Abstract
Phyllodes tumors of the breast are rare, accounting for less than 1% of the breast tumors. They are mostly seen in women between 45 and 49 years old. These are fast growing tumors with a large spectrum of behavior (from benign to metastatic) and can resemble fibroadenomas. Correct diagnosis mostly through core needle biopsy is important to decide whether a surgical excision has to be done. Here we report a case of a 57-year-old woman with a fast growing, ulcerated tumor in the left breast. Core needle biopsy suggested a malignant phyllodes tumor with heterologous liposarcomatous differentiation. Treatment with total mastectomy and adjuvant radiotherapy followed. Primary treatment is always surgery, whether radiotherapy or chemotherapy has to follow remains uncertain. There is a high-recurrence rate, especially when the surgical margins are narrow.
Key words: Phyllodes tumor, breast, mastectomy
Introduction
Phyllodes tumors of the breast are rare biphasic fibro-epithelial lesions that contain two types of breast tissue: stromal (connective) tissue and glandular (lobule and duct) tissue.1 There is a resemblance with the benign fibroadenomata and it’s important to differentiate between the two entities. Phyllodes tumors contain a large spectrum from benign to borderline to malignant according to features such as tumor margins, stromal overgrowth, tumor necrosis, cellular atypia, and number of mitosis per high power field.2
They account for less than 1% of all breast neoplasms, women between 35 and 55 are commonly affected and with the median age of presentation at 45 years.3 Often they present as a rapidly growing and painless mass, as in our case, where the patient presented with a breast of 30 cm in greatest dimension, which started growing a few months earlier. A tumor larger than 10 cm is called a giant tumor, these are found in 20% of the phyllodes tumors.4
Case Report
A 57-year-old patient presented on a Friday night in the emergency department with complaints of fluid loss through her left breast. Inspection showed a giant mass of the left breast with ulceration (Figure 1). The mass measured 30 cm in greatest dimension. The skin was stretched over the mass, had a purple color and there was a central ulceration supposedly where the nipple used to be. Palpation of axillary, mediastinal and clavicular lymph nodes were negative. The right breast appeared normal.
Figure 1.
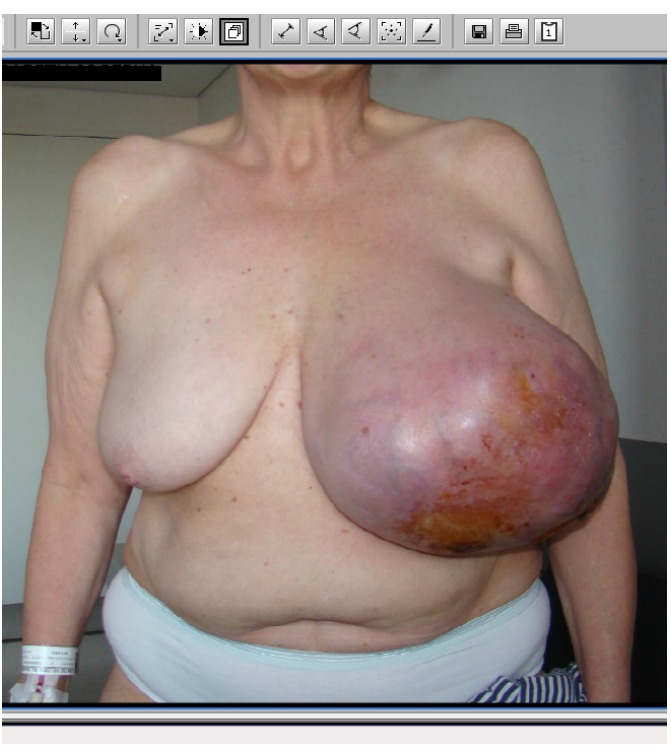
A giant phyllodes tumor of the breast with ulceration.
