Abstract
In this work, we presented a ratiometric electrochemical immunosensor based on redox substrate and immunoprobe. Carboxymethyl cellulose-Au-Pb2+ (CMC-Au-Pb2+) and carbon-Au-Cu2+ (C-Au-Cu2+) nanocomposites were firstly synthesized and implemented as redox substrate and immunoprobe with strong current signals at −0.45 V and 0.15 V, respectively. Human immunoglobulin G (IgG) was used as a model analyte to examine the analytical performance of the proposed method. The current signals of CMC-Au-Pb2+ (Isubstrate) and C-Au-Cu2+ (Iprobe) were monitored. The effect of redox substrate and immunoprobe behaved as a better linear relationship between Iprobe/Isubstrate and Lg CIgG (ng mL−1). By measuring the signal ratio Iprobe/Isubstrate, the sandwich immunosensor for IgG exhibited a wide linear range from 1 fg mL−1 to 100 ng mL−1, which was two orders of magnitude higher than other previous works. The limit of detection reached 0.26 fg mL−1. Furthermore, for human serum samples, the results from this method were consistent with those of the enzyme linked immunosorbent assay (ELISA), demonstrating that the proposed immunoassay was of great potential in clinical diagnosis.
Electrochemical immunoassay has become an effective analytical method for the determination of ultra-trace antibodies and antigens, owing to its advantages of high sensitivity, low cost, rapid detection, miniaturization1. For sensitive detection of ultra-trace target, signal amplification procedure is necessary, which is commonly realized by using nanomaterials and enzymes to promote the electron transport2,3,4. Generally, the current response of amperometric immunoassay is at tens of nanoamperes or several microamperes levels5,6, which makes the assay become vulnerable to surroundings, resulting in an issue of the reproducibility of electrochemical immunoassay.
The two-channel ratiometric detection has been introduced in fluorescence7,8, colorimetric9,10 and electrochemical analysis10,11, recording two parallel signals at different wavelengths or redox potentials. Compared to measurements performed with single signal, two-channel ratiometric strategy has been shown to increase accuracy and reproducibility of targets detection12,13. Up to now, ratiometric electrochemical detections of ultra-trace heavy metal ions10, small molecules14 and DNA15,16, which is commonly assisted by oligonucleotide, have attracted many attentions. Ratiometric detection based on two-channel method is promising for realizing ultrasensitive and highly reproducible electrochemical immunoassay.
Recently, some DNA-assisted two-channel ratiometric electrochemical immunoassays have been reported17,18. In these methods, DNA hybridization was employed, using the redox species-labeled oligonucleotides to functionalize antibodies and substrate, to monitor the immunoreaction. Although the DNA-assisted ratiometric electrochemical immunoassay exhibited good reproducibility and sensitivity, the limitation of this approach was that the complex, expensive and time-consuming preparation of the redox species-labeled oligonucleotides and the oligonucleotide-tagged antibodies was involved. If these obstacles were resolved by DNA-free method, the two-channel ratiometric immunoassay will be of great significance in clinical diagnosis.
Herein, a novel DNA-free ratiometric sandwich electrochemical immunoassay was firstly developed using redox substrate and immunoprobe. Carboxymethyl cellulose-Au-Pb2+ (CMC-Au-Pb2+) nanocomposites and carbon-Au-Cu2+ (C-Au-Cu2+) nanocomposites, as two new redox materials with powerful current signals at −0.45 V and 0.15 V, were synthesized by electrostatic adsorption and coordination effect, respectively. CMC-Au-Pb2+ and C-Au-Cu2+ were implemented as redox substrate and probe, respectively. The human immunoglobulin G (IgG) served as a model analyte6. By measuring the ratio of peak currents of immunoprobe and substrate (Iprobe/Isubstrate), the proposed immunosensor for IgG detection exhibited a wide linear detection range from 1 fg mL−1 to 100 ng mL−1, which was two orders of magnitude wider than other sandwich immunoassays. For human serum detection, the results of the proposed method were consistent with those of ELISA method, demonstrating its great potential in clinical diagnosis.
