Abstract
This study was designed to evaluate the prognostic value of platelet to lymphocyte ratio (PLR) in hepatocellular carcinoma (HCC). A comprehensive literature search for relevant studies was performed in Web of science, Embase and Pubmed. A total of nine studies with 2017 patients were included in this meta-analysis, and combined hazard ratio (HR) or odds ratio (OR) and 95% confidence intervals (95%CIs) were served as effect measures. Pooled results showed that elevated PLR was associated with poor overall survival (OS) (HR = 1.63, 95%CI: 1.42–1.88, p = 0.000; I2 = 0.0%, Ph = 0.637) and poor disease-free survival (DFS)/recurrence-free survival (RFS) (HR=1.32, 95%CI: 1.15–1.52, p = 0.000; I2 = 19.3%, Ph = 0.287) in HCC patients. In addition, high PLR was not significantly correlated with the presence of vascular invasion, tumor multifocality, poor tumor grade or high level of serum AFP (>400 ng/ml). In conclusion, elevated PLR indicated a poor prognosis for patients with HCC. PLR may be a reliable, easily-obtained, and low cost biomarker with prognostic potential for HCC.
Hepatocellular carcinoma (HCC) is one of the most common and aggressive malignancies. Meanwhile, it is the third leading cause of cancer-related deaths across the world1. Despite the major treatment methods of HCC, including surgical resection, liver transplantation, radiofrequency ablation, transarterial chemoembolization (TACE) and molecular therapy have achieved significant improvements, the prognosis of the patients remains unsatisfactory due to the distant metastasis and high fatality2. Several criteria have been proposed to predict patient prognosis, like functional liver reserve, Tumor Node Metastasis (TNM), Barcelona Clinic Liver Cancer (BCLC) score and Cancer of the Liver Italian Program (CLIP) staging score. However, since these criteria are cumbersome, they are rarely used in routine clinical practice. Thus, the identification of an efficient, simple and easily-obtained prognostic biomarker, especially serum biomarkers for prognosis and recurrence of HCC, is essential.
Recently, a variety of inflammatory indices such as C-reactive protein (CRP), neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and modified Glasgow prognostic score (mGPS) have demonstrated their prognostic value in multiple cancers3,4,5,6. Among these markers, elevated PLR was identified as an unfavourable prognostic factor in various cancers such as colorectal cancer7, breast cancer8 and gastric cancer9. The prognostic value of PLR in HCC has also been investigated10,11,12.
Nevertheless, conflicting data have emerged concerning the prognostic value of PLR to predict disease progression and overall survival (OS) in HCC. We therefore collected the available publications and conducted this meta-analysis to evaluate the impact of PLR for OS and disease-free survival (DFS)/recurrence-free survival (RFS) in HCC. In addition, the correlation between PLR and patients’ clinicopathological features was also examined.
Results
Selection and characteristics of studies
The literature searching progress was showed in Fig. 1. A total of 911,13,14,15,16,17,18,19,20 eligible studies published between 2012 and 2016 with 2017 patients were identified. The basic characteristics of the original studies were presented in Table 1. Of these studies, two studies11,16 were reported by the same center,but according to the inclusion and exclusion criteria, the patients included in these two studies were not overlapping. Five studies11,14,16,17,18 were conducted in China, the other four studies13,15,19,20 were conducted in Hong Kong China, USA, UK and Singapore, respectively. Surgical resection as initial treatment for HCC was reported in 6 studies13,14,16,18,19,20. Mix treatment (locoregional, systemic treatments and supportive care)15 and TACE11 were reported in one study.respectively. One study17 just demonstrated that all patients were not receiving sorafenib as systemic treatment instead of providing details of the treatment. Seven studies11,14,16,17,18,19,20 defined OS as the length of time from initial treatment to death or last follow up, while the other two studies13,15 generally described OS as overall survival. One study14 gave the definition of DFS as the time from treatment initiation until disease progression or death. Three studies16,18,20 defined DFS as the duration of time between the date of treatment and the date of first recurrence. One study19 provided the definition of RFS as the time from initial treatment to first recurrence and death without disease was censored. One study13 generally described RFS as recurrence-free survival. Sample sizes ranged from 80 to 367. The prognostic value of PLR for OS was reported or estimated in all studies, whereas the prognostic significance of PLR in DFS/RFS was only provided in six studies13,14,16,18,19,20 in which the included patients undertook surgical resection as initial treatment. The cut-off values used for PLR in these studies were determined by different methods and ranged from 112.3 to 300. The scores of study quality assessed by Newcastle-Ottawa quality assessment scale ranged from 5 to 8(with a mean of 7). A high value indicated better methodology.