The patient noticed this mass a few months ago; it significantly increased in size, and exhibited skin changes. There was no documented medical history. X-ray of the chest showed a small trace of pleural effusion on the left side. Computed tomography (CT) thorax, sonography of the liver and bone scan were negative for staging. The mass reached up to the thoracic wall muscles (m. pectoralis major and minor) with no clear arguments for invasion (Figure 2). Neither CT nor sonography showed axillary lymphadenopathies. There was no evidence for metastasis. A core needle biopsy of the tumor was performed and showed a benign spindle cell proliferation, presumably a fibro-epithelial tumor, suggestive for a phyllodes tumor. Given the dimensions of the mass, whether the core biopsy was representative of the lesion was questionable. A simple total mastectomy on the left side was performed. No axillary lymph node sampling was performed. Macroscopically the specimen was 30×21×18 cm; it was covered with some skin containing fragments of the nipple. The tumor was 22×17×17 cm and weighted 4.194 kg. Microscopic examination confirmed the diagnosis of phyllodes tumor varying from benign to borderline to malign. The malignant areas are composed of heterologous, sarcomatoid foci, which consist of a myxoïd liposarcoma (Figures 3 and 4). There were some additional foci of ductal carcinoma in situ of the breast (DCIS). Neither skin nor nipple showed pathological changes. Surgical margins were negative, but at some areas the margin was only 1 mm.
Figure 2.
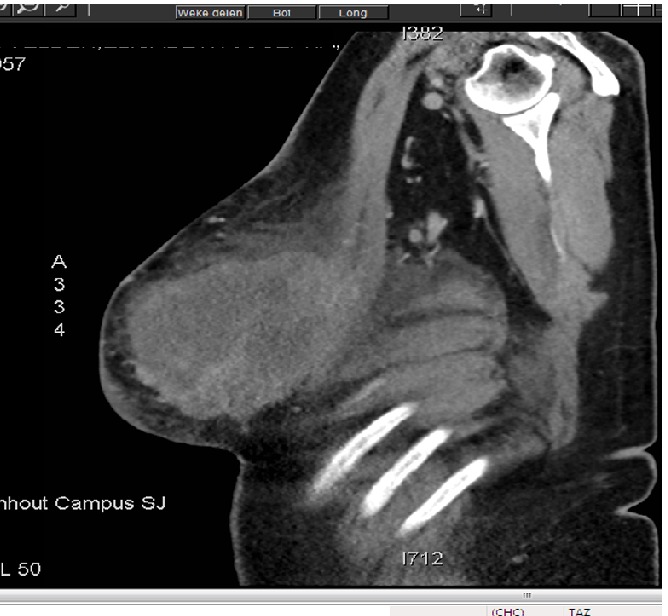
Computed tomography thorax showed expansion of the mass, reaching up to the thoracic wall muscles (m. pectoralis major and minor) with infiltration of the surrounded fatty tissue. There is no clear evidence for invasion of the tumor.
Figure 3.
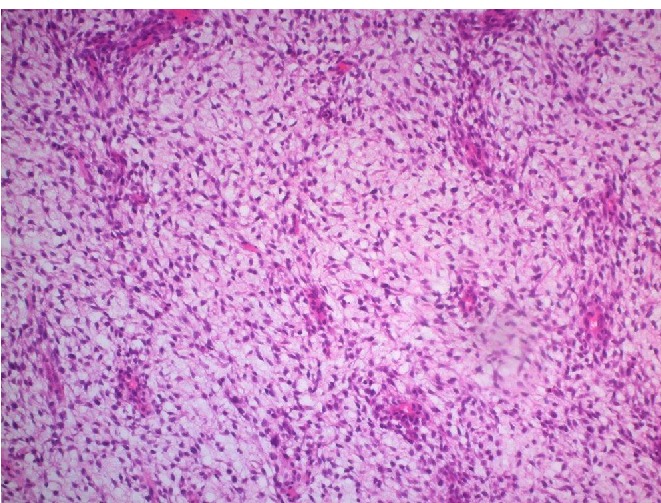
Panoramic view of the bifasic tumor showing tubular and stromal components with a leaf-like pattern. (Hematoxylin and eosin stain, ×50).
Figure 4.
