Abstract
Nephrolithiasis, secondary hyperparathyroidism (sHPT), and cardiovascular complications are associated with disturbances in Ca handling and contribute to morbidity/mortality during haemodialysis (HD). Calcimimetics, activators of the calcium-sensing receptor (CaSR), provide an effective means of reducing parathyroid hormone (PTH) secretion in sHPT. Polymorphism in CaSR gene (CASR) influences Ca-related parameters, however it was not shown in HD patients for CASR rs7652589. The minor allele at this polymorphism modifies the binding sites of transcription factors and CaSR expression. We hypothesized that CASR rs7652589 variants may also influence CaSR in end stage renal disease (ESRD). We aimed to determine the associations of rs7652589 with nephrolithiasis-related ESRD, Ca, P, ALP, PTH, response to treatment with cinacalcet, prevalence of coronary artery disease, and all-cause/cardiovascular mortality in HD patients (n = 1162). Healthy individuals (n = 918) were controls. This study shows that the A allele of rs7652589 is a risk allele for nephrolithiasis-related ESRD. The AA genotype is associated with more severe sHPT (higher Ca and PTH concentrations). The A allele is associated with reduced CaSR transcript level in peripheral blood mononuclear cells. According to computational analysis, potential binding sites for GLI3, AHR and TP53 are removed by the A allele, whereas binding sites for SOX18 and TP63 are created.
Calcium (Ca) stones contribute to nephrolithiasis-related end-stage renal disease (ESRD), which requires renal replacement therapy (RRT) in 26.7% of cases. Struvite stones (in humans, a mixture of struvite and carbonate-apatite1) account for a further 42.2% of cases2. Risk calcium-sensing receptor gene (CASR) genotypes in rs1042636, rs7652589, rs1501899, and rs6776158 were proposed as markers that could identify patients prone to developing Ca nephrolithiasis3,4. It was suggested that the appearance of the binding site for the octamer-binding transcription factor 1 (Oct-1) proximal to CASR promoters may downregulate calcium-sensing receptor (CaSR) expression in subjects with the minor allele in rs7652589 or rs15018995. It was also demonstrated that the minor allele in rs6776158 may predispose to Ca stones by decreasing transcriptional activity of the CASR promoter 1 and CaSR expression in kidney tubules4.
Mineral bone disorders, including secondary hyperparathyroidism (sHPT), are closely associated with cardiovascular complications and are the main cause of morbidity and mortality in haemodialysis (HD) subjects6. CaSR expression is decreased in human uremic parathyroid glands to nearly 60% of normal expressionα7. Calcimimetics, allosteric activators of CaSR, provide an effective means of reducing parathyroid hormone (PTH) secretion in such patients8. Serum PTH concentrations and other features of sHPT9,10, as well as treatment with cinacalcet11,12, seem to be associated with CASR (OMIM +601199) polymorphisms. The CASR A986S (rs1801725) polymorphism was also associated with coronary artery disease (CAD), myocardial infarction (MI), all-cause mortality, and cardiovascular mortality in non-dialyzed subjects13.
CaSR in parathyroid cells is sensitive to ionized Ca concentration and regulates PTH secretion14. Blood ionized Ca is associated with polymorphisms in CASR A986S (rs1801725), R990G (rs1042636), and Q1011E (rs1801726)15,16. In 2000, Yano et al.9 demonstrated the association of CASR alleles (codon 990) with PTH secretion in HD patients. In 2001, Yamauchi et al.17 reported that CASR polymorphisms of G990R and intron 5 were closely associated with the magnitude of PTH secretion and/or PTH degradation as well as the clinical severity in primary HPT patients. In 2002, the CASR R990G polymorphism was shown to influence the response of the parathyroid glands to changes in extracellular ionized Ca in HD patients. The glands of patients with the GG genotype were more sensitive to extracellular changes in ionized Ca10.
Studies of the association between CASR and the response to treatment with the calcimimetic agent cinacalcet included CASR R990G. These studies showed that CASR R990G influences the response to calcimimetics in patients with sHPT with an odds ratio of 2.612. Cinacalcet was also effective with a heterozygous mutation (R185Q, CGA > CAA) in exon 4 of CASR18. However, the CASR R990G polymorphism, which was investigated in small groups (n ≤ 20) of patients with primary HPT related to type 1 multiple endocrine neoplasia and in patients with sHPT, was not associated with the efficacy profile of cinacalcet19.
Vascular calcification is accelerated in the presence of elevated circulating Ca and phosphorus (P) concentrations20. Coronary artery calcification contributes to the development and progression of CAD21,22. Calcification is associated with the loss of functional CaSR in vascular smooth muscle cells, and this loss may be influenced by genetic factors (CASR, R185Q)23. Calcimimetics delayed the onset of calcification in vascular smooth muscle cells23.
In our study, the CASR promoter-near region single nucleotide polymorphism (SNP) rs7652589 was selected for genotyping. It has been shown that this variant and rs1501899 predispose individuals to idiopathic Ca nephrolithiasis5,24,25. Both of these SNPs were also associated with a specific phenotype in primary HPT patients characterized by an increased susceptibility to kidney stones and higher serum concentrations of ionized Ca and PTH25. We hypothesized that CASR rs7652589 variants may also influence CaSR in chronic kidney disease, particularly in ESRD. To the best of our knowledge, the CASR rs7652589 SNP has not been investigated in HD patients. The aim of the study was to determine the associations of the CASR rs7652589 SNP with nephrolithiasis-related ESRD, Ca, P, alkaline phosphatase (ALP), PTH, response to treatment with cinacalcet, prevalence of CAD, including MI, as well as all-cause and cardiovascular mortality in HD patients. Additionally, we aimed to investigate CaSR transcript level in peripheral blood mononuclear cells (PBMC) and to perform in silico prediction of transcription factor binding site (TFBS) overlapping CASR rs7652589.
Results
Patient characteristics
The demographic, clinical and laboratory data of the entire HD group as well as the demographic, clinical and laboratory data of subjects treated with cinacalcet are presented in Table 1. Among patients with nephrolithiasis-related ESRD (n = 108), there were 42 subjects with chronic infective tubulointerstitial nephritis (3.6% of all HD patients, 38.9% of nephrolithiasis-related ESRD subjects). Kidney stone composition was known in 11 patients. Calcium oxalate stones were diagnosed in all these cases. Primary gout or cystinuria were not diagnosed among the studied HD patients. In 10 out of 42 subjects who developed chronic infective tubulointerstitial nephritis in the course of renal stone disease, staghorn calculi were demonstrated after 1–3 decades since the detection of the first stone.
Table 1. Demographic, clinical, and biochemical characteristics of haemodialysis (HD) patients.
| Parameter | All HD patients n = 1162 | HD patients at the initiation of treatment with cinacalcet n = 162 |
|---|---|---|
| Demographic | ||
| Age at the last data actualization, years | 66.1, 17.1–95.9 | 59.1, 17.1–83.0 |
| Gender, males | 506 (56.4) | 87 (53.7) |
| Clinical | ||
| Main causes of end-stage renal disease | ||
| Diabetic nephropathy | 335 (28.8) | 22 (13.6) |
| Hypertensive nephropathy | 215 (18.5) | 27 (16.7) |
| Chronic glomerulonephritis | 170 (14.6) | 47 (29.0) |
| Nephrolithiasis related chronic kidney disease | 108 (9.3) | 19 (11.7) |
| Age at RRT onset, years | 60.9, 11.1–91.1 | 51.3, 11.1–77.5 |
| Renal replacement therapy vintage, years | 4.69, 0.07–29.5 | 6.89, 0.07–28.3 |
| Patients with coronary artery disease | 416 (35.8) | 46 (28.4) |
| Patients with myocardial infarction | 224 (19.3) | 32 (19.7) |
| Parathyroidectomized subjects | 33 (2.8) | 0 (0.0) |
| Patients treated with cinacalcet | 162 (13.9) | 162 (100.0) |
| Seruma | ||
| Total calcium, mg/dl | 8.85, 6.01–12.8 | 9.02 (8.80–11.7) |
| Phosphorus, mg/dl | 5.03, 1.75–12.0 | 5.40 (2.58–12.0) |
| Total alkaline phosphatase, U/l | 95.8, 13.5–1684 | 120 (41–1353) |
| Intact parathyroid hormone, pg/ml | 377, 7.33–3757 | 999 (518–3280) |
| 25-hydroxyvitamin D, ng/ml | 13.8, 4.51–30.1b | 13.7, 5.80–23.4c |
Results are presented as median and range (maximum–minimum) or number (percentage).
