Abstract
Solitary fibrous tumor is an uncommon mesenchymal neoplasm. Liver is a rare location of this tumor. We report a case of hepatic solitary fibrous tumor in a 56-year-old female, who presented with right upper abdominal pain. An extended right hepatectomy was performed. Histopathological and immunohistochemical examination revealed solitary fibrous tumor of the liver.
Key words: Solitary fibrous tumor, Liver, Immunohistochemistry
Introduction
Solitary fibrous tumor (SFT) is an uncommon mesenchymal neoplasm affecting the pleura and mediastinum.1 The other sites include the respiratory tract, orbit, peritoneum and central nervous system.1,2 Liver is a rare site of SFT.1,2 Pre-operative diagnosis of SFTs of liver is difficult because of non-specific clinical and radiological features. Moreover fine needle aspiration cytology (FNAC) may be misleading or inconclusive.2 The diagnosis of SFTs is mainly based on the histopathological examination of the resected specimen. Tumor size, cellularity, pleomorphism, mitosis and necrosis suggest its benign or malignant behavior.2 We report a case of SFT of liver in a 56-year-old female diagnosed post-operatively based on histomorphology and immunohistochemistry (IHC).
Case Report
A 56-year-old female presented with right upper abdominal pain for two weeks. There was no history of vomiting, jaundice or fever. There were no other significant medical or surgical illnesses in the past. She denied any drug intake or alcohol abuse. On palpation, there was a mass in the right hypochondrium. Laboratory investigations, including liver function tests and serum tumor markers, were normal in range. Ultrasonography revealed a space-occupying lesion measuring 24 cm in greatest dimension in the liver. Axial cut of contrast enhanced computed tomography (CECT) revealed heterogeneously enhancing mass measuring 23×22×10 cm in the right lobe and segment 4 (Figure 1A). The tumor showed an arterial phase enhancement in the periphery and central portal venous phase enhancement. An ultrasound-guided FNAC was attempted, which revealed only blood elements in the smears. Based on the clinical and radiological findings, a pre-operative diagnosis of hemangioma was made. An extended right hepatectomy was performed.
Figure 1.
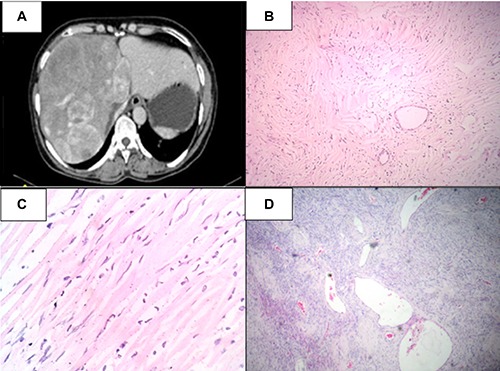
A) Contrast enhanced computed tomography abdomen showing heterogeneously enhancing mass occupying right lobe and segment 4; B) microscopically, the tumor had low to moderate cellularity comprised of spindle to fibroblast-like cells in a collagenous background [hematoxylin and eosin (H&E), 40×]; C) the cells had spindle to elongated nuclei with variable indistinct eosinophilic cytoplasm (H&E, 100×); D) many thin walled ectatic blood vessels were interspersed within the tumor cells and resembled hemangiopericytoma-like areas (H&E, 40×).
On gross examination, the specimen measured 23×22×10 cm. On cut section, a well-circumscribed tumor measuring 20×18×9 cm was seen. The tumor was grayish-white in color, firm in consistency and had whorled appearance. The adjacent liver parenchyma was grossly unremarkable with no evidence of cirrhosis. The resections margins were grossly free of tumor.
Microscopically, the tumor had low to moderate cellularity comprised of spindle to fibroblast-like cells in a collagenous background (Figure 1B). The cells had spindle to elongated nuclei with variable indistinct eosinophilic cytoplasm (Figure 1C). Many thin walled ectatic blood vessels were found interspersed within the tumor cells and resembled hemangiopericytoma-like areas (Figure 1D). There was no evidence of pleomorphism or increase in mitosis. On IHC, these tumor cells were positive for vimentin, CD34 and BCl2. (Figure 2A-C) desmin, smooth muscle actin (SMA), CD31, erythroblast transformation specific related gene (ERG), FVIII, CD117, S100, neuron-specific enolase (NSE), HepPar1, ALK1 and pancytokeratin were negative (Figure 2D). Based on light microscopy and IHC features, a final diagnosis of benign solitary fibrous tumor of liver was made.
Figure 2.
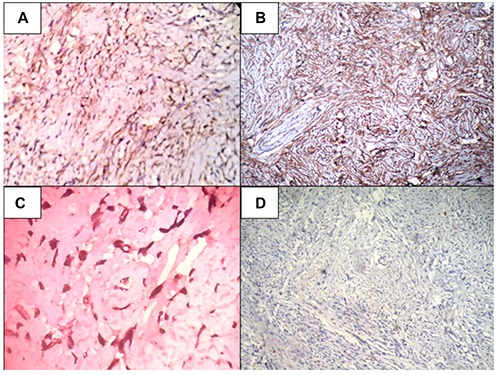
A) The tumor cells positive for vimentin (40×); B) the tumor cells positive for CD34 (100×); C) the tumor cells positive for BCl2 (400×); D) the tumor cells negative for pancytokeratin (100×).