Figure 1. Flow diagram of the study selection procedure.
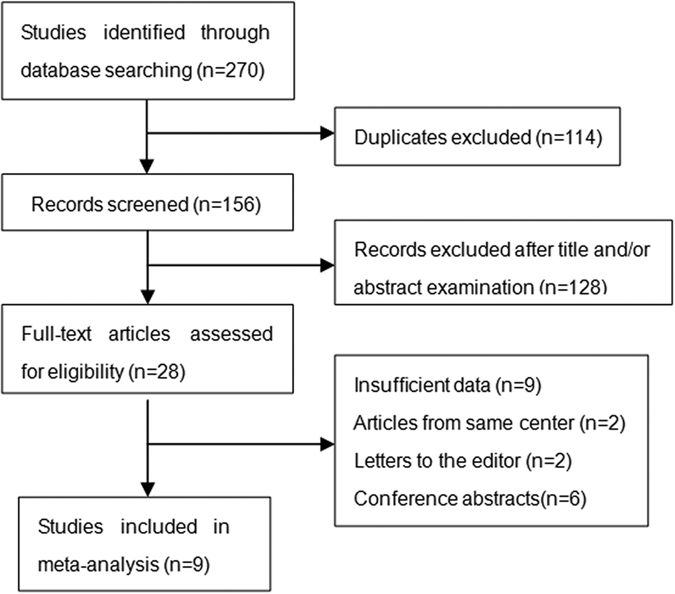
Table 1. Baseline characteristics of all the included studies.
| Study | Year | Country | Sample Size | Mean/median | Stage BCLC/TNM | PLR Cut-off | Treatment | Outcome | Hazard ratio | Follow-up (month) | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pinato18 | 2012 | UK | 112 | 65 | BCLC/A-D | 300 | Mix | OS | R | 10(median) | 7 |
| Sun17 | 2014 | China | 80 | 47 | TNM/I-III | 151.8 | Surgery | OS/DFS | E/R | NA | 7 |
| Li20 | 2014 | China | 243 | 57 | BCLC/C.D | 111.23 | No sorafenib | OS | E | 2.7(median) | 6 |
| Xue15 | 2015 | China | 291 | 53.05 | BCLC/B.C | 150 | TACE | OS | R | 9(median) | 8 |
| Wang16 | 2015 | US | 113 | 55.5 | NA | 118.5 | Surgery | OS/RFS | R/R | NA | 5 |
| Ni19 | 2015 | China | 367 | NA | BCLC/A-C | 150 | Surgery | OS/DFS | E/E | 24(median) | 6 |
| Chan23 | 2015 | Hong Kong | 324 | 56.8 | BCLC/0.A | 150 | Surgery | OS/DFS | R/R | 44.6 | 8 |
| Ji21 | 2016 | China | 321 | 51 | TNM/I-III | 115 | Surgery | OS/DFS | R/E | NA | 8 |
| Goh22 | 2016 | Singapore | 166 | 66 | NA | 290 | Surgery | OS/RFS | E/E | 23(median) | 8 |
BCLC: Barcelona Clinic Liver Cancer score; NA: not available; R: reported in article; E: estimated; OS: overall survival; DFS: disease-free survival; RFS: recurrence-free survival; NOS: Newcastle-Ottawa quality assessment scale.