Conversion factors to SI units are as follows: for calcium, 0.2495; 25-hydroxyvitamin D, 2.496; parathyroid hormone, 0.1061; and phosphorus, 0.3229.
aNormal serum ranges: total calcium, 8.80–10.20 mg/dl; phosphorus, 2.70–4.50 mg/dl; total alkaline phosphatase, 35–105 U/l; intact parathyroid hormone, 15.0–65.0 pg/ml; 25-hydroxyvitamin D, 30–80 ng/ml.
bn = 217.
cn = 32.
Frequencies of CASR rs7652589 variants in HD patients and controls
A power analysis predicted sufficient sample power (>90%) to detect associations between the CASR rs7652589 SNP and ESRD requiring RRT with a genetic effect of 1.25 or greater in the dominant, recessive or log-additive modes of inheritance (Supplementary Table 1). There were no significant differences in the frequencies of CASR rs7652589 polymorphic variants between the entire group of HD subjects and healthy controls (Supplementary Table 2).
Association of CASR rs7652589 SNP with HD patient data
Among HD patients, there were 959 (82.5%) subjects with sHPT (serum PTH > 150 pg/ml). Their genotype frequencies (GG 36%, AG 49%, AA 15%) did not differ from those of healthy subjects.
Comparisons of clinical and laboratory data of HD patients stratified by CASR rs7652589 genotypes suggested the possibility of associations (P < 0.05 without the Bonferroni correction) between CASR rs7652589 SNP and patient age at the start of RRT, nephrolithiasis-related ESRD, serum total Ca concentration, and prevalence of PTH results over 500 pg/ml (Supplementary Table 3).
Patients with the CASR rs7652589 AA genotype started RRT earlier than subjects with the GG genotype (Supplementary Table 3); however, patients who were aged < 40 years at the onset of RRT (n = 169) exhibited the GG genotype in 38% of cases, the AG genotype in 47% of cases, and the AA genotype in 15% of cases. These frequencies were not different from the respective frequencies (36%, 49%, and 15%) in patients starting RRT at age ≥ 40 years (n = 993).
Prevalence of nephrolithiasis-related ESRD significantly differed in patients stratified by CASR rs7652589 genotype (Supplementary Table 3). Compared to patients with the GG genotype, patients with the AA genotype (the additive model of inheritance) exhibited a higher frequency of nephrolithiasis-related ESRD, and the frequency of the A allele was higher in patients with nephrolithiasis-related ESRD than in other patients (Table 2). An association between the CASR rs7652589 SNP and nephrolithiasis remained significant after adjusting for gender and age at the onset of RRT (Supplementary Table 4). For the additive model of inheritance, the sample power was 99.8% with a genetic effect of 2.00 or greater, which was sufficient for the establishment of a significant association (Supplementary Table 5).
Table 2. CASR rs7652589 genotype and allele frequencies in HD patients with and without nephrolithiasis-related ESRD.
| CASR rs7652589 | HD patients with nephrolithiasis-related ESRD | HD patients without nephrolithiasis-related ESRD | Odds ratio (95% CI) | P value | Ptrend | Pgenotype |
|---|---|---|---|---|---|---|
| (n, frequency) | (n, frequency) | |||||
| n = 108 | n = 1054 | |||||
| GG | 29 (0.27) | 396 (0.38) | Reference | — | 0.007 | 0.02 |
| AG | 55 (0.51) | 509 (0.48) | 1.476 (0.923–2.358) | 0.1 | ||
| AA | 24 (0.22) | 149 (0.14) | 2.199 (1.240–3.900) | 0.01 | ||
| AA+AG vs GG | 79 (0.73) | 658 (0.62) | 1.639 (1.052–2.555) | 0.036a | ||
| AA vs AG+GG | 84 (0.78) | 905 (0.86) | 1.735 (1.068–2.821) | 0.035a | ||
| MAF | (0.48) | (0.38) | 1.469 (1.109–1.947) | 0.008 | ||
| P for HWE | 0.830 | 0.476 |
Abbreviations: ESRD, end stage renal kidney disease; HD, haemodialysis; HWE, Hardy-Weinberg equilibrium; MAF, minor allele frequency.
aNot significant after the Bonferroni correction.
Stratification of patients by CASR rs7652589 genotypes (Supplementary Table 3) suggested that the CASR rs7652589 A allele may be associated with higher serum concentrations of total Ca. Upon further analysis, HD patients were stratified by categories of serum calcemic status: hypocalcemic (Ca < 8.80 mg/dl), normocalcemic (Ca 8.80–10.20 mg/dl), and hypercalcemic (Ca > 10.20 mg/dl). If possible influences of Ca-containing drugs were not analyzed, the AA + AG patients showed higher serum Ca levels than the GG subjects (the dominant model of inheritance, P = 0.033, Supplementary Table 3). A significant trend for increasing frequencies of the A allele and the AA genotype was revealed among groups ordered as hypocalcemic, normocalcemic, and hypercalcemic (Fig. 1). Genetic comparisons between calcemic groups (Supplementary Tables 6–8) showed significant findings (Ptrend = 0.001, Pgenotype = 0.001) between hypercalcemic and hypocalcemic HD patients: hypercalcemic subjects exhibited a higher frequency of the A allele (OR 1.48, 95% CI 1.16–1.88, P = 0.002). The CASR rs7652589 genotype distribution yielded significance in the recessive (OR 2.28, 95% CI 1.45–3.58, P = 0.001) and the additive (OR 2.52, 95% CI 1.52–4.20, P = 0.001) models of inheritance (Supplementary Table 8). Taking into analysis the lowest Ca levels as possible less influenced by Ca-containing drug medication, we could observed significantly higher Ca concentrations in the AA patients compared with the GG (the additive model of inheritance, P = 0.000008) and the AA + AG (the recessive model of inheritance, P = 0.0000006) patients (Supplementary Table 3). In genetic comparisons, the most pronounced differences in distribution of CASR rs7652589 polymorphic variants were observed between hypocalcemic (n = 730) and normocalcemic (n = 396) subjects (Ptrend < 0.0001, Pgenotype < 0.0001): normocalcemic subjects showed a higher frequency of the A allele (OR 1.52, 95% CI 1.28–1.82, P < 0.0001). The significance in distribution of CASR rs7652589 was revealed in the recessive (OR 2.69, 95% CI 1.92–3.76, P < 0.0001) and the additive (OR 2.82, 95% CI 1.94–4.10, P < 0.0001) models of inheritance. Small number of hypercalcemic patients among subjects, in whom possible treatment with phosphate binders was analyzed (n = 36), could be reason for a weak significance of genetic comparisons between hypercalcemic and hypocalcemic patients (Ptrend = 0.169, Pgenotype = 0.013).
Figure 1. Association of CASR rs7652589 polymorphic variants with calcemic status of haemodialysis (HD) patients under conditions of taking Ca-containing phosphate binders.
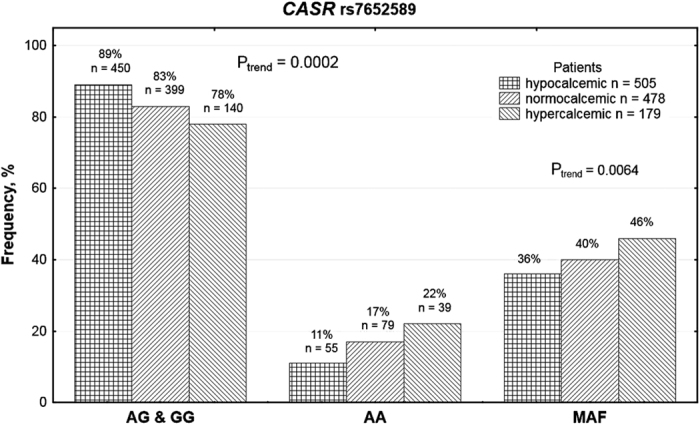
. HD patients were stratified by categories of serum calcemic status: hypocalcemic (Ca < 8.80 mg/dl), normocalcemic (Ca 8.80–10.20 mg/dl), and hypercalcemic (Ca > 10.20 mg/dl). A significant trend for increasing frequencies of the A allele and the AA genotype was revealed among groups ordered as hypocalcemic, normocalcemic, and hypercalcemic.