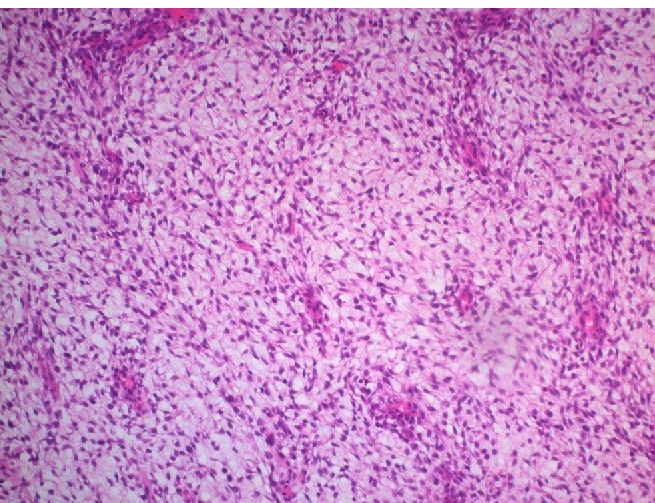
View of the sarcomateus overgrowth compatible with a myxoid liposarcoma: myxoid background with plexiform vascular pattern (crow’s foot) between monotonous spindle cell proliferation. (Hematoxylin and eosin stain, ×200).
Because of the malignant character of the tumor, the large size and the limited surgical margins adjuvant radiotherapy was given: 50 Gray in 25 sessions. Five-year therapy with Tamoxifen was started because of the foci DCIS. Follow-up after seven months showed that the patient was in good general condition with until now no evidence of recurrence.
Discussion
The phyllodes tumor was first described in 1827 by Chelius5 but Johannes Muller was in 1838 the first to use the term cystosarcoma phyllodes. Phyllon is the Greek word for leaf, the name has been chosen because the tumor displays characteristics of a large, malignant sarcoma, takes on a leaf like appearance when sectioned, and displays epithelial, cystlike spaces when viewed histologically.6 A phyllodes tumor has a wide range of biological behavior: from benign similar to fibroadenomas to borderline with local recurrence risks and malignant with metastatic potential.3 These categories are based on the degree of stromal cellular atypia, mitotic activity per 10 high-power fields, degree of stromal overgrowth (these three are the main criteria), tumor necrosis and margins.2 The behavior of the tumor is very difficult to predict. Liposarcomas, like in our case, may also develop as stromal components of phyllodes tumors and the finding of such a malignant heterologous element places the tumor into a malignant category.7 Three similar cases have already been described in the literature.7-9 Fibroadenoma and phyllodes tumor are often indistinguishable at first presentation because they are firm, mobile, well-defined, lobulated and painless masses.10 In contrast to fibroadenomas a phyllodes tumor grows quickly and the mean measure at presentation is 4-5 cm. The peak incidence is between 45 and 49 years old, this is about 20 years later than fibroadenomas and earlier than most invasive ductal and lobular cancers.11 Radiologic examinations like mammography, sonography and magnetic resonance imaging cannot differentiate between the two types of breast tumors. When there are indications of phyllodes tumor the diagnosis has to be made through core needle biopsy. This has only a sensitivity of 75% for differentiating phyllodes tumors from fibroadenomas so if clinical suspicion remains excisional biopsy is indicated for the correct differentiation. Because of tumor heterogeneity it is possible that core biopsies of phyllodes tumors show only fibroadenoma so excisional biopsy is always indicated.10,12 Since there are chances of false negative diagnosis (25-30%) there has to be a low threshold for total excision of the tumor. If the excisional biopsy shows a fibroadenoma observation is sufficient. When the result is a phyllodes tumor wide excision has to follow with intention of surgical margins of minimal 1 cm. Lumpectomy or partial mastectomy are the standard if the surgical margins can be respected otherwise a total mastectomy has to be done. Routinely lymphadenectomy is not indicated because phyllodes tumors spread hematogenously and rarely spread to the lymph nodes (<1% have pathological lymph nodes).3,13 Axillary staging is only necessary when the lymph nodes are pathologic on clinical examination. There are no straightforward guidelines about use of radiotherapy and chemotherapy. Literature on this topic is limited and does not contribute to decision-making. A phyllodes tumor has a high recurrence rate ranging from 10 to 40%.3 The surgical margins are the most important factor, histologic grade of the tumor and overgrowth of stromal components also have an impact. Most recurrences are local. Treatment of local recurrence is resection with wide surgical margins. Whether radiotherapy has to follow is controversial. A study with 37 women suggested that adjuvant radiotherapy could improve the disease free survival of malignant phyllodes tumor.14 The likelihood of distant metastasis depends on the tumor biology and not on the surgical margins.3 Of patients, 10% with a phyllodes tumor and 20% with malignant tumors develop distant metastases.3 The most common sites are lungs, following by bones, brain and rarely liver and heart. The exact role of hormonal therapy remains to be defined3 because the practical significance of the observed expression of hormone receptors remains unclear.2
Conclusions
A phyllodes tumor has a wide range of behaviors: from benign to borderline to malignant. It is important to make a correct diagnosis and to treat according the guidelines because they have a high recurrence rate and possibility to metastasize.
