategies for patients with urachal carcinoma.
Acknowledgements
The authors would like to thank Dr Mary Coghlan for statistical help and the input of the National Cancer Registry in Ireland.
References
- 1.NCCN. Guidelines in Oncology (NCCN Guidelines). Bladder Cancer 2013. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- 2.Sheldon CA, Clayman RV, Gonzalez R, et al. Malignant urachal lesions. J Urol 1984;131:1-8. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi T, Yuasa T, Uehara S, et al. Clinical outcome of urachal cancer in Japanese patients. Int J Clin Oncol 2016;21:133-8. [DOI] [PubMed] [Google Scholar]
- 4.Hue L, Jacquin M. Cancer colloide de la lombille et de paroi abdominale anterieure ayant envahi la vessie. Union Med de la Siene-Inf Rouen 1863;6:418-20. [Google Scholar]
- 5.Siefker-Radtke AO, Gee J, Shen Y, et al. Multimodality management of urachal carcinoma: the M.D. Anderson Cancer Center experience. J Urol 2003;169:1295-8. [DOI] [PubMed] [Google Scholar]
- 6.Johnson DE, Hodge GB, Abdul-Karim FW, Ayala AG. Urachal carcinoma. Urology 1985;26:218-21. [DOI] [PubMed] [Google Scholar]
- 7.Grignon DJ, Ro JY, Ayala AG, et al. Primary adenocarcinoma of the bladder: a clinicopathologic analysis of 72 cases. Cancer 1991;67:2165-72. [DOI] [PubMed] [Google Scholar]
- 8.Bruins HM, Visser O, Ploeg M, et al. The clinical epidemiology of urachal carcinoma: results of a large, population based study. J Urol 2012;188:1102-7. [DOI] [PubMed] [Google Scholar]
- 9.Kakizoe T, Matsumoto K, Andoh M, et al. Adenocarcinoma of urachus. Report of 7 cases and review of literature. Urology 1983;21:360-6. [DOI] [PubMed] [Google Scholar]
- 10.Kumar N, Khosla D, Kumar R, et al. Urachal carcinoma: clinicopathological features, treatment and outcome. J Cancer Res Ther 2014;10:571-4. [DOI] [PubMed] [Google Scholar]
- 11.Herr HW, Bochner BH, Sharp P, et al. Urachal carcinoma: contemporary surgical outcomes. J Urol 2007;178:74-8. [DOI] [PubMed] [Google Scholar]
- 12.Tomita K, Tobisu K, Kume H, et al. Long survival with extended surgery for urachal carcinoma involving adjacent organs. J Urol 1998;159:1298. [PubMed] [Google Scholar]
- 13.Lane V. Prognosis in carcinoma of the urachus. Eur Urol 1976;2:282-3. [DOI] [PubMed] [Google Scholar]
- 14.Ashley RA, Inman BA, Sebo TJ, et al. Urachal carcinoma: clinicopathological features and long-term outcomes of an aggressive malignancy. Cancer 2006;107:712-20. [DOI] [PubMed] [Google Scholar]
- 15.Pinthus JH, Haddad R, Trachtenberg J, et al. Population based survival data on urachal tumors. J Urol 2006;175:2042-7. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi K, Kawai T, Suzuki M, Torikata C. Prognostic factors in urachal adenocarcinoma. A study in 41 specimens of DNA status, proliferating cell-nuclear antigen immunostaining, and argyrophilic nuclear-organizer region counts. Hum Pathol 1996;27:240-7. [DOI] [PubMed] [Google Scholar]
- 17.Molina JR, Quevedo JF, Furth AF, et al. Predictor of survival from urachal cancer. A Mayo Clinic study of 49 cases. J Urol 2007;110:2434-40. [DOI] [PubMed] [Google Scholar]
- 18.Kojima Y, Yamada Y, Kamisawa H, et al. Complete response of a recurrent advanced urachal carcinoma treated by S-1/cisplatin combination chemotherapy. Int J Urol 2006;13:1123-5. [DOI] [PubMed] [Google Scholar]
- 19.Tran B, McKendrick J. Metastatic urachal cancer responding to FOLFOX chemotherapy. Can J Urol 2011;17:5120-3. [PubMed] [Google Scholar]
- 20.Kawakami S, Kageyama Y, Yonese J, et al. Successful treatment of metastatic adenocarcinoma of the urachus: report of a case with more than 10-year survival. Urology 2001;58:462. [DOI] [PubMed] [Google Scholar]
- 21.Jung HA, Sun JM, Park SH, et al. Treatment outcomes and relevance of palliative chemotherapy in urachal cancer. Chemotherapy 2014;60:73-80. [DOI] [PubMed] [Google Scholar]
- 22.Siefker-Radtke A. Urachal adenocarcinoma: a clinician’s guide for treatment. Semin Oncol 2012:39:619-24. [DOI] [PubMed] [Google Scholar]
- 23.Galsky MD, Iasanos A, Mironov S, et al. Prospective trial of ifosfamide, paclitaxel, and cisplatin in patients with advanced non-transitional cell carcinomas of the urothelial tract. Urology 2007;69:255-9. [DOI] [PubMed] [Google Scholar]
- 24.Logothetis CJ, Samuels ML, Ogden S. Chemotherapy for adenocarcinomas of bladder and urachal origin: 5-fluorouracil, doxorubicin, and mitomycin-C. Urology 1985;26:252-5. [DOI] [PubMed] [Google Scholar]
- 25.Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009;27:2091-6. [DOI] [PubMed] [Google Scholar]
- 26.Grothey A, Galanis E. Targeting angiogenesis: progress with anti- VEGF- treatment with large molecules. Nat Rev Clin Oncol 2009;6:507-18. [DOI] [PubMed] [Google Scholar]
- 27.Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol 2005;23:3502-8. [DOI] [PubMed] [Google Scholar]
- 28.Testa I, Verzoni E, Grassi P, et al. Response to targeted therapy in urachal adenocarcinoma. Rare Tumors 2014; 6:124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen D, Li Y, Yu Z, et al. Investigating urachal carcinoma for more than 15 years. Oncol Lett 2014;8:2279-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Brien K, Comber H, Sharp L. Completeness of case ascertainment at the Irish National Cancer Registry. Ir J Med Sci 2014;183:219-24. [DOI] [PubMed] [Google Scholar]