Results and Discussion
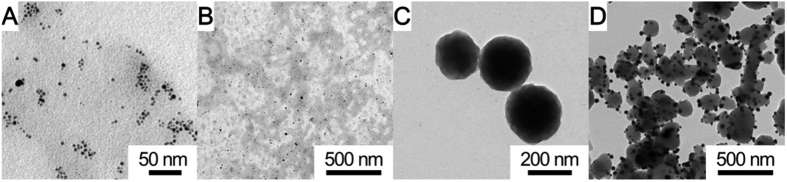
Transmission electron microscopy (TEM) images revealed that Au nanoparticle (AuNP) about 10 nm in diameter was densely distributed on the as-synthesized carboxymethyl cellulose-Au (CMC-Au) film (Fig. 1A,B and S1A). Scanning electron microscopy (SEM) images indicated that an even CMC-Au film turned out to be rough after coating Pb2+ (Fig. S1B). The obtained CMC-Au-Pb2+ film was insoluble and could firmly adhere to the electrode, resulting from the chelating properties between CMC and lead ions19,20.
Figure 1.
TEM images of CMC-Au (A,B), CNP (C) and CNP-Au (D).
After the stepwise microwave-assisted reactions, the carbon nanoparticle (CNP) approximately 200 nm in size was obtained (Fig. 1C), and AuNPs about 30 nm in diameter were distributed on the carbon nanoparticle-Au (CNP-Au) nanocomposites (Fig. 1D). X ray photoelectron spectra (XPS) were performed to investigate the composition of CMC-Au-Pb2+ and C-Au-Cu2+ (Fig. S2). C1s and O1s corresponded to CMC (Fig. S2A) and CNP (Fig. S2D). The Au4f peaks (83.9 eV and 87.5 eV; 84.4 eV and 87.6 eV) were consistent with Au0,21 demonstrating the existence of AuNP (Fig. S2B,E). For CMC-Au-Pb2+, the Pb4f peaks (139.1 eV and 143.8 eV) were consistent with Pb2+22, indicating that Pb2+ was successfully adsorbed by CMC-Au (Fig. S2A,C). While the Cu2p peaks (935.9 eV, 943.4 eV, 955.5 eV and 963.3 eV) consistent with Cu2+ proved that Cu2+ was adsorbed by CNP-Au (Fig. S2D,F)23.
CMC-Au and CNP-Au were characterized by UV-visible (UV-vis) spectra (Fig. S3). No adsorption peaks were observed for CMC and CNP. After the reduction of HAuCl4, two peaks at 516 nm and 536 nm were observed for CMC-Au and CNP-Au, respectively. These adsorption peaks were mainly derived from Au nanoparticles, suggesting that CMC-Au and CNP-Au were obtained, and their peak position differences were mainly caused by the size of Au nanoparticles3. After CNP-Au was mixed with Cu2+, the adsorption peak shifted to 536 nm. Furthermore, CMC-Au-Pb2+ and CNP-Au-Cu2+ were characterized by Fourier transform infrared spectra (FT-IR) (Fig. S4A,B). The peaks at 2980 cm−1 corresponded to methylene and methyl groups. The peaks at 1384 cm−1 and 1639 cm−1 were from methyl groups and carbonyl groups, respectively. No specific peaks were observed for CNP at <1500 cm−1, resulting from the carbonation of glucose. The peaks at 3500 cm−1 were derived from carboxyl and hydroxyl groups.