PLR and OS in HCC
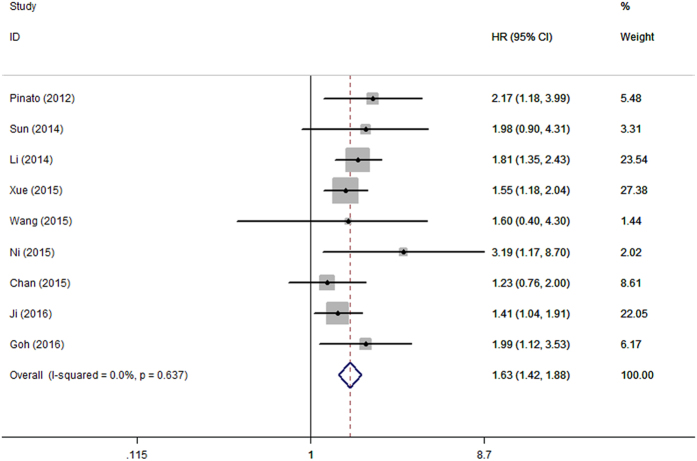
Pooled data from all the nine studies revealed that elevated PLR was significantly associated with poor OS with a pooled HR of 1.63 (95%CI: 1.42–1.88, p = 0.000; Fig. 2), and without significant heterogeneity in the data (I2 = 0.0%, Ph = 0.637).
Figure 2. Forrest plot of hazard ratio (HR) for the association of PLR with OS in patients with HCC.
Subgroup analysis of six studies13,14,16,18,19,20 with 1371 patients who underwent surgery only showed that elevated PLR predicted poor OS (HR = 1.54, 95%CI: 1.24–1.91, p = 0.000; I2 = 0.0%, Ph = 0.499).
PLR and DFS/FRS in HCC
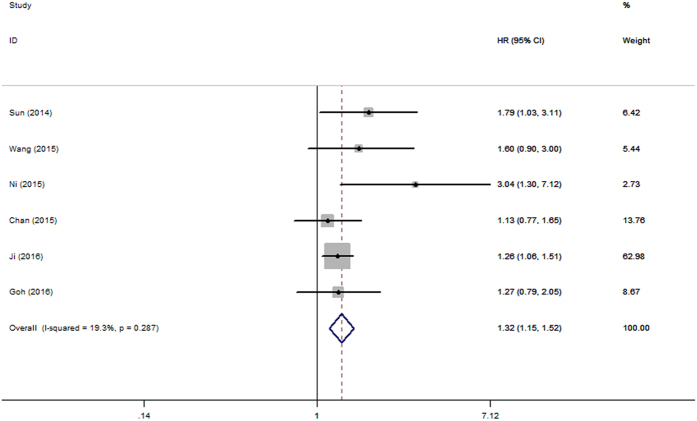
There were six studies13,14,16,18,19,20 with 1371 patients investigated the association between PLR and DFS/RFS. The combined data revealed that elevated PLR was correlated with shortened DFS/RFS (HR = 1.32, 95%CI: 1.15–1.52, p = 0.000; I2 = 19.3%, Ph = 0.287; Fig. 3).
Figure 3. Forrest plot of hazard ratio (HR) for the association of PLR with DFS/RFS in patients with HCC.
Associations between PLR and clinicopathologic features in HCC
The associations between PLR and clinicopathologic parameters were summarized in Table 2. Four studies11,14,15,16 reported data about the correlation between elevated PLR and high level of serum AFP (>400 ng/ml). Three studies suggested no correlation, while one study reported statistical significance. Pooled data from all the four studies did not support a correlation (OR = 1.24, 95%CI: 0.87–1.75, p = 0.229; I2 = 16.1%, Ph = 0.311). As for the other three clinicopathologic features: vascular invasion, tumor multifocality and poor tumor grade, combined data did not show statistical significance either.
Table 2. Associations between PLR and clinicopathologic features.
| Clinicopathologic feature | Study | No. of patients | OR (95%CI) | P | Effects model | Heterogebeity | |
|---|---|---|---|---|---|---|---|
| I2% | Ph | ||||||
| AFP > 400 ng/ml | Pinato18, Sun17, Xue15, Ni19 | 843 | 1.24(0.87–1.75) | 0.229 | F | 16.1 | 0.311 |
| Vascular invasion | Xue15, Ni19, Goh22 | 824 | 1.03(0.70–1.53) | 0.878 | F | 37.1 | 0.204 |
| Tumor multifocality | Pinato18, Ni19, Goh22 | 643 | 1.10(0.58–2.05) | 0.777 | F | 0 | 0.92 |
| Poor tumor grade | Sun17, Ni19, Goh22 | 613 | 1.18(0.73–1.91) | 0.493 | F | 0 | 0.925 |
OR: odds ratio; F: fixed-effects models; Ph: p value of Q test for heterogeneity.
