Abstract
Gastric metastasis is rare but it can be the initial symptom of cancer. The second leading cause of this type of metastasis is breast cancer. A lack of clinical signs and nonspecific side effects of the treatment of primary tumors can lead to the misdiagnosis of metastatic gastric cancer. Upper gastrointestinal endoscopy with biopsy and immunohistochemistry should be used for diagnosis. Treatment is palliative; it includes chemo, endocrine, and radiation therapies. Four patients with breast cancer and gastric metastasis were identified. All the patients tested positive for estrogen and progesterone receptors, and received chemotherapy and hormone therapy. One patient underwent surgery and two received radiation therapy. Patients with breast cancer and gastrointestinal symptoms should be investigated for gastric metastasis, given its morbidity and negative impact on quality of life.
Key words: Breast cancer, gastric metastasis, immunohistochemistry, chemotherapy, hormonal therapy
Introduction
Breast cancer is the most frequent malignancy in women and the leading cause of death among this population.1 The estimated number of new cases of breast cancer in Brazil in 2014 is 57,120, with an estimated risk of 57.09 cases per 100,000 women.1 Gastric metastasis is rare and few cases have been described. Breast cancer is the second most frequent malignancy which presents with gastric metastasis, surpassed only by melanoma.2,3 The most frequent sites of breast cancer metastasis are local and distant lymph nodes, brain, lungs, liver, and bones.
It has been described by Ciulla and colleagues that 6% of women with breast cancer develop gastric metastasis during the course of the disease.2 Symptoms of gastric metastasis are often nonspecific and can be confused with side effects of the cancer treatment.4 Diagnosis is established after performing biopsy through endoscopy followed by histological and immunohistochemical analysis. It can be difficult to differentiate primary gastric cancer from gastric metastasis. Women with breast cancer metastatic to the stomach have a worse prognosis and higher chance of disease progression to other sites. According to a Dutch case series, patients in this situation had median overall survival of two years. Only 22% of the patients survived more than two years.4 The aim of treatment is palliative and includes chemotherapy alone or combined with biologic agents, hormone therapy, and radiation therapy. Surgery is indicated in selected cases when perforation, massive bleeding or obstruction occurs. Nevertheless, surgery has low impact on survival.4
Four breast cancer patients with gastric metastasis were reported.
Case Report
Four patients with breast cancer metastatic to the stomach have been identified. The mean age at diagnosis of cancer was 56 years old (ranging from 47 to 67 years old). The average age at diagnosis of gastric metastasis was 61.5 years (ranging from 51 to 74 years old). The patients’ characteristics are listed in Table 1.
Table 1.
Patients’ characteristics.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Date of birth | 07 Jun 1949 | 20 Oct 1961 | 15 Jul 1947 | 31 Jul 1939 |
| Date of diagnosis | May 1999 | Aug 2009 | May 2009 | Jan 2007 |
| Type of tumor | Lobular invasive | Lobular invasive | Ductal invasive | Ductal invasive |
| First line treatment | Carboplatin + Docetaxel | Capecitabine e Radiotherapy | Docetaxel + Capecitabine | Fulvestrant + Zoledronic acid |
| Second line treatment | Tamoxifen | Letrozole | Letrozole | Capecitabine |
| Third line treatment | Paclitaxel + Bevacizumab | Ciclofosfamide e Metotrexate | Did not receive | Did not receive |
| Immunihistochemical profile | RE/RP 100%, Her-2 negative, Ki 67 10% | RE/RP 100%, Her-2 negative, ki 67 60% | RE/RP 100%, Her-2 negative, ki 67 30% | RE/RP 100%, Her-2 inconclusive, ki 67 15% |
| Gastric metastasis diagnosis | May 2005 | Mar 2013 | Jul 2012 | May 2013 |
| Metastasis site | Only gastric metastasis | Lymph nodes, peritoneum | Peritoneum, stomach, colon, abdominal lymph nodes | Bones, lymph nodes and gastric metastasis |
| Metastasis endoscopy results | Ulcerated lesion | Diffuse Infiltration (linitis) | Infiltration and ulcerated lesion, stenosing in distal antrum and pylorus | Flat erosions |
| Metastasis pathology results | RE/PR 100% Her-2 negative | RE/PR 100% Her-2 negative | RE/RP 100% Her-2 negative | RE 95%, RP 45, HER-2 negative |
| Local treatment for metastasis | Surgery | Radiotherapy | None | Radiotherapy |
| Gastrectomy | Yes | No | No | No |
| Date of death | Jan 2013 | Nov 2014 | No | Mar 2014 |
| Meningeal infiltration | Yes | Yes | No | Yes |
Patients 1 and 2 had invasive lobular carcinoma and patients 3 and 4 had invasive ductal carcinoma. The mean time between the diagnosis of the disease and the presentation of gastric metastasis was 57 months (ranging from 38 to 76 months). Non-specific symptoms such as epigastric pain, dyspepsia and vomiting were reported. Patients 1 had estrogen receptor 100%, progesterone receptor 100%, Ki 67 10% and human epidermic growth factor 2 (HER-2) negative on the immunohistochemistry of the primary lesion and gastric metastasis. Patients 2 had estrogen receptor 100%, progesterone receptor 100%, Ki 67 60% and HER-2 negative on the immunohistochemistry of the primary lesion and gastric metastasis. Patients 3 had estrogen receptor 100%, progesterone receptor 100%, Ki 67 30% and HER-2 negative on the immunohistochemistry of the primary lesion and gastric metastasis. Patient 4 had estrogen receptor 100%, progesterone receptor 100%, Ki 67 25% and HER-2 inconclusive on the primary lesion and estrogen receptor 95%, progesterone receptor 45% and HER-2 negative on the gastric metastasis. Figure 1 demonstrates histological samples stained by hematoxylin and eosin in patient 1. Endoscopic findings were ulcerated lesion on patient 1; diffuse infiltration (linitis) on patient 2; infiltrative, ulcerated, stenotic lesion in distal antrum and pylorus on patient 3; and flat erosion lesions on patient 4. Patient 1 underwent total gastrectomy due to major bleeding; patient 2 and 4 received chemoradiotherapy to local control and patient 3 received chemotherapy only. Although the development of gastric metastasis means poor prognosis, patient 1 survived for eight years after the diagnosis of gastric metastasis
Figure 1.