In subjects stratified by CASR rs7652589 genotype, a higher prevalence of PTH results over 500 pg/ml was shown in patients harboring the AA genotype compared to patients bearing the G allele (Supplementary Table 3). Serum PTH levels over 500 pg/ml were associated with the allele A (Table 3).
Table 3. CASR rs7652589 genotype and allele frequencies in HD patients with serum PTH >500 pg/mL and remaining HD patients.
| CASR rs7652589 | HD patients with serum PTH >500 pg/ml | HD patients serum PTH ≤ 500 pg/ml | Odds ratio (95% CI) | P value | Ptrend | Pgenotype |
|---|---|---|---|---|---|---|
| (n, frequency) | (n, frequency) | |||||
| n = 442 | n = 720 | |||||
| GG | 153 (0.35) | 272 (0.38) | Reference | — | 0.035 | 0.035 |
| AG | 208 (0.47) | 356 (0.49) | 1.039 (0.800–1.350) | 0.8 | ||
| AA | 81 (0.18) | 92 (0.13) | 1.565 (1.094–2.240) | 0.018a | ||
| AA+AG vs GG | 289 (0.65) | 448 (0.62) | 1.147 (0.896–1.468) | 0.3 | ||
| AA vs AG+GG | 361 (0.82) | 628 (0.87) | 1.532 (1.106–2.121) | 0.01 | ||
| MAF | (0.42) | (0.38) | 1.200 (1.011–1.424) | 0.041 | ||
| P for HWE | 0.486 | 0.141 |
Abbreviations: HD, haemodialysis; HWE, Hardy-Weinberg equilibrium; MAF, minor allele frequency; PTH, parathyroid hormone.
aNot significant after the Bonferroni correction.
There were no significant differences between groups stratified by CASR rs7652589 genotype in respect to serum phosphorus concentration (without or with possible influence of phosphate binders taken into the analysis), PTX rate or frequencies in cinacalcet administration, CAD or MI (Supplementary Table 3).
Cytokine genes in nephrolithiasis-related ESRD
Among polymorphic variants of cytokine-associated genes tested in HD patients (Supplementary Table 9), borderline associations with nephrolithiasis-related ESRD were shown for the chemokine (C-C motif) ligand 2 gene (CCL2 -2518 A > G) rs1024611 (Ptrend = 0.059) and the interleukin (IL) 18 gene (IL18) rs360719 (Ptrend = 0.085). The interaction analysis revealed a significant epistatic interaction between CASR rs7652589 and CCL2 rs1024611 polymorphisms (OR for interaction 0.565; Chi-square statistic, 1df, 5.19; P = 0.023) (Supplementary Table 10).
Vitamin D signaling pathway genes in nephrolithiasis-related ESRD
No significant differences in frequency distributions of polymorphic variants in vitamin D signaling pathway genes between HD patients with and without nephrolithiasis-related ESRD were observed (Supplementary Table 11). Associations between vitamin D signaling pathway genes and nephrolithiasis-related ESRD as well as interactions with CASR rs7652589 were also not observed (Supplementary Table 12).
Genotypes as independent determinants of specific phenotypes
Gender, age, serum concentrations of Ca and PTH, the G allele of CCL2 -2518 A > G (rs1024611), and the A allele of CASR rs7652589 were entered into the multivariate stepwise linear regression model as possible determinants of nephrolithiasis-related ESRD. Among these factors, only the A allele of CASR rs7652589 was an independent determinant of nephrolithiasis-related ESRD (B 0.09, 95% CI 0.01–0.17, P = 0.020).
Gender, RRT vintage, serum PTH level, serum P concentration, serum ALP activity, and the A allele of CASR rs7652589 were chosen as possible determinants of serum Ca concentration exceeding 10.2 mg/dl (the upper normal range) under conditions of taking Ca-containing phosphate binders. High serum Ca levels were determined in HD patients by serum concentrations of PTH (B 0.16, 95% CI 0.07–0.24, P = 0.0004) and P (B 0.11, 95% CI 0.04–0.19, P = 0.002), but not by the A allele of CASR rs7652589. A replacement of the A allele in this model by the AA genotype of CASR rs7652589 did not change the significance of the determinants. When serum Ca as a continuous variable was taken to the analysis, there were also no association of the CASR rs7652589 A allele or AA genotype with Ca level. The same abovementioned 6 parameters were applied as possible determinants of serum Ca concentration under conditions diminishing possible influences of Ca-containing drugs. Significant independent positive determinants of serum Ca remained female gender (B 0.08, 95% CI 0.02–0.15, P = 0.017) and the AA genotype of CASR rs7652589 (B 0.14, 95% CI 0.07–0.21, P = 0.00008), but not the A allele if used instead of the AA genotype.
Gender, RRT vintage, serum P concentration, serum ALP activity, and the A allele of CASR rs7652589 were chosen as possible determinants of serum PTH levels exceeding 500 pg/ml. These PTH levels were determined in HD patients by RRT vintage (B 0.21, 95% CI 0.14–0.27, P < 0.000001), serum P concentration (B 0.18, 95% CI 0.11–0.24, P < 0.000001), and serum ALP activity (B 0.25, 95% CI 0.17–0.32, P < 0.000001), but not by the A allele of CASR rs7652589. However, if the A allele was replaced in this model by the AA genotype of CASR rs7652589, the AA genotype was significantly related to serum PTH levels exceeding 500 pg/ml (B 0.09, 95% CI 0.02–0.015, P < 0.008). The remaining determinants maintained their significance.
Response to treatment with cinacalcet
CASR genotypes of HD patients treated with cinacalcet were disclosed after collection of all data related to administration of this agent.
Initial PTH concentrations and maximum doses of cinacalcet were similar in the genotype-stratified groups. There was no significant difference in prevalence of responders to cinacalcet with respect to the CASR rs7652589 polymorphism (Supplementary Table 13). Among the collected clinical/laboratory variables, significant determinants of sensitivity to treatment with cinacalcet were lower ALP activity (B −0.19, 95% CI −0.35–0.03, P = 0.023) and lower maximal doses of cinacalcet used for treatment (B −0.19, 95% CI −0.36 – −0.03, P = 0.019).
Association of the CASR rs7652589 SNP with clinical outcomes of HD patients
A total of 449 HD patients died, and 270 underwent renal transplantation between January 2nd, 2009 and April 20th, 2015.
Using the Kaplan-Meier curve estimate in the entire cohort of HD patients, CASR rs7652589 genotypes did not have an effect on all-cause mortality (Fig. 2). Significance was also not revealed when the analysis was performed using models of inheritance (Supplementary Figures 1–3). Neither cardiac mortality nor cardiovascular mortality rates were associated with the CASR rs7652589 SNP (data not shown, P > 0.05).
Figure 2. The Kaplan-Meier survival analysis of the studied haemodialysis patients since the initiation of renal replacement therapy stratified by CASR rs7652589 genotypes.
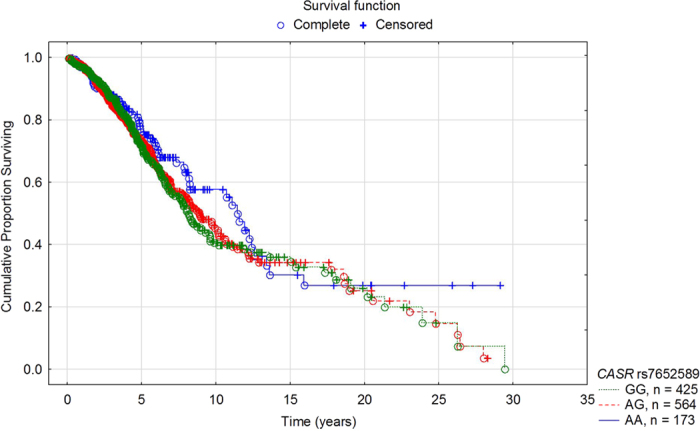
Patients’ survival was not dependent on the tested genotypes (the log-rank test P = 0.449).