Sensitivity analysis
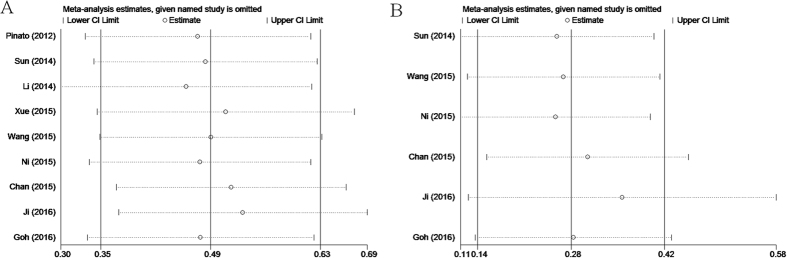
Each individual study was omitted every time to estimate the influence of individual data sets on the pooled HR. The results showed that the corresponding HRs for OS and DFS/RFS were not markedly changed (Fig. 4), indicating the robustness of presented results.
Figure 4.
Sensitivity analysis on the relationship between PLR and (A) OS and (B) DFS/RFS in HCC.
Publication bias
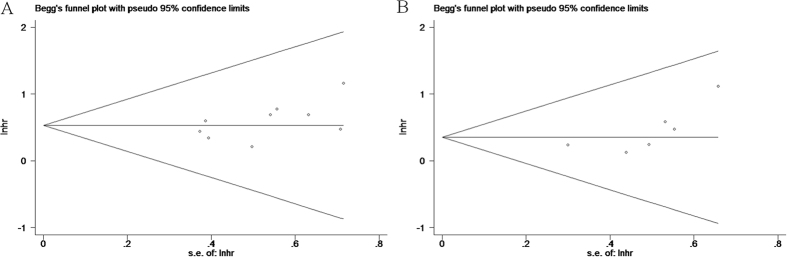
The results of Begg’s test suggest no evidence of publication bias (p = 0.175 for OS and P = 0.060 for DFS/RFS, respectively)(Fig. 5).
Figure 5.
Begg’s funnel plot of publication bias test for (A) OS and (B) DFS/RFS in HCC.
Discussion
In the present study, we mainly investigated the prognostic impact of pretreatment PLR on OS and DFS/RFS in patients with HCC by the method of meta-analysis. The pooled outcomes from nine primary studies with 2017 patients demonstrated that elevated PLR predicted poor OS (HR = 1.63, 95%CI: 1.42–1.88, p = 0.000; I2 = 0.0%, Ph = 0.637) and poor DFS/RFS (HR = 1.32, 95%CI: 1.15–1.52, p = 0.000; I2 = 19.3%, Ph = 0.287) in HCC. It should be noted that the result was not substantially changed in sensitivity analysis. Furthermore, stratified analysis revealed that elevated PLR was also significantly correlated with shortened OS(HR = 1.54, 95%CI: 1.24–1.91, p = 0.000; I2 = 0.0%, Ph = 0.499) in HCC patients treated by surgical resection.
The clinicopathologic features of HCC, especially tumor multifocality and vascular invasion were reported to be associated with the prognosis and survival of HCC21. In this circumstance, we conducted pooled analysis to evaluate the associations between pretreatment elevated PLR and clinicopathologic features in HCC. However, the result indicated that elevated PLR was not significantly associated with the presence of vascular invasion, tumor multifocality (satellite nodule), high level of serum AFP (>400 ng/ml) or poor tumor grade(Edmonson grade 3 or 4).