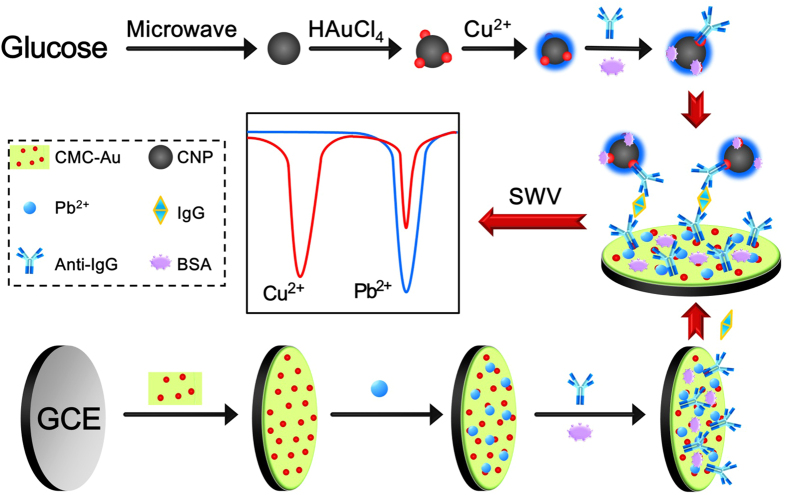
Conventionally, in sandwich immunoassay, the capture antibodies and labeled antibodies were immobilized on the substrate and the probes, respectively. Antigens were subsequently recognized by the substrate and probe24,25,26. If the substrate was fabricated by redox material27,28, the analyte can be detected simultaneously by the signals of substrate and probe, respectively. In this case, the DNA-free ratiometric immunoassay was presented and its principle was illustrated in Fig. 2. CMC-Au-Pb2+ and C-Au-Cu2+ were simply synthesized by mixing CMC-Au with Pb2+ and CNP-Au with Cu2+, which were used as redox substrate and probes, respectively. After immunoreaction and electrochemical measurement, two parallel signals of Cu2+ and Pb2+ were obtained. Based on the linear relation between Iprobe/Isubstrate and Lg CIgG (ng mL−1), the quantity of IgG was determined.
Figure 2. Schematic illustration of the function principle and the fabrication procedure of the proposed immunosensor.
The cross-talk level of redox peaks of CMC-Au-Pb2+ and C-Au-Cu2+ was investigated by square wave voltammetry (SWV) (Fig. S5). No current peaks for CMC-Au and CNP-Au appeared in the potential range of −1.3 V to 0.5 V. In contrast, two strong signals at −0.45 V and 0.15 V for CMC-Au-Pb2+ and C-Au-Cu2+ were observed, which were derived from the enriched Pb2+ and Cu2+. In this case, CMC-Au-Pb2+ and C-Au-Cu2+ were used as the redox substrate and immunoprobe.
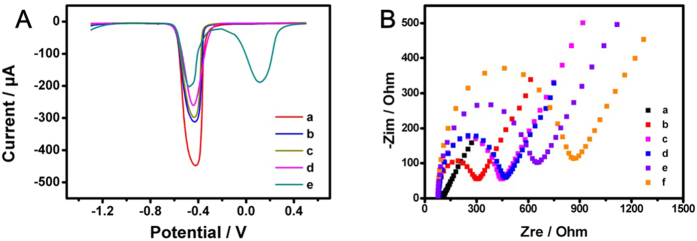
The stepwise fabrication procedures of this immunosensor were monitored by SWV (Fig. 3A) and electrochemical impedance spectroscopy (EIS) (Fig. 3B), respectively. After Pb2+ incubated on the CMC-Au modified glassy carbon electrode (GCE), a strong current peak at −0.45 V was observed, suggesting that Pb2+ was successfully adsorbed by CMC-Au (curve a). The current signal of substrate gradually decreased when goat-anti-human immunoglobulin G (anti-IgG), bovine albumin (BSA) and IgG were subsequently incubated on the substrate (curve b-d), indicated that the immunosensor was obtained and IgG was recognized. The signal decrease was caused by anti-IgG, BSA and IgG, which can hinder the electron transport29. After the incubation of immunoprobe, the signal decrease of substrate was observed and the signal of probe appeared (curve e). EIS was performed in 5 mM [Fe(CN)6]4−/3− aqueous solution containing 0.1 M KCl. The Nyquist plots were shown in Fig. 3B. Compared with bare GCE (curve a), a larger semicircle was obtained for electrode covered with CMC-Au-Pb2+ film (curve b). The diameter of semicircle gradually increased when anti-IgG and BSA were subsequently coated, suggesting that the immunosensor was obtained (curve c and d). Then the resistance increased with the incubation of IgG, indicating that IgG immune reacted with anti-IgG (curve e). The resistance further increased when the probes were coated, demonstrating that the probe was fixed (curve f). These resistance increases were derived from that the proteins hindered the electron transport30,31.
Figure 3.