Effect of CASR rs7652589 G>A polymorphism on CaSR transcript levels in PBMC from HD patients
We found a reduced CaSR transcript level in the PBMC from carriers of the CASR rs7652589 A allele as compared with carriers of the CASR GG genotype. The median of CaSR transcript level in PBMC was 2.00 (range 0.86–4.49) for the AA genotype, 3.90 (range 0.03–9.98) for the AG genotype, and 11.59 (range 6.34–31.44) for the GG genotype (Fig. 3).
Figure 3. Effect of the CASR rs7652589 G>A polymorphism on CaSR transcript levels in PBMC obtained from HD patients.
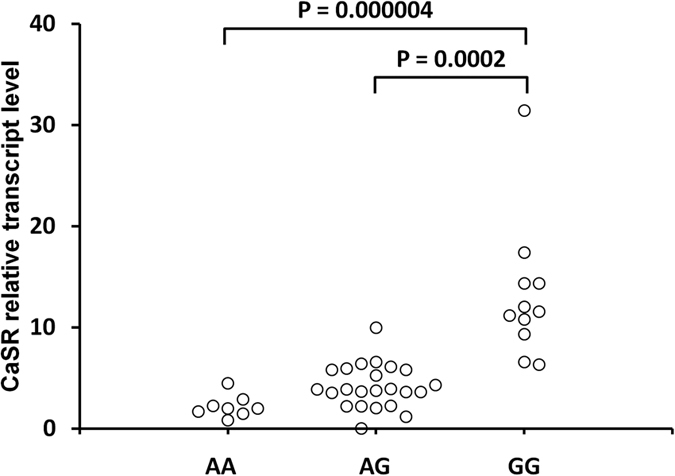
The PBMC from whole venous blood were isolated by Ficoll-Hypaque centrifugation (density, 1.077 g/cm3) followed by total RNA isolation. Quantitative analyses of CaSR transcript levels were performed by RT and Q-PCR SYBR Green I system. The quantity of CaSR transcript in each sample was standardized by the geometric mean of reference PBGD and B2M cDNA levels.
In silico TFBS prediction
ENCODE TFBS ChiP-seq dataset reports 5 transcription factors (CTCF, ELK4, KAP1, POLR2A, and TCF7L2) tested in the HEK293 cells with none having DNA-binding sites overlapping rs7652589 position. Furthermore, none of the 161 ChiP-seq analyzed transcription factors in all ENCODE cell lines contains a DNA-binding site overlapping rs7652589 (Table 4).
Table 4. Results of the transcription factor binding site prediction by software FIMO.
| Allele | Matched sequence | Transcription factor | Modification (in presence of the A allele) | Strand | P value | Q value |
|---|---|---|---|---|---|---|
| G | ttgcGtgttct | GLI3 | Removed | + | 6.59E-05 | 0.0152 |
| G | attgcGtgt | AHR | Removed | + | 8.53E-05 | 0.0216 |
| G | gcGtgttcttgcagg | TP53 | Removed | + | 0.000376 | 0.0435 |
| A | CATGCAATT | PO2F1_f1 | − | − | 0.000449 | 0.119 |
| A | gcaacagttcaattgcAtg | SOX18 | Added | + | 0.000101 | 0.0232 |
| A | ttgcAtgttcttgcagggag | TP63 | Added | + | 0.000214 | 0.0448 |
The computational analysis predicted removal of binding sites for the transcriptional activator GLI3, aryl hydrocarbon receptor (AHR), and cellular tumor antigen p53 (TP53) at the applied statistical significance cut off in the presence of the A allele. Binding sites for the transcription factor SOX18 and tumor protein 63 (TP63) however, were added in the presence of the A allele. Binding site for the PO2F1 motif, of which Oct-1 is a member, was not found in the presence of the A allele at the applied statistical significance cut off (Table 4).
Discussion
In this study, we have shown that an epistatic interaction between the CASR rs7652589 SNP (risk allele A) and CCL2 rs1024611 (risk allele G) is associated with nephrolithiasis-related ESRD requiring regular dialysis treatment. The A allele of CASR rs7652589 was also a significant determinant of nephrolithiasis-related ESRD among other possible determinants such as gender, age, serum concentrations of Ca and PTH, and the G allele of CCL2 rs1024611. The AA genotype of CASR rs7652589 SNP appears to be independently related to higher concentrations of serum total Ca and higher frequency of serum PTH concentrations exceeding 500 pg/ml, but not to serum P concentration and serum total ALP activity. Prevalence of CAD, including MI, response to treatment with cinacalcet hydrochloride, as well as all-cause and cardiovascular mortality since the onset of RRT were not associated with the CASR rs7652589 SNP. These results were not influenced by the possible association of the CASR rs7652589 polymorphism with end-stage renal failure as a general phenotype.
Nephrolithiasis was reported in 6.6% of the elderly Polish population26, and severe nephrolithiasis leading to infective tubulointerstitial nephritis was shown in 2.4% of dialysis patients in Poland27. In as many as 9.3% of the studied HD patients, a long-term presence of nephrolithiasis and recurrent formation of calculi, as well as related complications, became a cause of progressive chronic kidney disease (chronic infective tubulointerstitial nephritis, obstructive nephropathy, and pyonephrosis with subsequent nephrectomy) leading to RRT. Prevalence of nephrolithiasis related ESRD in the studied group was higher than that previously described (up to 4.7% in the period 1989–1991 in France)2. This difference may be due to inclusion in our nephrolithiasis related ESRD group not only patients with chronic or recurrent obstruction of the urinary tract (obstructive nephropathy) but also subjects in whom chronic infective tubulointerstitial nephritis was diagnosed as related to stone formation without obstruction of the urinary tract as the main clinical problem.
HD patients having nephrolithiasis as a cause of ESRD differed in the CASR rs7652589 SNP compared with other HD patients. The A allele of CASR rs7652589 was recognized in our study as a risk allele for severe nephrolithiasis, among other possible factors, such as gender, age, serum concentrations of Ca and PTH, and the G allele of CCL2 rs1024611.
It should be noted that CCL2 rs1024611 and IL18 rs360719 yielded borderline associations with nephrolithiasis-related ESRD simultaneously with a significant association with the CASR rs7652589 SNP.
The G allele of CCL2 rs1024611, which is known to be a risk allele for inflammatory infective and non-infective diseases28,29, could simultaneously participate in progressing chronic kidney disease in nephrolithiasis patients as indicated by the interaction of CCL2 rs1024611 with CASR rs7652589 in the studied HD subjects. Moreover, CCL2 expression was found to be induced by calcium oxalate monohydrate in renal epithelial cells30. Suppression of CCL2 mRNA expression and downregulation of the expression of CCL2-related proteins including transforming growth factor beta-1 by resveratrol in oxalate-treated human renal epithelial cells significantly reduced the number of urine calcium oxalate crystals31. Patients harboring the G allele of CCL2 rs1024611, which was also associated with increased CCL-2 production32 in HD patients33, could be particularly prone to increased CCL2 expression contributing to interstitial inflammation and fibrosis34 and, therefore, to the initiation of chronic kidney disease and the progression of this disease to ESRD. A significant epistatic interaction between CASR rs7652589 and CCL2 rs1024611 polymorphisms is in accordance with this concept.
IL18 rs360719 did not exhibit an epistatic interaction with CASR rs1501899. This SNP was associated with chronic infective tubulointerstitial nephritis as a cause of ESRD in our previous study35. HD patients with nephrolithiasis-related ESRD showed chronic infective tubulointerstitial nephritis in only 38.9% of subjects. This fact can explain the borderline association of IL18 rs360719.
The CASR rs7652589 SNP, together with the CASR rs1501899 SNP, was involved in kidney stone production in primary HPT patients; however, the allele frequency at the rs7652589 SNP was similar in primary HPT patients and controls25. HD patients generally exhibited sHPT–82.5% in the current study. The first calculi were identified in our patients long before the development of sHPT. Patients with sHPT did not differ from healthy subjects in their distribution of the CASR rs7652589 SNP.