Accumulated evidence has showed that systematic inflammatory response plays an important role in tumor initiation and progression22,23. The exact mechanism is still unknown. While a large amount of studies demonstrated that the inflammation of microenvironment could influence the proliferation and survival of tumor cells24,25. Since most of the HCC patients are related to the chronic HBV or HCV infection, the patients would experience inflammation chronically. Platelets, a participant of inflammatory response, have been reported to protect tumor cells from natural killer-mediated lysis, thus supporting the tumor metastasis26. Additionally, the platelet-derived growth factor, basic fibroblast growth factor and hepatocyte growth factor could enhance tumor cells’ capability to metastasis27. Furthermore, thrombocytosis was shown to be an independent prognostic factor for epithelial ovarian cancer28. On the other hand, lymphocytes play a significant role in anti-tumor immune response. In HCC patients, increased infiltration of CD4+ T lymphocytes at the tumor margins has been reported to be associated with a lower recurrence rate and better prognosis29. However, Schreiber et al.3reported that tumor cells could reduce cytotoxic T lymphocyte (CTL) infiltration by secreting immunosuppressive cytokines such as vascular endothelial growth factor (VEGF), transforming growth factor–β (TGF-β), IL-10 and by consuming IL-2, a cytokine that is critical for maintaining CTL function. Thus, platelets and lymphocytes are tightly correlated with tumor progression. Previous meta-analyses31,32,33 have demonstrated the negative impact of elevated PLR on the prognosis of esophageal cancer and nonsmall cell lung cancer. Our study was the first study investigating the prognostic significance of PLR for HCC patients and the results were consistent with previous reports. Due to the convenience to obtain and low cost, the PLR could be a promising biomarker for clinical use.
There still existed several limitations of this study. First, HRs and 95%CIs were not directly provided in some studies, and we had to use Tierney’s method34 to calculate the value from supplied data. Second, due to the insufficient data to obtain or calculate HRs and 95%CIs of PLR for OS and DFS/RFS, none of studies concerning the prognostic value of PLR on HCC patients treated by liver transplantation were included in this meta-analysis. And this may limit the usefulness of PLR in clinical practice. Third, all of the included studies were retrospective and published in English. Finally, we analyzed the correlations between the elevated PLR and clinicopathological parameters of patients. However, for each clinicopathological feature, there were only 3 or 4 studies reporting the relevant information.
In conclusion, our results indicated that elevated pretreatment PLR might be an unfavorable prognostic factor for OS and DFS/RFS in patients with HCC, which could be useful in stratifying patients and determining individual treatment plan. However, considering the limitations listed above, more well-designed and large-scale investigations are warranted to better understand the value of PLR in the prognosis of HCC.
Methods
Search strategy
The following databases were systematically searched until April 2016 without time restrictions: Web of science, Embase and Pubmed. The search strategy was based on combination of following terms: “PLR”, ” platelet to lymphocyte ratio”, “platelet-lymphocyte ratio”, “hepatocellular carcinoma”, ”HCC”, “liver carcinoma”, ”liver cancer ”. Reports in English were eligible for inclusion. Reviews and reference lists in each identified publications were also manually retrieved for additional publications.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) HCC was diagnosed on pathology or the diagnostic criteria of the American Association for the Study of Liver Disease; (2) PLR was measured by serum-based methods before formal treatment; (3) HRs and 95%CIs for PLR in OS and/or DFS/RFS were described in the study or could be calculated from the supplied data.
The exclusion criteria were as follows: (1) reviews, conference abstract, letter, full text not available in English.; (2) overlapping or duplicate data; (3) did not provide the cut-off value for elevated PLR; (4) nonhuman studies.
Data extraction and quality assessment
All data extractions were performed separately by two independent investigatoras (W.C.M. and Y.J.) and disagreements were resolved by joint discussion. The following data were recorded for each eligible study: family name of the first author, year of the publication, country of the origin, sample size, treatment methods, cut-off values of PLR, survival data and clinicopathologic parameters. The quality of the included studies was assessed according to the Newcastle-Ottawa Quality Assessment Scale (NOS) by two independent investigators (W.C.M. and Y.J.). The NOS consists of three aspects: selection (four points), comparability (two points), and outcome assessment (three points). NOS scores ≥6 were regarded as high-quality studies.
Statistical Analysis
The impact of PLR on OS and DFS/RFS was measured by combined HRs and their 95% CIs which were directly extracted from each eligible articles or estimated according to the methods reported by Tierney et al.3 As for the impact of PLR on clinicopathologic features, pooled ORs and their 95% CIs were used. The Cochrane Q test and I2 statistic were used to assess the heterogeneity of the pooled results. Cochran Q test’s p value < 0.10 or I2 >50% was considered as large heterogeneity between studies and random effect model (DerSimonian Laird method) was performed to calculate the pooled HR and 95% CI. In other cases, fixed effects model (Mantel-Haenszel method) was adopted.
