Abstract
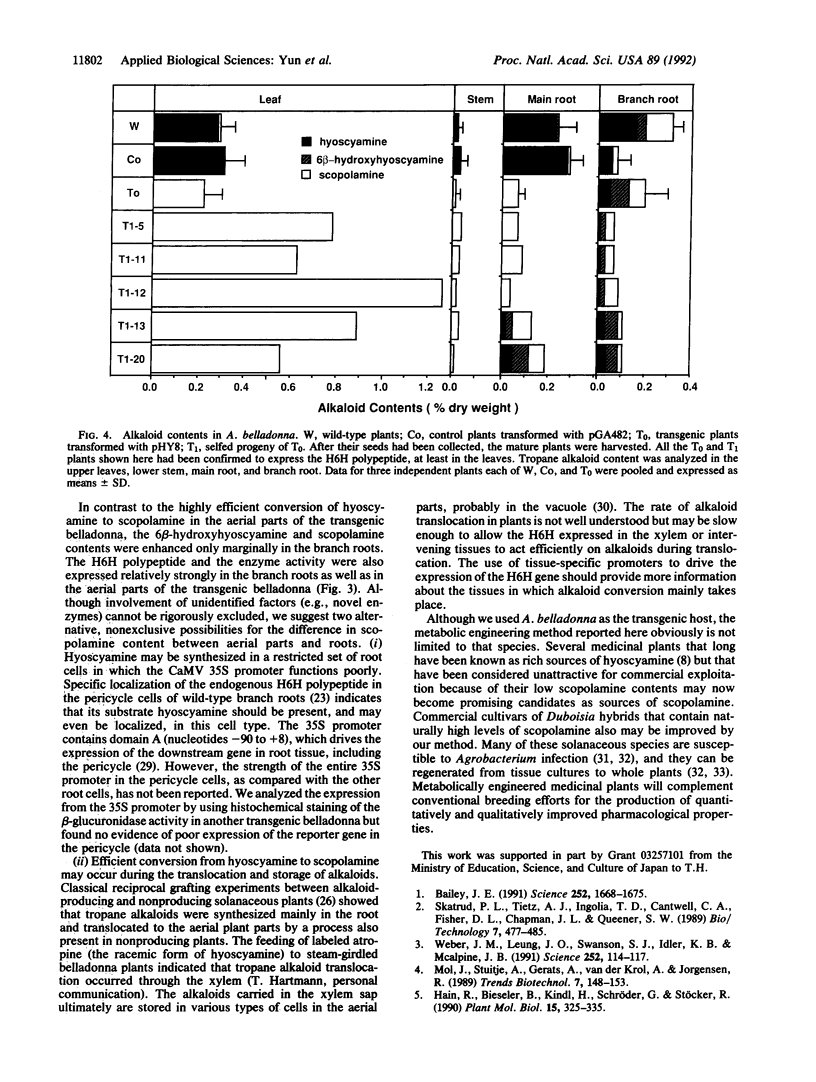
The tropane alkaloid scopolamine is a medicinally important anticholinergic drug present in several solanaceous plants. Hyoscyamine 6 beta-hydroxylase (EC 1.14.11.11) catalyzes the oxidative reactions in the biosynthetic pathway leading from hyoscyamine to scopolamine. We introduced the hydroxylase gene from Hyoscyamus niger under the control of the cauliflower mosaic virus 35S promoter into hyoscyamine-rich Atropa belladonna by the use of an Agrobacterium-mediated transformation system. A transgenic plant that constitutively and strongly expressed the transgene was selected, first by screening for kanamycin resistance and then by immunoscreening leaf samples with an antibody specific for the hydroxylase. In the primary transformant and its selfed progeny that inherited the transgene, the alkaloid contents of the leaf and stem were almost exclusively scopolamine. Such metabolically engineered plants should prove useful as breeding materials for obtaining improved medicinal components.