HD patients harboring the CASR rs7652589 AA genotype more frequently exhibited serum PTH levels exceeding 500 pg/ml; therefore, they were prone to more severe sHPT than the remaining subjects. Moreover, they presented with higher serum levels of total calcium. If possible influences of Ca-containing drugs were at least partially eliminated from the analysis, the AA genotype appeared significant independent determinant of higher Ca levels. Similarly, subjects with primary HPT having the AA haplotypes of the rs7652589 and rs1501899 SNPs demonstrated higher ionized Ca concentrations than patients harboring the other CASR haplotypes25. Hypercalcemic sHPT usually indicates tertiary HPT. HD patients bearing the CASR rs7652589 AA genotype seem to be more prone to this severe form of sHPT. Generally, our results have shown that associations of the CASR rs7652589 SNP with serum concentrations of Ca and PTH that were observed in primary HPT patients25 are also present in ESRD patients regularly treated with HD, in whom sHPT is a common finding.
No association of the CASR rs7652589 SNP with serum activity of total ALP was found in the tested HD patients. Serum total ALP activity reflects alkaline phosphohydrolases of tissue-specific origins such as bone, liver, and intestine36. A lack of association, if any exists, may be due to total ALP non-specificity.
Recently, Babinsky et al.37 did not reveal differences in CASR rs7652589 genotype distribution in kidney transplant recipients showing low or high levels of aortic calcification and coronary artery calcification. The CASR rs7652589 SNP was not associated with cardiovascular outcomes or mortality in this cohort37. Our study did not indicate any associations between this SNP and prevalence of CAD, including myocardial infarction, as well as all-cause and cardiovascular mortality rates in HD patients.
Calcimimetics increase CaSR expression38 and are used to reduce PTH secretion by parathyroid cells in sHPT patients8. A decrease in serum PTH concentration in response to administration of cinacalcet varies between HD patients but, according to our results, these differences in responsiveness are not dependent on the CASR rs7652589 SNP. However, our study group was too small to allow us to draw definitive conclusions.
It was demonstrated that Ca nephrolithiasis is associated with a decreased CaSR expression in kidney tubules4, and uremic parathyroid glands show a decreased CaSR expression6. We observed significantly reduced CaSR transcript levels in the PBMC from carriers of the CASR rs7652589 A allele as compared to carriers of the CASR GG genotype. Lower expression of CaSR was associated with higher levels of Ca and PTH in the CASR rs7652589 AA genotype carriers in our study. Although we were able to examine a CaSR expression only in PBMC, an association of reduced transcript levels with A allele indicates that CASR rs7652589 may be involved in Ca-related disturbances occurring due to CaSR downregulation.
The tested CASR rs7652589 variant is located 13 kbp upstream from the TATA box of promoter 1 on human chromosome 3. Our in silico analysis confirmed that the minor allele at this polymorphism is able to modify the binding sites of specific transcription factors and, consequently, CASR expression. We have shown that potential binding sites for GLI3, AHR and TP53 are removed by the A allele, whereas binding sites for SOX18 and TP63 are added. We performed an analysis of the PO2F1 motif, of which Oct-1 is a member. In contrast to Vezzoli et al.5, our analysis does not support appearance of Oct-1 in presence of the A allele. This finding likely stems from the software differences between studies, as we find, that p-value alone of the PO2F1 motif is statistically highly significant. However applied in our work, false discovery rate filter marked PO2F1 motif above cut-off criteria. Therefore, our study suggests that Oct-1 may not be a down-regulator of CaSR expression in the A allele harbouring subjects as it was postulated by Vezzoli et al.5. It is still unsolved how binding sites for the transcription factor SOX18 and tumor protein 63, that are added in the presence of the A allele, may influence a CaSR expression.
Limitations of the study
Associations of CASR rs7652589 with selected phenotypes were analyzed under conditions of conventional treatment targeting sHPT. Ca-containing drugs were administered the most commonly, serving as phosphate binders and Ca supplements. These drugs were usually started still prior to HD therapy, and their discontinuation was not possible due to ethical reasons. However, evaluations of patient compliance reveal poor adherence with drugs prescriptions in HD subjects: half were noncompliant with medication taking39. Poor compliance could be a reason of decreases in serum Ca levels and increases in P concentrations during the prescribed treatment.
It has to be pointed out that we have not applied the Bonferroni corrected P value in analyses of the CASR association with 8 main phenotypes tested in this study (nephrolithiasis-related ESRD, Ca, P, ALP, PTH, response to treatment with cinacalcet, prevalence of coronary artery disease, and survival probability). Associations of CASR, presented in our paper as significant, were obtained at P value below 0.05 but in some analyses over 0.006. However, a significance of polymorphic variant as a determinant of a specific phenotype was verified among other clinically important variables, and only these CASR variants which remained significant were considered as significantly associated. Although the Bonferroni correction is still widely used, there is a criticism to this method40 and not all authors are willing to use it in genetic studies41,42,43, especially when the established associations are investigated in more detail or in other uniform cohort like in our study. When several phenotypes is simultaneously tested, the use of Bonferroni correction may exclude some important associations.
Patients and Methods
Patients
ESRD patients whose current method of RRT was intermittent HD were enrolled in the study. Patient enrollment began in January, 2009 and ended in May, 2014. At enrollment, patients were dialyzed in 21 dialysis centers in the Greater Poland region of Poland. The patient group included 1162 individuals. Nephrolithiasis-related ESRD was diagnosed if calculus formation caused obstructive nephropathy and/or chronic infective tubulointerstitial nephritis. CAD was diagnosed based on a medical history, electrocardiograms, exercise stress test, and, in some cases, coronary angiography or computed tomography. MI was diagnosed based on a medical history and evidence showing characteristic electrocardiographic abnormalities and elevated levels of cardiac markers of cardiomyocyte damage.
In all HD patients, therapeutic efforts were aimed at reaching the normal serum levels of Ca and P and maintaining serum intact PTH levels at a range of approximately 2 to 9 times the upper normal limit for the assay in accordance with the Kidney Disease: Improving Global Outcomes (KDIGO) Work Group clinical practice guidelines44. Patients with serum PTH levels equal to or exceeding 500 pg/ml could receive treatment with cinacalcet hydrochloride (reimbursed by the National Health Fund - NHF). HD patients with sHPT who failed to respond to pharmacological treatment presented with PTH levels exceeding 1000 pg/ml in repeated evaluations and were tested for parathyroid gland adenoma on 99mTc-MIBI scintigraphy. Parathyroidectomy (PTX) was performed if possible (no clinical contraindications, written informed consent obtained).
Treatment with cinacalcet (Mimpara, Amgen Europe, Breda, Netherlands) at a daily dose of 30 mg was started in 162 patients with PTH exceeding 500 pg/ml. Patients who showed serum PTH of 1000 pg/ml or greater before the planned treatment with cinacalcet underwent 99mTc-MIBI scintigraphy. Only those without scintigraphic suspicion of parathyroid gland adenoma qualified for this treatment. Serum PTH was checked in the treated group every 4 weeks, and cinacalcet doses were increased up to 180 mg per day, if well tolerated, until the PTH concentration decreased at least by 40% of the initial level, which was considered as a satisfactory response to treatment. Patients who did not exhibit sufficiently decreased serum PTH were referred to as non-responders to cinacalcet.
The principal dietary and pharmacological treatment of all patients was based on a standard of care according to the physician. Guidelines for this treatment based on recommendations of the Polish Society of Nephrology and the principal country consultants in nephrology. Nephrologists had been prescribing calcium carbonate or calcium acetate in 1031 cases due to hyperphosphataemia without hypercalcaemia or due to hypocalcaemia. Sevelamer hydrochloride was used in 24 hypercalcaemic/hyperphosphatemic patients. Frequency of patients receiving phosphate binders did not differ in groups stratified by CASR polymorphic variants (Supplementary Table 3). Among vitamin D supplements, alfacalcidol (Alfadiol, GlaxoSmithKline Pharmaceuticals SA, Poland) was the most frequently used. None of patients was administered intravenous vitamin D analogues. Patients were using erythropoietin stimulating agents, iron and water-soluble vitamins. Co-morbid conditions were treated as needed.