Sensitivity analysis was conducted by removing each study and recalculating the combined HRs. Begg’s test was used to evaluate the publication bias. All statistical analyses were performed using Stata 12 (Stata Corp., College Station, Texas). P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Ma, W. et al. Prognostic value of platelet to lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. Sci. Rep. 6, 35378; doi: 10.1038/srep35378 (2016).
Footnotes
Author Contributions W.C.M., P.Z. and Y.J. conceived and designed this study; W.C.M., Y.J. and J.Q. searched databases and collected the data; Y.J., L.T.G., H.C.Y. and X.J.S. performed the statistical analysis, interpretation of data; W.C.M., Y.J., M.C.Z. and C.L.W. wrote the manuscript. All authors reviewed the final manuscript.
References
- El-Serag H. B. & Rudolph K. L. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132, 2557–2576, 10.1053/j.gastro.2007.04.061 (2007). [DOI] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D. & Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians 66, 7–30, 10.3322/caac.21332 (2016). [DOI] [PubMed] [Google Scholar]
- Szkandera J. et al. Validation of the prognostic relevance of plasma C-reactive protein levels in soft-tissue sarcoma patients. British journal of cancer 109, 2316–2322, 10.1038/bjc.2013.595 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez D., Morris-Stiff G., Toogood G. J., Lodge J. P. & Prasad K. R. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. Journal of surgical oncology 97, 513–518, 10.1002/jso.21001 (2008). [DOI] [PubMed] [Google Scholar]
- Hashimoto K. et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer 103, 1856–1864, 10.1002/cncr.20976 (2005). [DOI] [PubMed] [Google Scholar]
- Miyata H. et al. Prognostic value of an inflammation-based score in patients undergoing pre-operative chemotherapy followed by surgery for esophageal cancer. Experimental and therapeutic medicine 2, 879–885, 10.3892/etm.2011.308 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H. C. et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals 17, 216–222, 10.3109/1354750x.2012.656705 (2012). [DOI] [PubMed] [Google Scholar]
- Azab B. et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Medical oncology (Northwood, London, England) 30, 432, 10.1007/s12032-012-0432-4 (2013). [DOI] [PubMed] [Google Scholar]
- Aliustaoglu M. et al. The effect of peripheral blood values on prognosis of patients with locally advanced gastric cancer before treatment. Medical oncology (Northwood, London, England) 27, 1060–1065, 10.1007/s12032-009-9335-4 (2010). [DOI] [PubMed] [Google Scholar]
- Fan W. et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of survival and metastasis for recurrent hepatocellular carcinoma after transarterial chemoembolization. PloS one 10, e0119312, 10.1371/journal.pone.0119312 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue T. C. et al. The platelet-to-lymphocyte ratio predicts poor survival in patients with huge hepatocellular carcinoma that received transarterial chemoembolization. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine 36, 6045–6051, 10.1007/s13277-015-3281-x (2015). [DOI] [PubMed] [Google Scholar]
- Lai Q., Castro Santa E., Rico Juri J. M., Pinheiro R. S. & Lerut J. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transplant international: official journal of the European Society for Organ Transplantation 27, 32–41, 10.1111/tri.12191 (2014). [DOI] [PubMed] [Google Scholar]
- Wang Q. et al. The Severity of Liver Fibrosis Influences the Prognostic Value of Inflammation-Based Scores in Hepatitis B-Associated Hepatocellular Carcinoma. Annals of surgical oncology 22 Suppl 3, S1125–S1132, 10.1245/s10434-015-4598-9 (2015). [DOI] [PubMed] [Google Scholar]
- Sun Q., Jiao S.-C., Wu J.-Y., Long Y.-Y. & Chen L. Pretreatment haematological laboratory values: the new prognostic factors in patients undergoing hepatectomy for hepatocellular carcinoma. Biomedical Research-India 25, 580–587 (2014). [Google Scholar]