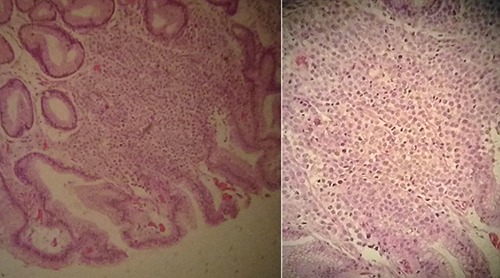
Histological sample stained by hematoxylin and eosin of patient 1.
Of the 4 patients, 2 died of metastatic disease in multiple organs; 1 died 13 months after the diagnosis and the other died 103 months after diagnosis. It is worth noting that the patient who had the longest survival rate presented gastric disease as her only disease site, and was initially treated with surgery. In our evaluation of clinical variables, the most notable feature was the incidence of meningeal disease, which was symptomatic in patients 1, 2 and 4 and was the cause of death of patients 1 and 2.
Discussion
The majority of breast tumors arise in the ductal epithelium. The lobular subtype represents the second most frequent breast neoplasia.1 Metastatic dissemination to the upper gastrointestinal tract is related mainly to lobular infiltrative breast carcinoma.2,3 In this series, half of the patients had lobular carcinoma according to the finding of Pectasides and colleagues series, in which 5 of 8 patients had the lobular subtype. In a series published by Taal and colleagues, 83% of the 51 patients with gastric metastasis had a lobular subtype.4,5 Since de the ductal subtype is the most common breast neoplasm, the proportion of our series seems reasonable.
Endoscopic findings varied, which is consistent with the literature. Gastric metastasis can be presented with benign characteristics, diffuse infiltrations, nodules and/or ulcerations.6 The most common presentation is plastic linitis with diffuse infiltration to the submucosa and muscularis propria.5 The lesions are usually present in the fundus and gastric body, which can be isolated.6
Since gastric cancer and gastric metastasis of breast cancer present with practically the same clinical, endoscopic and radiological characteristics,7 the immunohistochemical profile is the most reliable method to distinguish them and therefore establish diagnosis. Often, the markers cytokeratin (CK) 7, CK19, carcinoembryonic antigen (CEA), estrogen receptor (RE), and progesterone receptor (RP) are present in breast cancer metastasis. The intense positivity of the RE and RP allows the definite diagnosis of gastric metastasis of breast cancer.6 The estrogen receptor is an important biomarker since its positivity predicts the response to hormonal therapy, which means a better prognosis. The presence of the progesterone receptor is intimately related to the estrogen receptor, and isolated expression of the RP is rare (1% of the breast cancer cases). The best response to endocrine therapy occurs when both are present.
The overexpression of the HER-2 is related to greater tumor proliferation, since it codes one of the tumor cell protein that accelerates cellular proliferation. CK7 is present in 90% of breast cancer and 50 to 64% of primary gastric cancer. In our series, none of the patients overexpressed HER-2. CEA is usually found in gastrointestinal and breast neoplasms. However, it can be present in both benign and malignant cases.8 Patients 1 and 2 had expression of CEA in immunohistochemistry.