References
- 1.Chulia MT, Paya A, Niveiro M, et al. Phyllodes tumor in ectopic breast tissue of the vulva. Int J Surg Pathol 2001;9:81-3. [DOI] [PubMed] [Google Scholar]
- 2.Tan BY, Acs G, Apple SK, et al. Phyllodes tumours of the breast: a consensus review. Histopathology 2016;68:5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker SJ, Harries SA. Phyllodes tumours. Postgrad Med J 2001;77:428-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang MI, Ramaswamy B, Patterson CC, et al. Giant breast tumors: surgical management of phyllodes tumors, potential for reconstructive surgery and a review of literature. World J Surg Oncol 2008;6:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chelius M. Neue jahrbucher der deutschen medicin und chirurgie. Naegele and Puchelt: Heidelberg, Germany; 1827. [Google Scholar]
- 6.Tavassoli FA. Phyllodes tumours. Tavassoli FA, Devilee P. World Health Organization Classification of Tumours. Pathology and genetics of tumours of the breast and female genital organs. Lyon, Frankrijk: IARC Press; 2003. pp 100-3. [Google Scholar]
- 7.Uriev L, Maslovsky I, Vainshtein P, et al. Malignant phyllodes tumor with heterologous liposarcomatous differentiation and tubular adenoma-like epithelial component. Int J Med Sci 2006;3:130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Argáez Cimé NL, Gutiérrez Vega P, López Cruz J. [Malignant phyllodes tumor with differentiation to liposarcoma. A report of a case and bibliographic review]. Ginecol Obstet Mex 2005;73:145-50. [Article in Spanish] [PubMed] [Google Scholar]
- 9.Satou T, Matsunami N, Fujiki C, et al. Malignant phyllodes tumor with liposarcomatous components: a case report with cytological presentation. Diagn Cytopathol 2000;22:364-9. [DOI] [PubMed] [Google Scholar]
- 10.Lee AH, Hodi Z, Ellis IO, Elston CW. Histological features useful in the distinction of phyllodes tumour and fibroadenoma on needle core biopsy of the breast. Histopathology 2007;51:336-44. [DOI] [PubMed] [Google Scholar]
- 11.Rosen PP. Fibroepithelial neoplasms Rosen PP. Rosen’s breast pathology, 3rd ed Philadelphia, USA: Wolters Kluwer; 2008. pp 187-229. [Google Scholar]
- 12.Dillon MF, Quinn CM, McDermott EW, et al. Needle core biopsy in the diagnosis of phyllodes neoplasm. Surgery 2006;140:779-84. [DOI] [PubMed] [Google Scholar]
- 13.Gradishar WJ, Anderson BO, Balassanian R, et al. Breast cancer version 2.2015. J Natl Compr Canc Netw 2015;13:448-75. [DOI] [PubMed] [Google Scholar]
- 14.Pandey M, Mathew A, Kattoor J, et al. Malignant phyllodes tumor. Breast J 2001;7:411-6. [DOI] [PubMed] [Google Scholar]