(A) SWV responses of the modified electrodes: CMC-Au-Pb2+/GCE (a), Anti-IgG/CMC-Au-Pb2+/GCE (b), BSA/Anti-IgG/CMC-Au-Pb2+/GCE (c), IgG/BSA/Anti-IgG/CMC-Au-Pb2+/GCE (d) and C-Au-Cu2+-IgG/IgG/BSA/Anti-IgG/CMC-Au-Pb2+/GCE (e). (B) EIS of the modified electrodes: bare GCE (a), CMC-Au-Pb2+/GCE (b), Anti-IgG/CMC-Au-Pb2+/GCE (c), BSA/Anti-IgG/CMC-Au-Pb2+/GCE (d), BSA/Anti-IgG/CMC-Au-Pb2+/GCE (e) and C-Au-Cu2+-IgG/BSA/Anti-IgG/CMC-Au-Pb2+/GCE (f).
The pH of the detection solution can significantly affect the sensitivity of the immunosensor. The effect of pH on the current response was investigated as shown in Fig. S6. After immunoassay, the Isubstrate generally increased from pH 4.0 to 5.5 and then slightly decreased from pH 5.5 to 6.0. The change trend of Iprobe caused by pH was similar with that of Isubstrate. This indicated that pH 5.5 was optimal for detecting IgG.
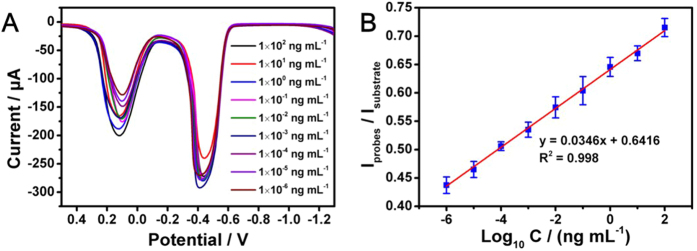
Under the optimized pH, the analytical property of the proposed immunosensors for IgG was investigated by detecting a series of standard IgG samples (Fig. 4A). Isubstrate was irregularly changed with adding IgG, which was consistent with that of Iprobe. Obviously, no satisfactory calibration curves would be obtained between single current signal (Isubstrate or Iprobe) and logarithm of IgG concentration. Unexpectedly, a good linear relation between Iprobe/Isubstrate and logarithm of IgG concentration existed (Fig. 4B). The linear regression equation was Iprobe/Isubstrate = 0.0346Lg C (ng mL−1) + 0.6416 with a correlation coefficient of 0.998. The proposed immunosensor for IgG exhibited a wide linear detection range (LDR) from 1 fg mL−1 to 100 ng mL−1 with an ultralow limit of detection (LOD) of 0.26 fg mL−1 (Fig. 4B). The results indicated that the two-channel ratiometric method was effective for ultrasensitive electrochemical immunoassay. The comparison of this method with some previous works was illustrated in the Table 1. It can be seen that present method possessed a wider LDR and lower LOD.
Figure 4.
(A) SWV responses of the immunosensor for different concentrations of IgG. (B) Calibration curves between the Isubstrate/Iprobe and logarithm of IgG concentration.
Table 1. Comparison of the performances of the present and referenced electrochemical biosensors for IgG.
| Method* | LDR | LOD | Refs |
|---|---|---|---|
| (ng mL−1) | (ng mL−1) | ||
| DPV | 0.01–10.0 | 6.9 × 10−3 | 33 |
| DPV | 0.1–1 × 105 | 0.05 | 34 |
| DPV | 0.01–500 | 9.7 × 10−3 | 35 |
| DPV | 0.01–100 | 1 × 10−3 | 36 |
| EIS | 0.5–125 | 0.02 | 37 |
| i-t | 5 × 10−5–5 | 3.2 × 10−6 | 38 |
| LSV | 1 × 10−6–1 | 5 × 10−7 | 39 |
| PC | 1 × 10−5–1.0 | 6 × 10−6 | 40 |
| SWV | 30-1 × 103 | 25 | 41 |
| SWV | 1 × 10−3–0.1 | 5 × 10−5 | 42 |
| SWV | 1 × 10−5–100 | 2.6 × 10−6 | This Work |
*Differential pulse voltammetry (DPV), amperometric i-t curve (i-t), photocurrent analysis (PC), linear sweep voltammetry (LSV).