Blood samples for Ca, P, ALP, and PTH were routinely taken from all HD patients at the onset of RRT. Periodical measurements were conducted every 1–3 months for Ca and P and every 6–12 months for ALP and PTH. Differences in the frequency of measurements were dependent on the intrinsic regulations of the respective dialysis centers.
A majority of the HD patients enrolled in the current study were previously genotyped for T helper (Th) cell cytokine-associated genes, including CCL2 -2518 A>G rs1024611 that is also referred to as the monocyte chemotactic protein 1 gene (MCP1). Patients were also genotyped for vitamin D signaling pathway genes35,45,46. The frequency distributions of polymorphic variants of these genes and their associations with the tested phenotypes, as well as their epistatic interactions with CASR rs7652589, were analyzed in HD patients in the ongoing study.
Controls
Healthy individuals (blood donors and healthy volunteers unrelated to the patients and to each other) from the same region of Poland served as controls for the frequency distribution of the CASR rs7652589 SNP. The control group included 918 subjects.
All study subjects were Caucasians of Polish origin.
Patient Data
Demographic and clinical parameters including date of birth, gender, chronic kidney disease causing ESRD and requiring RRT, date of the start of RRT, presence of CAD, a history of MI and PTX were recorded by a review of the medical charts and by personal communications with the nephrologists responsible for the treatment of patients in the dialysis centers. The patient data were evaluated every year from the initiation to the end of the enrollment period with respect to the diagnosis of CAD and the history of MI and PTX.
Laboratory parameters included serum concentrations of total Ca, P, and intact PTH, as well as serum activity of total ALP; all serum concentrations were determined using routine laboratory methods by laboratories under agreement with the dialysis centers. The laboratory data were evaluated in our database every year: the last four results obtained while the patient was in stable condition were collected as long as their number was not limited by a short HD vintage. For analysis, each laboratory parameter was represented as a mean value of up to 4 results obtained during the last period of HD vintage. In patients who underwent PTX or were treated with cinacalcet, laboratory data obtained before those treatments were included in the analysis. Analyses of the CASR association with serum Ca were also performed using the lowest Ca levels among up to 4 last collected values, if patients were receiving Ca-containing drugs. The CASR association with circulating P were also explored using the highest P levels among up to 4 last collected values, if patients were taking phosphate-binding drugs.
Additionally, plasma 25(OH)D levels were determined in 217 blindly selected HD patients. Blood samples for 25(OH)D were taken in the winter to avoid differences in sunlight exposure between patients who sunbathed and those who did not. Plasma 25(OH)D concentrations were measured in HD patients who had not been treated with vitamin D or had stopped vitamin D treatment at least 3 weeks earlier.
In patients treated with cinacalcet, serum PTH concentrations preceding the initiation of cinacalcet administration and those measured 1–2 months after implementation of the maximum cinacalcet dose were collected; however, serum PTH concentrations were collected no longer than 12 months from the beginning of treatment.
For the survival analysis, the dates of kidney transplantation, movement to a non-collaborating dialysis center, or death were recorded. The causes of death were recorded and categorized as cardiac (reported as myocardial infarction, sudden cardiac death, severe arrhythmias, cardiomyopathies, or cardiac failure), vascular (reported as cerebrovascular events, cerebral stroke, or generalized atherosclerosis), or other. The last evaluation of patient data was performed between March 27th and April 20th, 2015.
Genotyping the CASR rs7652589 polymorphism
The CASR rs7652589 SNP was genotyped using leukocyte DNA obtained from 1162 HD patients and 918 controls. High-resolution melting curve analysis (HRM) was performed using a LightCycler 480 system (Roche Diagnostics, Mannheim, Germany). The 178 bp PCR product was amplified using the following primers: F: 5′-ACTGCCCTCATCATTCCTTC-3′ and R: 5′-ATCATCCTCCCTGCAAGAAC-3′. The annealing temperature was 61 °C. Amplified DNA fragments were subjected to HRM with temperatures ranging from 77 °C to 92 °C in 0.1 °C increments. Quality control was ensured by running 10% of the samples in duplicate.
Real-time quantitative PCR (Q-PCR) analysis of CaSR transcript levels in PBMC
Blood samples (5 ml each) from 42 HD patients was collected into tubes containing EDTA. The study group included 8 individuals with AA, 23 with AG, and 11 with GG genotype. PBMC were separated by Ficoll-Hypaque centrifugation (density, 1.077 g/cm3) followed by total RNA isolation. Total RNA was isolated according to the method of Chomczyński and Sacchi47. RNA samples were treated with DNase I, quantified and reverse-transcribed (RT) into complimentary DNA (cDNA). Quantitative analysis of CaSR cDNA was performed by Light Cycler®480 II Real-Time PCR System (Roche Diagnostics GmbH, Mannheim, Germany), using SYBR Green I as detection dye. CaSR cDNA was quantified using the relative quantification method with a calibrator. The calibrator was prepared as a cDNA mix from all cDNA samples and consecutive dilutions were used to create a standard curve. For amplification, 1 μl of cDNA solution was added to 9 μl of LightCycler 480 SYBR Green I Master Mix (Roche Diagnostics GmbH, Mannheim, Germany) and primers. The quantity of CaSR transcript in each sample was standardized by the geometric mean of porphobilinogen deaminase (PBGD) and beta-2-microglobulin (B2M) transcript levels. The PCR amplification efficiency for target and reference cDNA was determined by different standard curves created by consecutive dilutions of the cDNA template mixture. The CaSR cDNA 96 bp amplicon was amplified using the primer pair: (forward 5′ ATGCCAAGAAGGGAGAAAGAC 3′) and (reverse 5′ GGAGGACAGACAGCACAAAG 3′). The PBGD 160 bp and B2M cDNA 106 bp amplicons was amplified using the primer pair: (forward 5′ GCC AAG GAC CAG GAC ATC 3′), (reverse 5′ TCA GGT ACA GTT GCC CAT C 3′) and (forward 5′ CACCCCCACTGAAAAAGATG 3′), (reverse 5′ CCTCCATGATGCTGCTTACA 3′), respectively. The CaSR mRNA levels were expressed as multiples of these cDNA concentrations in the calibrator.
Statistical analysis
The results are presented as percentages for categorical variables or as the median and range (minimum - maximum) for continuous variables because the majority of continuous variables were non-normally distributed as tested by the Shapiro–Wilk test.
Clinical and laboratory data were compared in HD subjects stratified by CASR rs7652589 genotype. The results were analyzed using three inheritance models (dominant, recessive and additive). Continuous variables were compared using the Mann-Whitney test. For comparison of categorical variables, Chi-square test with Yates correction was used. Parameters for which this preliminary analysis suggested an association with CASR rs7652589 in at least one model of inheritance (P < 0.05 without the Bonferroni correction) were chosen for detailed statistical analysis as described below.
Statistical analysis of CaSR transcript level comparison between the AA vs GG and AG vs GG genotype carriers was evaluated by the Kruskal-Wallis test.
In this study, the power for detection of genetic associations was determined using Quanto v.1.2.4 software48. Departure from the Hardy-Weinberg equilibrium was determined by Chi-squared analysis. Polymorphisms were tested for trends in association using the Chi-square test if two groups were compared or the Cochran-Armitage test if three groups were compared (Ptrend). Genotype distributions were compared between the tested groups using the standard Chi-square test (Pgenotype). Odds ratios (OR) and 95% confidence intervals (CIs) for OR were calculated to assess the strength of the association with categorical variables. Chi-square test with Yates correction was used for statistical evaluation of OR. Additionally, gender and age at the onset of RRT (<40 years vs. ≥ 40 years) were adjusted for in analyses conducted using Cochran-Mantel-Haenszel statistics. All probabilities were two-tailed, and a Bonferroni corrected P value lower than 0.017 (1 SNP, 3 models, 1 phenotype) was considered significant.
Multiple regression analysis was performed to determine whether genotypes that were associated with specific phenotypes were also independent determinants of those phenotypes among other variables. The multivariable models included clinical characteristics of HD patients, laboratory parameters related to Ca and sHPT, and a CASR polymorphic variant tested for association. The chosen variables were entered into a stepwise multivariate linear regression model, and an assessment of confounding variables was performed. The results of the multivariate analysis are presented as the regression coefficient (B) values ± 95% CI.