- Pinato D. J. et al. A novel and validated prognostic index in hepatocellular carcinoma: the inflammation based index (IBI). Journal of hepatology 57, 1013–1020, 10.1016/j.jhep.2012.06.022 (2012). [DOI] [PubMed] [Google Scholar]
- Ni X. C. et al. Prognostic Value of the Modified Glasgow Prognostic Score in Patients Undergoing Radical Surgery for Hepatocellular Carcinoma. Medicine 94, e1486, 10.1097/md.0000000000001486 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. et al. Platelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine 36, 2263–2269, 10.1007/s13277-014-2833-9 (2015). [DOI] [PubMed] [Google Scholar]
- Ji F. et al. A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection: the neutrophil to lymphocyte ratio (NLR) combined with the aspartate aminotransferase/platelet count ratio index (APRI). BMC cancer 16, 137, 10.1186/s12885-016-2189-1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh B. K. et al. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and prognostic nutrition index as preoperative predictors of early mortality after liver resection for huge (>/=10 cm) hepatocellular carcinoma. Journal of surgical oncology, 10.1002/jso.24197 (2016). [DOI] [PubMed] [Google Scholar]
- Chan A. W. et al. Prognostic Nutritional Index (PNI) Predicts Tumor Recurrence of Very Early/Early Stage Hepatocellular Carcinoma After Surgical Resection. Annals of surgical oncology 22, 4138–4148, 10.1245/s10434-015-4516-1 (2015). [DOI] [PubMed] [Google Scholar]
- Bruix J. & Sherman M. Management of hepatocellular carcinoma. Hepatology (Baltimore, Md.) 42, 1208–1236, 10.1002/hep.20933 (2005). [DOI] [PubMed] [Google Scholar]
- Grange J. M., Krone B. & Mastrangelo G. Infection, inflammation and cancer. International journal of cancer 128, 2240–2241, 10.1002/ijc.25533 (2011). [DOI] [PubMed] [Google Scholar]
- Proctor M. J. et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. European journal of cancer (Oxford, England: 1990) 47, 2633–2641, 10.1016/j.ejca.2011.03.028 (2011). [DOI] [PubMed] [Google Scholar]
- Moore M. M., Chua W., Charles K. A. & Clarke S. J. Inflammation and cancer: causes and consequences. Clinical pharmacology and therapeutics 87, 504–508, 10.1038/clpt.2009.254 (2010). [DOI] [PubMed] [Google Scholar]
- Mantovani A., Romero P., Palucka A. K. & Marincola F. M. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet (London, England) 371, 771–783, 10.1016/s0140-6736(08)60241-x (2008). [DOI] [PubMed] [Google Scholar]
- Nieswandt B., Hafner M., Echtenacher B. & Mannel D. N. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer research 59, 1295–1300 (1999). [PubMed] [Google Scholar]
- Sierko E. & Wojtukiewicz M. Z. Platelets and angiogenesis in malignancy. Seminars in thrombosis and hemostasis 30, 95–108, 10.1055/s-2004-822974 (2004). [DOI] [PubMed] [Google Scholar]
- Asher V., Lee J., Innamaa A. & Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico 13, 499–503, 10.1007/s12094-011-0687-9 (2011). [DOI] [PubMed] [Google Scholar]
- Wada Y., Nakashima O., Kutami R., Yamamoto O. & Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology (Baltimore, Md.) 27, 407–414, 10.1002/hep.510270214 (1998). [DOI] [PubMed] [Google Scholar]
- Schreiber R. D., Old L. J. & Smyth M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science (New York, N.Y.) 331, 1565–1570, 10.1126/science.1203486 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang H., Gao L., Zhang B., Zhang L. & Wang C. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: a systematic review and meta-analysis. Scientific reports 6, 22618, 10.1038/srep22618 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X. et al. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: evidence from 3,430 patients. Scientific reports 6, 23893, 10.1038/srep23893 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yodying H. et al. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis. Annals of surgical oncology 23, 646–654, 10.1245/s10434-015-4869-5 (2016). [DOI] [PubMed] [Google Scholar]
- Tierney J. F., Stewart L. A., Ghersi D., Burdett S. & Sydes M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8, 16, 10.1186/1745-6215-8-16 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]