To investigate the repeatability, 10 ng mL−1 IgG was determined by the proposed immunosensor for three times. The results showed small deviation of ±2.35%, proving its fantastic repeatability. In order to testify the specificity, the 10 ng mL−1 IgG samples containing 100 ng mL−1 interfering substances, such as glucose (GC), dopamine (DA), uric acid (UA), ascorbic acid (AA), glutamic acid (Glu), lactic acid (LA) and BSA, were analyzed. As shown in Fig. S7, the obtained current signals were not affected by the interference substances, demonstrating the admirable specificity of this immunosensor. The stability of the immunosensor was examined by determining the IgG. After 14 days, the capacity of the immunosensor remained 85%, suggesting the good stability of the immunosensor. Compared with some recently reports, results indicated that the proposed immunosensor was more sensitive (Table 1).
Generally, for a normal adult, the concentration of IgG is about 7–16 mg mL−1 in serum. The human serum was firstly determined by ELISA method. After being diluted 1:106, the received human serum samples were investigated by the proposed immunosensor. The results were listed in the Table S1, and relative error (RE) was less than 10%. The results of this immunoassay were consistent with the ELISA method, indicating its great potentials in clinic diagnosis.
Conclusion
In summary, CMC-Au-Pb2+ and C-Au-Cu2+ firstly synthesized as redox species, with parallel current signals. A novel ratiometric electrochemical immunosensor was successfully developed using CMC-Au-Pb2+ and C-Au-Cu2+ to construct the redox substrate and probe, respectively. The linear range of the proposed immunoassay (1 fg mL−1 to 100 ng mL−1), two orders of magnitude higher than other sandwich electrochemical immunoassay. This immunosensor exhibited excellent sensitivity, reproducibility, specificity, stability and practicability. This ratiometric method could be easily extended to the detection of other targets.
Methods
Materials
CMC (M.W. 250000) was purchased from Aladdin Industrial Corporation (Shanghai, China). Pb(NO3)2 and Cu(NO3)2·3H2O were bought from Sinopharm Chemical Reagents Co., Ltd. (Beijing, China). AA (99.7%), UA, HAuCl4·3H2O (99.9%), GC, LA, Glu and dopamine hydrochloride were commercially obtained from Alfa Aesar (Beijing, China). IgG, anti-IgG and BSA (standard grade) were obtained from Xinjingke Biotechnology Co., Ltd. (Beijing, China). Clinical human serum samples were provided by Capital Normal University Hospital (Beijing, China). All other reagents were used as received. Deionized-distilled water was purified by Olst ultrapure K8 apparatus (Olst, Ltd., resistivity > 18 MΩ cm).
Apparatus
TEM was performed with a JEOL-100CX electron microscope under 80 kV accelerating voltage (H7650, Hitachi, Japan). SEM was performed with a Hitachi SU8010 SEM. X-ray photoelectron spectroscopy (XPS) analysis was conducted on an ESCALAB 250 X-ray Photoelectron Spectroscope (Thermofisher, American). The UV-vis spectroscopy and Fourier transform infrared spectroscopy (FT-IR) were recorded by a UV-2550 spectrophotometer (Shimadzu, Japan) and a FT-IR spectrophotometer, respectively. EIS measurements were performed using Princeton PARSTAT 2273 (America). SWV was performed on a CHI832 electrochemical work station (Chenhua Instruments Co., Shanghai, China) with a three-electrode system which was consisted of a platinum wire as the auxiliary electrode, an Ag/AgCl electrode (saturated KCl) as the reference electrode and a GCE as the working electrode.
Synthesis of C-Au-Cu2+ immunoprobe
The stepwise fabrication procedures of immunoprobe were shown in Fig. 2. CNP was synthesized according to the literature32. In brief, a homogeneous solution was produced by 1% glucose mixed with 10% sodium citrate. Further, the prepared solution was heated to 170 °C for 30 min in microwave reaction instrument (250 W). CNP was centrifuged, dispersed. After mixing CNP with 100 μL 4% HAuCl4, a microwave reaction (100 °C, 10 min) was carried out. The CNP-Au nanocomposite was centrifuged and dispersed to 5 mL water. For adsorbing Cu2+, 1 mL CNP-Au was mixed with 1 mL100 mM Cu2+ for 3 h. Then the obtained C-Au-Cu2+ was centrifuged, washed and dispersed to 1 mL water. 5 mL C-Au-Cu2+ was mixed with 500 μL anti-IgG with stirring gently for 8 h. After that, the obtained C-Au-Cu2+/anti-IgG was blocked by 1% BSA for 1 h, and CNP-Au-Cu2+/anti-IgG/BSA was centrifuged, washed for several times, and dispersed to 5 mL water.