Full text
PDF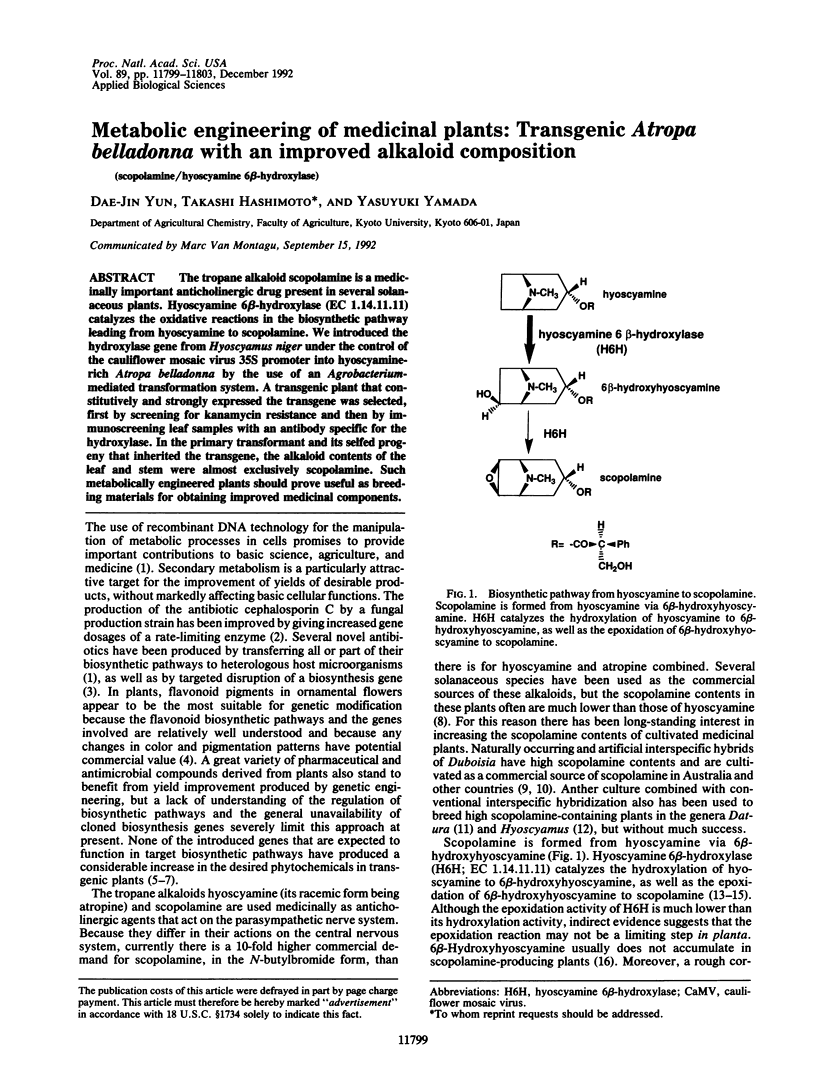



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. E. Toward a science of metabolic engineering. Science. 1991 Jun 21;252(5013):1668–1675. doi: 10.1126/science.2047876. [DOI] [PubMed] [Google Scholar]
- Benfey P. N., Ren L., Chua N. H. The CaMV 35S enhancer contains at least two domains which can confer different developmental and tissue-specific expression patterns. EMBO J. 1989 Aug;8(8):2195–2202. doi: 10.1002/j.1460-2075.1989.tb08342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Hain R., Bieseler B., Kindl H., Schröder G., Stöcker R. Expression of a stilbene synthase gene in Nicotiana tabacum results in synthesis of the phytoalexin resveratrol. Plant Mol Biol. 1990 Aug;15(2):325–335. doi: 10.1007/BF00036918. [DOI] [PubMed] [Google Scholar]
- Hamill J. D., Robins R. J., Parr A. J., Evans D. M., Furze J. M., Rhodes M. J. Over-expressing a yeast ornithine decarboxylase gene in transgenic roots of Nicotiana rustica can lead to enhanced nicotine accumulation. Plant Mol Biol. 1990 Jul;15(1):27–38. doi: 10.1007/BF00017721. [DOI] [PubMed] [Google Scholar]
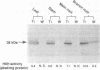
- Hashimoto T., Hayashi A., Amano Y., Kohno J., Iwanari H., Usuda S., Yamada Y. Hyoscyamine 6 beta-hydroxylase, an enzyme involved in tropane alkaloid biosynthesis, is localized at the pericycle of the root. J Biol Chem. 1991 Mar 5;266(7):4648–4653. [PubMed] [Google Scholar]
- Hashimoto T., Kohno J., Yamada Y. Epoxidation in vivo of hyoscyamine to scopolamine does not involve a dehydration step. Plant Physiol. 1987 May;84(1):144–147. doi: 10.1104/pp.84.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Yamada Y. Hyoscyamine 6beta-hydroxylase, a 2-oxoglutarate-dependent dioxygenase, in alkaloid-producing root cultures. Plant Physiol. 1986 Jun;81(2):619–625. doi: 10.1104/pp.81.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Yamada Y. Purification and characterization of hyoscyamine 6 beta-hydroxylase from root cultures of Hyoscyamus niger L. Hydroxylase and epoxidase activities in the enzyme preparation. Eur J Biochem. 1987 Apr 15;164(2):277–285. doi: 10.1111/j.1432-1033.1987.tb11055.x. [DOI] [PubMed] [Google Scholar]
- Hohn T. M., Ohlrogge J. B. Expression of a fungal sesquiterpene cyclase gene in transgenic tobacco. Plant Physiol. 1991 Sep;97(1):460–462. doi: 10.1104/pp.97.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuda J., Okabe S., Hashimoto T., Yamada Y. Molecular cloning of hyoscyamine 6 beta-hydroxylase, a 2-oxoglutarate-dependent dioxygenase, from cultured roots of Hyoscyamus niger. J Biol Chem. 1991 May 25;266(15):9460–9464. [PubMed] [Google Scholar]
- Weber J. M., Leung J. O., Swanson S. J., Idler K. B., McAlpine J. B. An erythromycin derivative produced by targeted gene disruption in Saccharopolyspora erythraea. Science. 1991 Apr 5;252(5002):114–117. doi: 10.1126/science.2011746. [DOI] [PubMed] [Google Scholar]