The post-operative period was uneventful and the patient was discharged on seventh post-operative day. At 6-months post-operative follow-up, the patient is doing well.
Discussion and Conclusions
First described in the year 1931, SFT is a rare neoplasm primarily occurring in pleura.1,3 Although it has been reported in other extrathoracic sites, the occurrence in liver is particularly rare.1-3 Bejarano and colleagues have enlisted 51 cases of hepatic SFT.3 Single case report has also been reported by Patra and colleagues.4 In liver it may arise from the inner layer of the liver capsule or from the intrahepatic fibrous connective tissue.3
SFT of liver has a female preponderance with a male:female ratio of 1:2.3 The mean age of presentation is 55 years.4 The clinical presentations of hepatic SFT are non-specific and may range from asymptomatic to pain abdomen, nausea, vomiting and weight loss.4
Radiologically, these tumors are mildly hyperechoic on sonography and appear as a heterogeneous mass with irregular central enhancement during the portal venous phase on CECT.5 On T1-weighted magnetic resonance images this tumor present as low or intermediate intensity and on T2 sequences both hypointense and hyperintense areas are seen.1,4 These findings are suggestive but not diagnostic of hepatic SFTs.1,4,5
Role of FNAC as a diagnostic modality is still not well-established and sometimes may be misleading or inconclusive.2,4,5 Percutaneous trucut biopsy is not favored as it carries the risk of tumor growth.1 The definitive diagnosis of hepatic SFT is based on histopathological examination of the resected specimen.3 Therefore these tumors should not be biopsied prior to surgery if the patient is a candidate for surgical resection. Histologically, the tumor has variable cellularity with spindle shaped cells along with fibrocollagenous stroma. Hemangiopericytoma-like pattern of vessels is characteristic.3 Morphologically, Hepatic SFT has varied differential diagnosis and high on the list are sclerosed hemangioma and leiomyoma.4,5 The other differentials include gastrointestinal stromal tumor, inflammatory pseudotumor, epithelioid hemangioendothelioma, fibrolamellar hepatocellular carcinoma and neurogenic tumors.4,5 Considering the large number of differentials, IHC has an important role in the diagnosis. On IHC, it is consistently positive for vimentin, CD34 and BCl2.3,4 A positive CD34 expression in SFT helps in distinguishing it from leiomyoma (SMA positive), fibrolamellar hepatocellular carcinoma (HepPar1 positive) and neurogenic tumors (S100 and NSE positive).3 Negative expression of CD31, FVIII and ERG helps to distinguish SFT from sclerosed hemangioma and epithelioid hemangioendothelioma, which show CD34 positivity in the endothelial cells.4 CD117 and ALK1 negativity help to rule out gastrointestinal stromal tumor and inflammatory pseudotumor respectively. In the present case, vimentin, CD34 and BCl2 positivity, and SMA, CD31, FVIII, ERG, S100, NSE, HepPar1, CD117 and ALK1 negativity helped in confirming the diagnosis of SFT.
SFT of liver is often a benign tumor with a good prognosis.4 However large size, cellular pleomorphism, atypia, high mitotic activity and central necrosis suggest its malignant behavior.2,3 Local recurrence and distant metastasis is more frequent in tumors with mitotic index of more than 4 per 10 high power fields. Also a factor suggestive of malignancy as reported in literature is the presence of metastatic lesions.2,3 Surgical removal of the tumor with clear margins is the treatment of choice. In case of incomplete resections or histopathological features of malignancy adjuvant chemotherapy or radiotherapy is required.2-4
Although SFT of liver is a rare tumor, it should be considered in the differentials of hepatic mass. Morphologically it may mimic other hepatic spindle cell neoplasms. Thus IHC plays a pivotal role in its diagnosis. Surgical resection with free margins is the mainstay of treatment.
References
- 1.Liu Q, Liu J, Chen W, et al. Primary solitary fibrous tumors of liver: a case report and literature review. Diagn Pathol 2013;8:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korkolis DP, Apostolaki K, Aggeli C, et al. Solitary fibrous tumor of the liver expressing CD34 and vimentin: a case report. World J Gastroenterol 2008;14:6261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bejarano González N, García Borobia FJ, Romaguera Monzonís A, et al. Solitary fibrous tumor of the liver. Case report and review of the literature. Rev Esp Enferm Dig 2015;107:633-9. [DOI] [PubMed] [Google Scholar]
- 4.Patra S, Vij M, Venugopal K, Rela M. Hepatic solitary fibrous tumor: report of a rare case. Indian J Pathol Microbiol 2012;55:236-8. [DOI] [PubMed] [Google Scholar]
- 5.Peng L, Liu Y, Ai Y, et al. Skull base metastases from a malignant solitary fibrous tumor of the liver. A case report and literature review. Diagn Pathol 2011;6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]