Synthesis of CMC-Au
After a homogeneous solution was prepared by 1 mL 1% CMC and 10 μL 4% HAuCl4, 40 μL 1.5% NaBH4 was quickly injected with vortex mixing for 1 min. The solution turned from light yellow to red. Then, the CMC-Au was obtained and diluted to 16 mL prior to use.
Fabrication of immunosensor
The schematic illustration of the fabrication procedure of the proposed immunosensor was shown in Fig. 2. GCE (Φ = 4 mm) was prepared by polishing with the alumina powders and successively sonication washing with water. After 20 μL CMC-Au was dropped and dried at 37 °C, the obtained GCE was dipped in 10 mM Pb2+ for 10 min. After that, a thin CMC-Au-Pb2+ film was formed and washed with plenty of deionized water. 80 μL 100 μg mL−1 anti-IgG was incubated on CMC-Au-Pb2+/GCE for 12 h at 4 °C. The obtained electrode was incubated with 1% BSA at 37 °C for 1 h to block the remaining active points. Therefore, the immunosensor was accomplished and stored at 4 °C.
Electrochemical measurement
The immunosensor was incubated with 80 μL IgG for 1 h at 37 °C. Next, 20 μL C-Au-Cu2+/anti-IgG/BSA was incubated for 1 h at 37 °C. The modified GCE was carefully washed with water after each step. Subsequently, SWV was performed from −1.3 V to 0.6 V in acetate buffer with pulse amplitude of 25 mV and an increase E of 4 mV s−1.
Additional Information
How to cite this article: Tang, Z. and Ma, Z. Ratiometric ultrasensitive electrochemical immunosensor based on redox substrate and immunoprobe. Sci. Rep. 6, 35440; doi: 10.1038/srep35440 (2016).
Supplementary Material
Acknowledgments
This research was financed by grants from the National Natural Science Foundation of China (21273153, 21673143), Natural Science Foundation of Beijing Municipality (2132008), and the Project of the Construction of Scientific Research Base by the Beijing Municipal Education Commission.
Footnotes
Author Contributions Z.M. proposed and supervised the project. Z.T. carried out the whole experiment and wrote the main manuscript text.
References
- Chikkaveeraiah B. V., Bhirde A. A., Morgan N. Y., Eden H. S. & Chen X. Y. Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano 6, 6546–6561 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C. Z., Yang G. H., Li H., Du D. & Lin Y. H. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 87, 230–249 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha K., Agasti S. S., Kim C., Li X. N. & Rotello V. M. Gold nanoparticles in chemical and biological sensing. Chem.Rev. 112, 2739–2779 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Feng H. B. & Li J. H. Graphene oxide: preparation, functionalization, and electrochemical applications. Chem. Rev. 112, 6027–6053 (2012). [DOI] [PubMed] [Google Scholar]
- Wang Q. et al. Electrochemical immunosensor for detecting the spore wall protein of Nosema bombycis based on the amplification of hemin/G-quadruplex DNAzyme concatamers functionalized Pt@Pd nanowires. Biosens.Bioelectron. 60, 118–123 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang S. et al. A double signal electrochemical human immunoglobulin G immunosensor based on gold nanoparticles-polydopamine functionalized reduced graphene oxide as a sensor platform and AgNPs/carbon nanocomposite as signal probe and catalytic substrate. Biosens. Bioelectron. 77, 1078–1085 (2016). [DOI] [PubMed] [Google Scholar]
- Royzen M., Dai Z. H. & Canary J. W. Ratiometric displacement approach to Cu(II) sensing by fluorescence. J. Am. Chem. Soc. 127, 1612–1613 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang X. L., Xiao Yi. & Qian X. H. A ratiometric fluorescent probe based on FRET for imaging Hg2+ ions in living cells. Angew. Chem. Int. Ed. 47, 8025–8029 (2008). [DOI] [PubMed] [Google Scholar]