The patients presented with epigastric pain, dyspepsia, bloating, and vomiting, compatible with upper gastrointestinal disease. The non-specificity of symptoms can be misunderstood with adverse effects of treatment, leading to a delay in diagnosis, which invariably has a negative impact on patient survival. This fact is supported by autopsy data, which show gastric metastases in 18% of patients, while only 6% of patients received diagnosis during life.4 The mean time between breast cancer diagnosis and its progression to gastric metastasis was 31.5 months (ranging from 10 to 53 months). In a Dutch trial, the mean time to progression was 27 months (ranging from 0 to 176 months). In another series published by Pectasides and colleagues, the mean time to progression was 41 months (ranging from 2 to 82 months). Systemic therapy is indicated for gastric metastasis from breast cancer and it can involve chemotherapy, radiation therapy or hormone therapy, alone or in combination. Surgery is reserved for selected cases, mostly when complications such as perforations, hemorrhage or obstruction occur. To treat obstructions, an endoluminal stent can be indicated. When bleeding occurs, endoscopic or endovascular therapy is suggested. Surgical treatment has no impact on global survival according to various published studies.4,5,9
Systemic therapy with chemotherapy is also routinely used in these situations.9 RE-positive gastric metastases have shown a good response to hormonal therapy and chemotherapy with significant increase of overall survival.2 Patients with invasive lobular carcinoma usually have good response to hormone therapy.9 In this series, all patients received chemotherapy as first line treatment. One of the patients received hormone therapy in second line treatment. Only one patient received radiation therapy combined with chemotherapy as an upfront treatment. Another one underwent radiation therapy after failure of the first line. A patient that had isolated gastric metastasis underwent total gastrectomy. In patients with breast cancer, the finding of a gastric lesion may represent metastasis instead of a second malignancy. The incidence of leptomeningeal carcinomatosis varies from 3 to 8% of solid tumors. The most common causes of leptomeningeal carcinomatosis are breast cancer, lung cancer and malignant melanoma.10 In our series, three patients had meningeal infiltration, so we hypothesized that there could be some similarity between meningeal and gastric tumor microenvironment favorable to tumor cell implantation
Conclusions
Although rare, gastric metastasis can have a negative impact on survival, and must be investigated in patients with breast cancer who present with dyspepsia and other upper gastrointestinal symptoms. Diagnostic confirmation involves comparison of the histology of the primary tumor and the metastasis, with immunohistochemistry as an essential tool. In this context, the treatment should be individualized and comprise local or systemic therapy, depending on the progression of the disease. Since survival rates constantly increase with the development of new treatments, one must pay attention to metastasis occurring in sites where it was previously unusual.5,11 The presentation as a single metastasis favors a more positive prognosis. The association with meningeal dissemination suggests that there is some biological similarity between these types of lining epithelium that favors the implantation of tumor cells via hematogeneous pathway.
References
- 1.Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA). Estimativa 2014: Incidência de câncer no Brasil. Rio de Janeiro; 2014. Available from: http://www.saude.sp.gov.br/resources/ses/perfil/gestor/homepage/outros-destaques/estimativa-de-incidencia-de-cancer-2014/estimativa_cancer_24042014.pdf
- 2.Ciulla A, Castronovo G, Tomasello G, et al. Gastric metastases originating from occult breast lobular carcinoma: diagnostic and therapeutic problems. World J Surg Oncol 2008;6:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taal BG, Peterse H, Boot H. Clinical presentation, endoscopic features, and treatment of gastric metastases from breast carcinoma. Cancer 2000;89:214-21. [PubMed] [Google Scholar]
- 4.Taal BG, den Hartog JFC, Steinmetz R, Peterse H. The spectrum of gastrointestinal metastases of breast carcinomaI: stomach. Gastrointest Endosc 1992;38:130-5. [DOI] [PubMed] [Google Scholar]
- 5.Pectasides D, Psyrri A, Pliarchopoulou K, et al. Gastric metastases originating from breast cancer: report of 8 cases and review of the literature. Anticancer Res 2009;29:4759-63. [PubMed] [Google Scholar]
- 6.Yamamoto D, Yoshida H, Sumida K, et al. Gastric tumor from metastasis of breast cancer. Anticancer Res 2010;30:3705-8. [PubMed] [Google Scholar]
- 7.Bognel C, Lasser P, Zimmermann P. [Gastric metastases: apropos of 17 cases]. Ann Chir 1992;46:436-41. [Article in French] [PubMed] [Google Scholar]
- 8.Ambroggi M, Stroppa EM, Mordenti P, et al. Metastatic breast cancer to the gastrointestinal tract: report of five cases and review of the literature. Int J Breast Cancer 2012;2012:439023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis MC, Mason T, Barnett J, et al. Gastric malignancies in breast cancer survivors: pathology and outcomes. Am J Surg 2009;197:633-6. [DOI] [PubMed] [Google Scholar]
- 10.Park KK, Yang SI, Seo KW, et al. A case of metastatic leptomeningeal carcinomatosis from early gastric carcinoma World J Surg Oncol 2012;10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones GE, Strauss DC, Forshaw MJ, et al. Breast cancer metastasis to the stomach may mimic primary gastric cancer: report of two cases and review of literature. World J Surg Oncol 2007;5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]