Survival probability since the onset of RRT was analyzed with respect to CASR genotypes. This analysis was also performed using dominant, recessive and additive models of inheritance. The Kaplan-Meier method with the log-rank test was used to estimate significant differences in the cumulative proportion of survival curves characterizing the genotype groups in each model of inheritance.
The previously mentioned statistical analyses were performed using Graph-Pad InStat 3.10, 32 bit for Windows, created July 9, 2009 (GraphPad Software, Inc., San Diego, California, United States), CytelStudio version 10.0, created January 16, 2013 (CytelStudio Software Corporation, Cambridge, Massachusetts, United States), and Statistica version 12, 2014 (Stat Soft, Inc., Tulsa, Oklahoma, United States).
Epistatic interactions between CASR rs7652589 and Th cell cytokine-associated genes and vitamin D signaling pathway genes were analyzed in patients with and without nephrolithiasis-related ESRD using the logistic regression and epistasis option in the PLINK software (http://pngu.mgh.harvard.edu/purcell/plink/).
Values of P < 0.05 were considered significant in the epistatic interaction, multiple regression and survival probability analyses.
In silico TFBS prediction
Postulated rs7652589 (G>A) regulatory impact on CASR expression through transcription factor binding sites motifs disruption5 was assessed with an analysis of the experimental ENCODE TFBS ChIP-seq data and in silico prediction of DNA-binding sites collected in HOCOMOCO version 949 and JASPAR CORE version 5.0 ALPHA 201650 databases with FIMO software version 4.11.151. FIMO scans DNA sequences utilizing position-specific frequency matrices of the given motifs and reports the output as the P value computed from log-likelihood ratio score for each database motif and applies false discovery rate analysis to estimate q values.
Since ENCODE TFBS ChIp-seq experiments contain data for only 5 out of the total 161 TFs for the relevant HEK293 cell line, we bona fide expanded our DNA-binding sites search in rs7652589 containing region to the all ChIP-seq’ed cell lines.
We carried out the computational analysis on the GenBank DNA sequences (contig NT-005612.16) adjacent to rs7652589 positions. Two FASTA sequences, each with one of the rs7652589 alleles, were used as an input for the FIMO. To minimize both false positive and false negative rates, the background file was calculated directly from used input sequences with MEME-suit script52.
Results were analyzed via a self-developed Python script to find predicted transcription factor binding sites ranges containing rs7652589. A P value < 0.0005 and q value < 0.05 were selected as the cut-off values for reliable predictions. Motifs matching to both strands were also analyzed whether or not they form perfect reverse complements, therefore making the match more probable. We obtained information about identified transcription factors from the SwissProt database, and filtered statistically significant hits present in Homo sapiens53. Details of in silico analysis are presented in Supplementary Methods.
Ethical approval
The research design was approved by the Institutional Review Board of Poznan University of Medical Sciences, Poland. Informed consent was obtained from all study participants. The study was carried out in accordance with the approved guidelines.
Additional Information
How to cite this article: Grzegorzewska, A. E. et al. Associations of the calcium-sensing receptor gene CASR rs7652589 SNP with nephrolithiasis and secondary hyperparathyroidism in haemodialysis patients. Sci. Rep. 6, 35188; doi: 10.1038/srep35188 (2016).
Supplementary Material
Acknowledgments
We would like to express our gratitude to the physicians of the dialysis centers for their consent in collecting the participants’ data. This study was funded in part by the scientific grant of the Baxter company allocated by the Chapter of the Polish Society of Nephrology as the blindly reviewed award winning project, grant number 504-04-02225363-00013-03071 (granted to AEG and PPJ). Additional funding was provided by the Poznan University of Medical Sciences, Poznań, Poland, grant numbers 502-01-02225363-03679 and 502-01-01124182-07474.Results of this study were presented as oral communication at the 53rd ERA-EDTA Congress, 21-24.05.2016, Vienna, Austria.
Footnotes
Author Contributions A.E.G. conceived the study. A.E.G. and M.P. contributed to the design of the research. A.E.G., M.P. and A.M. were involved in the data collection. A.E.G. and W.W. analyzed the data. A.M. and P.P.J. were responsible for the genotyping. B.F. performed analysis of CaSR transcript. I.S. and M.F. did in silico analyses. A.E.G. and P.P.J. participated in funding for the project. All authors edited and approved the final version of the manuscript.
References
- Griffith D. P. Struvite stones. Kidney Int. 13, 372–382 (1978). [DOI] [PubMed] [Google Scholar]
- Jungers P., Joly D., Barbey F., Choukroun G. & Daudon M. ESRD caused by nephrolithiasis: prevalence, mechanisms, and prevention. Am. J. Kidney Dis. 44, 799–805 (2004). [PubMed] [Google Scholar]
- Vezzoli G., Terranegra A. & Soldati L. Calcium-sensing receptor gene polymorphisms in patients with calcium nephrolithiasis. Curr. Opin. Nephrol. Hypertens. 21, 355–361 (2012). [DOI] [PubMed] [Google Scholar]
- Vezzoli G. et al. Decreased transcriptional activity of calcium-sensing receptor gene promoter 1 is associated with calcium nephrolithiasis. J. Clin. Endocrinol. Metab. 98, 3839–3847; doi: 10.1210/jc.2013-1834 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzoli G. et al. Calcium kidney stones are associated with a haplotype of the calcium-sensing receptor gene regulatory region. Nephrol. Dial. Transplant. 25, 2245–2252 (2010). [DOI] [PubMed] [Google Scholar]
- Renal U. S. Data System, USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD (2013).
- Kifor O. et al. Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J. Clin. Endocrinol. Metab. 81, 1598–1606 (1996). [DOI] [PubMed] [Google Scholar]
- Debelle F. et al. Cinacalcet for managing secondary hyperparathyroidism in dialysis patients in clinical practice in Belgium: a 16-month observational study (ECHO-B). Acta Clin. Belg. 68, 275–281 (2013). [DOI] [PubMed] [Google Scholar]
- Yano S. et al. Association of polymorphic alleles of the calcium-sensing receptor gene with parathyroid hormone secretion in hemodialysis patients. Nephron. 85, 317–323 (2000). [DOI] [PubMed] [Google Scholar]
- Yokoyama K. et al. Calcium-sensing receptor gene polymorphism affects the parathyroid response to moderate hypercalcemic suppression in patients with end-stage renal disease. Clin. Nephrol. 57, 131–135 (2002). [DOI] [PubMed] [Google Scholar]
- Rothe H. M., Shapiro W. B., Sun W. Y. & Chou S. Y. Calcium-sensing receptor gene polymorphism Arg990Gly and its possible effect on response to cinacalcet HCl. Pharmacogenet. Genomics. 15, 29–34 (2005). [DOI] [PubMed] [Google Scholar]
- Rothe H., Shapiro W. B., Sun W. Y. & Matalon A. Calcium-sensing receptor gene polymorphism Arg990Gly influences the response to calcimimetic agents in end-stage kidney disease patients with secondary hyperparathyroidism. Personalized Med. 5, 109–116 (2008). [DOI] [PubMed] [Google Scholar]
- Marz W. et al. Alanine to serine polymorphism at position 986 of the calcium-sensing receptor associated with coronary heart disease, myocardial infarction, all-cause, and cardiovascular mortality. J. Clin. Endocrinol. Metab. 92, 2363–2369 (2007). [DOI] [PubMed] [Google Scholar]
- Brown E. M. et al. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 366, 575–580 (1993). [DOI] [PubMed] [Google Scholar]
- Kapur K. et al. Genome-wide meta-analysis for serum calcium identifies significantly associated SNPs near the calcium-sensing receptor (CASR) gene. PLoS Genet. 6, e1001035 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scillitani A. et al. Blood ionized calcium is associated with clustered polymorphisms in the carboxyl-terminal tail of the calcium-sensing receptor. J. Clin. Endocrinol. Metab. 89, 5634–5638 (2004). [DOI] [PubMed] [Google Scholar]