- Liu J. C. et al. Highly sensitive colorimetric detection of 17β-estradiol using split DNA aptamers immobilized on unmodified gold nanoparticles. Sci. Rep. 4, 7571 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., She S., Zhang J. W., Bayaguud A. & We Y. G. Label-free colorimetric detection of mercury via Hg2+ ions-accelerated structural transformation of nanoscale metal-oxo clusters. Sci. Rep. 5, 16316 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong E. H. et al. A ratiometric electrochemical biosensor for sensitive detection of Hg2+ based on thymine-Hg2+-thymine structure. Anal. Chim. Acta 85, 3242–3248 (2015). [DOI] [PubMed] [Google Scholar]
- Deng W. P. et al. Diagnosis of schistosomiasis japonica with interfacial co-assembly based multi-channel electrochemical immunosensor arrays. Sci. Rep. 3, 1789 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz M. D., Hsu S. H., Reinhoudt D. N., Velders A. H. & Huskens J. Ratiometric fluorescent detection of an anthrax biomarker at molecular printboards. Angew. Chem. Int. Ed. 49, 5938–5941 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang X., Wu L., Zhou J. W., Zhang X. H. & Chen J. H. A new ratiometric electrochemical sensor for sensitive detection of bisphenol A based on poly-β-cyclodextrin/electroreduced graphene modified glassy carbon electrode. J. Electroanal. Chem. 742, 97–103 (2015). [Google Scholar]
- Du Y. et al. Reagentless, ratiometric electrochemical DNA sensors with improved robustness and reproducibility. Anal. Chem. 86, 8010–8016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Zhang X. H., Liu W., Xiong E. H. & Chen J. H. Sensitive electrochemical aptasensor by coupling “signal-on” and “signal-off” strategies. Anal. Chem. 85, 8397–8402 (2013). [DOI] [PubMed] [Google Scholar]
- Ren K. W., Wu J., Yan F. & Ju H. X. Ratiometric electrochemical proximity assay for sensitive one-step protein detection. Sci. Rep. 4, 4360 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K. W., Wu J., Yan F., Zhang Y. & Ju H. X. Immunoreaction-triggered DNA assembly for one-step sensitive ratiometric electrochemical biosensing of protein biomarker. Biosens. Bioelectron. 66, 345–349 (2015). [DOI] [PubMed] [Google Scholar]
- Kargl R. et al. Adsorption of carboxymethyl cellulose on polymer surfaces: Evidence of a specific interaction with cellulose. Langmuir 28, 11440–11447 (2012). [DOI] [PubMed] [Google Scholar]
- Hokkanen S., Bhatnagar A. & Sillanpää M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 91, 156–173 (2016). [DOI] [PubMed] [Google Scholar]
- Thomas T. D. & Weightman P. Valence electronic structure of AuZn and AuMg alloys derived from a new way of analyzing Auger-parameter shifts. Phys. Rev. B 33, 5406–5413 (1986). [DOI] [PubMed] [Google Scholar]
- Osaka A., Wang Y. H., Miura Y. & Tsugaru T. X-ray photoelectron spectroscopy of lead fluorosilicate glasses. J. Mater. Sci. 26, 2778–2782 (1991). [Google Scholar]
- Jolley J. G., Geesey G. G., Hankins M. R., Wright R. B. & Wichlacz P. L. Auger electron and X-ray photoelectron spectroscopic study of the biocorrosion of copper by alginic acid polysaccharide. Appl. Surf. Sci. 37, 469–480 (1989). [Google Scholar]
- Wang L. Y., Feng F. & Ma Z. F. Novel electrochemical redox-active species: one-step synthesis of polyaniline derivative-Au/Pd and its application for multiplexed immunoassay. Sci. Rep. 5, 16855 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. et al. An ultrasensitive electrochemical immunosensor based on the catalytical activity of MoS2-Au composite using Ag nanospheres as labels. Sens. Actuators B 206, 30–36 (2015). [Google Scholar]
- Rong Q. F., Feng F. & Ma Z. F. Metal ions doped chitosan–poly(acrylic acid) nanospheres: Synthesis and their application in simultaneously electrochemical detection of four markers of pancreatic cancer. Biosens. Bioelectron. 75, 148–154 (2016). [DOI] [PubMed] [Google Scholar]
- Han J. M., Ma J. & Ma Z. F. One-step synthesis of graphene oxide-thionine-Au nanocomposites and its application for electrochemical immunosensing. Biosens. Bioelectron. 47, 243–247 (2013). [DOI] [PubMed] [Google Scholar]
- Li F. Y. et al. An ultrasensitive label-free electrochemical immunosensor based on signal amplification strategy of multifunctional magnetic graphene loaded with cadmium ions. Sci. Rep. 6, 21281 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X. L., Chen X., Han J. M., Ma J. & Ma Z. F. Triple signal amplification using gold nanoparticles, bienzyme and platinum nanoparticles functionalized graphene as enhancers for simultaneous multiple electrochemical immunoassay. Biosens. Bioelectron. 53, 65–70 (2014). [DOI] [PubMed] [Google Scholar]
- Wang S. W. et al. 3D microfluidic origami electrochemiluminescence immunodevice for sensitive point-of-care testing of carcinoma antigen 125. Sens. Actuators B 176, 1–8 (2013). [Google Scholar]
- Ge L. et al. Three-dimensional paper-based electrochemiluminescence immunodevice for multiplexed measurement of biomarkers and point-of-care testing. Biomaterials 33, 1024–1031 (2012). [DOI] [PubMed] [Google Scholar]
- Xu T., Liu N., Yuan J. & Ma Z. F. Triple tumor markers assay based on carbon-gold nanocomposite. Biosens. Bioelectron. 70, 161–166 (2015). [DOI] [PubMed] [Google Scholar]
- Lai G. S., Zhang H. L., Yong J. & Yu A. M. In situ deposition of gold nanoparticles on polydopamine functionalized silica nanosphere for ultrasensitive nonenzymatic electrochemical immunoassay. Biosens. Bioelectron. 47, 178–183 (2013). [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zheng Y. Q., Kong R. M., Xia L. & Qu F. L. Ultrasensitive electrochemical immunosensor based on horseradish peroxidase (HRP)-loaded silica-poly(acrylic acid) brushes for protein biomarker detection. Biosens. Bioelectron. 75, 383–388 (2016). [DOI] [PubMed] [Google Scholar]
- Li R. et al. 4-amino-1-(3-mercapto-propyl)-pyridine hexafluorophosphate ionic liquid functionalized gold nanoparticles for IgG immunosensing enhancement. Anal. Chem. 86, 1789–1793 (2014). [DOI] [PubMed] [Google Scholar]
- Cui Y. L., Chen H. F., Tang D. P., Yang H. H. & Chen G. N. Au(III)-promoted polyaniline gold nanospheres with electrocatalytic recycling of self-produced reactants for signal amplification. Chem. Commun. 48, 10307–10309 (2012). [DOI] [PubMed] [Google Scholar]
- Tabrizi M. A. & Shamsipur M. & Mostafaie, A high sensitive label-free immunosensor for the determination of human serum IgG using overoxidized polypyrrole decorated with gold nanoparticle modified electrode. Mat. Sci. Eng. C 59, 965–969 (2016). [DOI] [PubMed] [Google Scholar]
- Wang Y. L. et al. Novel signal amplification strategy for ultrasensitive sandwich-type electrochemical immunosensor employing Pd–Fe3O4-GS as the matrix and SiO2 as the label. Biosens. Bioelectron. 74, 59–65 (2015). [DOI] [PubMed] [Google Scholar]
- Ma L. N., Ning D. L., Zhang H. F. & Zheng J. B. Au@Ag nanorods based electrochemical immunoassay for immunoglobulin G with signal enhancement using carbon nanofiberspolyamidoamine dendrimer nanocomposite. Biosens. Bioelectron. 68, 175–180 (2015). [DOI] [PubMed] [Google Scholar]
- Wang G. L., Shu J. X., Dong Y. M., Wu X. M. & Li Z. J. An ultrasensitive and universal photoelectrochemical immunoassay based on enzyme mimetics enhanced signal amplification. Biosens. Bioelectron. 66, 283–289 (2015). [DOI] [PubMed] [Google Scholar]
- Zarei H., Ghourchian H., Eskandari K. & Zeinali M. Magnetic nanocomposite of anti-human IgG/COOH-multiwalled carbon nanotubes/Fe3O4 as a platform for electrochemical immunoassay. Anal. Biochem. 421, 446–45 (2012). [DOI] [PubMed] [Google Scholar]
- Zhou M. et al. Application of hydrogel prepared from ferrocene functionalized amino acid in the design of novel electrochemical immunosensing platform. Biosens. Bioelectron 49, 243–248 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.