- Yamauchi M. et al. Association of polymorphic alleles of the calcium-sensing receptor gene with the clinical severity of primary hyperparathyroidism. Clin. Endocrinol. (Oxf). 55, 373–379 (2001). [DOI] [PubMed] [Google Scholar]
- Reh C. M., Hendy G. N., Cole D. E. & Jeandron D. D. Neonatal hyperparathyroidism with a heterozygous calcium-sensing receptor (CASR) R185Q mutation: clinical benefit from cinacalcet. J. Clin. Endocrinol. Metab. 96, E707–E712 (2011). [DOI] [PubMed] [Google Scholar]
- Filopanti M. et al. MEN1-related hyperparathyroidism: response to cinacalcet and its relationship with the calcium-sensing receptor gene variant Arg990Gly. Eur. J. Endocrinol. 167, 157–164 (2012). [DOI] [PubMed] [Google Scholar]
- Reynolds J. L. et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J. Am. Soc. Nephrol. 15, 2857–2867 (2004). [DOI] [PubMed] [Google Scholar]
- Nakamura S. et al. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin. J. Am. Soc. Nephrol. 4, 1892–1900 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler L. et al. Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications. A statement for health professionals from the American Heart Association. Writing Group. Circulation. 94, 1175–1192 (1996). [DOI] [PubMed] [Google Scholar]
- Alam M. U. et al. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc. Res. 81, 260–268 (2009). [DOI] [PubMed] [Google Scholar]
- Vezzoli G. et al. Calcium-sensing receptor and calcium kidney stones. J. Transl. Med. 9, 201 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzoli G. et al. Polymorphisms at the regulatory regions of the CASR gene influence stone risk in primary hyperparathyroidism. Eur. J. Endocrinol. 164, 421–427 (2011). [DOI] [PubMed] [Google Scholar]
- Chudek J. et al. The prevalence of chronic kidney disease and its relation to socioeconomic conditions in an elderly Polish population: results from the national population-based study PolSenior. Nephrol. Dial. Transplant. 29, 1073–1082 (2014). [DOI] [PubMed] [Google Scholar]
- Rutkowski B. et al. Report on the renal replacement therapy in Poland–2010. [Polish]. Drukonsul, Gdańsk (2013). [Google Scholar]
- Ben—Selma W., Harizi H. & Boukadida J. MCP—1 —2518 A/G functional polymorphism is associated with increased susceptibility to active pulmonary tuberculosis in Tunisian patients. Mol. Biol. Rep. 38, 5413—5419 (2011). [DOI] [PubMed] [Google Scholar]
- Aguilar F., González-Escribano M. F., Sánchez-Román J. & Núñez-Roldán A. MCP-1 promoter polymorphism in Spanish patients with systemic lupus erythematosus. Tissue Antigens. 58, 335–338 (2001). [DOI] [PubMed] [Google Scholar]
- Liu Z. et al. Calcium oxalate monohydrate crystals stimulate monocyte chemoattractant protein-1 and transforming growth factor β1 expression in human renal epithelial cells. Mol. Med. Rep. 5, 1241–1244 (2012). [DOI] [PubMed] [Google Scholar]
- Hong S. H. et al. Anti-nephrolithic potential of resveratrol via inhibition of ROS, MCP-1, hyaluronan and osteopontin in vitro and in vivo. Pharmacol. Rep. 65, 970–979 (2013). [DOI] [PubMed] [Google Scholar]
- Rovin B. H., Lu L. & Saxena R. A novel polymorphism in the MCP-1 gene regulatory region that influences MCP-1 expression. Biochem. Biophys. Res. Commun. 259, 344–348 (1999). [DOI] [PubMed] [Google Scholar]
- Buraczyńska M., Bednarek—Skublewska A., Buraczyńska K. & Książek A. Monocyte chemoattractant protein—1 (MCP—1) gene polymorphism as a potential risk factor for cardiovascular disease in hemodialyzed patients. Cytokine. 44, 361—365 (2008). [DOI] [PubMed] [Google Scholar]
- Umekawa T., Iguchi M., Uemura H. & Khan S. R. Oxalate ions and calcium oxalate crystal-induced up-regulation of osteopontin and monocyte chemoattractant protein-1 in renal fibroblasts. BJU Int. 98, 656–660 (2006). [DOI] [PubMed] [Google Scholar]
- Grzegorzewska A. E., Ostromecki G., Zielińska P., Mostowska A. & Jagodziński P. P. T-cell cytokine gene polymorphisms and vitamin D pathway gene polymorphisms in end-stage renal disease due to type 2 diabetes mellitus nephropathy: comparisons with health status and other main causes of end-stage renal disease. J. Diabetes Res. 2014, 120317; doi: 10.1155/2014/120317 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posen S. Alkaline phosphatase. Ann Intern Med. 67, 183–203 (1967). [DOI] [PubMed] [Google Scholar]
- Babinsky V. N. et al. Association studies of calcium-sensing receptor (CaSR) polymorphisms with serum concentrations of glucose and phosphate, and vascular calcification in renal transplant recipients. PLoS One. 10, e0119459; doi: 10.1371/journal.pone.0119459 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hénaut L. et al. Calcimimetics increase CaSR expression and reduce mineralization in vascular smooth muscle cells: mechanisms of action. Cardiovasc. Res. 101, 256–265 (2014). [DOI] [PubMed] [Google Scholar]
- Bame S. I., Petersen N. & Wray N. P. Variation in hemodialysis patient compliance according to demographic characteristics. Soc. Sci. Med. 37, 1035–1043 (1993). [DOI] [PubMed] [Google Scholar]
- Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav. Ecol. 15, 1044–1045; doi: 10.1093/beheco/arh107 (2004). [DOI] [Google Scholar]
- Wang C. et al. HLA and cytokine gene polymorphisms are independently associated with responses to hepatitis B vaccination. Hepatology 39, 978–988; doi: 10.1002/hep.20142 (2004). [DOI] [PubMed] [Google Scholar]
- Potaczek D. P. et al. Different FCER1A polymorphisms influence IgE levels in asthmatics and non-asthmatics. Pediatr. Allergy Immunol. 24, 441–449 (2013). [DOI] [PubMed] [Google Scholar]
- Sharma V. et al. Fine-mapping of IgE-associated loci 1q23, 5q31, and 12q13 using 1000 Genomes Project data. Allergy. 69, 1077–1084 (2014). [DOI] [PubMed] [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD–MBD). Kidney Int. 76 (Suppl 113), S1–S130 (2009). [DOI] [PubMed] [Google Scholar]
- Grzegorzewska A. E., Pajzderski D., Sowińska A., Mostowska A. & Jagodziński P. P. IL4R and IL13 polymorphic variants and development of antibodies to surface antigen of hepatitis B virus in hemodialysis patients in response to HBV vaccination or infection. Vaccine. 31, 1766–1770 (2013). [DOI] [PubMed] [Google Scholar]
- Grzegorzewska A. E., Jodłowska E., Mostowska A., Sowińska A. & Jagodziński P. P. Single nucleotide polymorphisms of vitamin D binding protein, vitamin D receptor and retinoid X receptor alpha genes and response to hepatitis B vaccination in renal replacement therapy patients. Expert Rev. Vaccines. 13, 1395–1403 (2014). [DOI] [PubMed] [Google Scholar]
- Chomczynski P. & Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 162, 156–159 (1987). [DOI] [PubMed] [Google Scholar]
- QUANTO 1.2.4. Sample size and power calculations for genetic-epidemiology studies. University of Southern California, Los Angeles, USA. http://biostats.usc.edu/software (2009).
- Kulakovskiy I. V. et al. HOCOMOCO: a comprehensive collection of human transcription factor binding sites models. Nucleic Acids Res. 41(Database issue), D195–D202 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathelier A. et al. JASPAR 2014: an extensively expanded and updated open-access database of transcription factor binding profiles. Nucleic Acids Res. 42(Database issue), D142–D147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C. E., Bailey T. L. & Noble W. S. FIMO: scanning for occurrences of a given motif. Bioinformatics. 27, 1017–1018 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey T. L., Johnson J., Grant C. E. & Noble W. S. The MEME Suite. Nucleic Acids Res. 43(W1), W39–W49 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- The UniProt Consortium. The universal protein resource (UniProt). Nucleic Acids Res. 36, D190–D195 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


