Abstract
Background
Major (above-knee or below-knee) amputation is a complication of diabetes and is seen more common among black and Hispanic patients. While amputation rates have declined for patients with diabetes in the last decade, it remains unknown if these improvements have equitably extended across racial groups and if measures of diabetic care, such as hemoglobin A1c testing, are associated with these improvements. We set out to characterize secular changes in amputation rates among black, Hispanic, and white patients, and to determine associations between hemoglobin A1c testing and amputation risk.
Methods
We identified 11,942,840 Medicare patients (55% female) with diabetes over the age of 65 years between 2002 and 2012 and followed them for a mean of 6.6 years. Of these, 86% were white, 11.5% were black, and 2.5% were Hispanic. We recorded the occurrence of major amputation and hemoglobin A1c testing during this time period and studied secular changes in amputation rate by race (black, Hispanic, and white). Finally, we examined associations between amputation risk and hemoglobin A1c testing. We measured both the presence of any testing and testing consistency using 3 categories: poor consistency (hemoglobin A1c testing in 0–50% of years), medium consistency (testing in 50–90% of years), and high consistency (testing in >90% of the years in the cohort).
Results
Between 2002 and 2012, the average major lower-extremity amputation rate in diabetic Medicare patients was 1.78 per 1,000 per year for black patients, 1.15 per 1,000 per year for Hispanic patients, and 0.56 per 1,000 per year for white patients (P < 0.001). Over the study period, the incidence of major amputation in Medicare patients with diabetes declined by 54%, from 1.15 per 1,000 in 2002 to 0.53 per 1,000 in 2012 (rate ratio = 0.53, 95% CI = 0.51–0.54). The reduction in amputation rate was similar across racial groups: 52% for black patients, 61% for Hispanic patients, and 55% for white patients. In multivariable analysis adjusting for patient characteristics, including race, any use of hemoglobin A1c testing was associated with a 15% decline in amputation risk (hazard ratio, 0.85; 95% CI, 0.83–0.87; P < 0.001). High consistency hemoglobin A1c testing was associated with a 39% decline in amputation (hazard ratio, 0.61; 95% CI, 0.59–0.62; P < 0.0001).
Conclusions
Although more frequent among racial minorities, major lower-extremity amputation rates have declined similarly across black, Hispanic, and white patients over the last decade. Hemoglobin A1c testing, particularly the consistency of testing over time, may be an effective component metric of longitudinal quality measures toward limiting amputation in all races.
INTRODUCTION
Amputation rates have declined by more than 50% among patients with diabetes in the last decade.1–3 Investigators at the Centers for Disease Control (CDC) hypothesize that this decline is a result of changes in health care delivery for patients with diabetes. They cited examples such as better acute clinical care, better health promotion and awareness, and improvements in the health care system itself.4–6 They argue that the procedural aspects of diabetic care—especially broader use of hemoglobin A1c testing and other preventive measures have been responsible for the declines in amputation rate for patients with diabetes.7–9
While a decline in amputation among patients with diabetes is certainly welcome news, certain aspects of this improvement deserve closer consideration. First, black and Hispanic patients have historically had much higher risks of amputation than white patients, nearly 4-fold higher in many reports.10,11 It is unclear if the decline in amputations described in the CDC’s report has equitably extended across racial and ethnic groups. Better understanding of this question is important because if the decline in amputation has occurred primarily in white patients, these improvements could potentially worsen this disparity.12 This circumstance is certainly plausible, given that black and Hispanic patients often have poorer access to the same preventive health measures, such as hemoglobin A1c testing, credited with improving amputation risk.13
The purpose of this project is to better understand relationships between amputation rates, race, and the utilization of preventive measures in patients with diabetes. To accomplish this goal, we examined trends in amputation and hemoglobin A1c testing across 3 racial groups—black, Hispanic, and white patients—using Medicare claims between 2002 and 2012. We hypothesized that a clearer understanding of the declines in amputation by race would help better inform future health policy directed at preventing amputation among Medicare patients with diabetes.
METHODS
Analytic Overview
We created a cohort of Medicare patients with diabetes between 2002 and 2012. For each of these years, we used the current year and up to 3 years of preexisting Medicare claims for each patient to assess for the occurrence of a major (above-knee or below-knee) leg amputation. We recorded only the first amputation per patient during this time interval. We defined this 3-year antecedent period because Medicare claims were available for 3 years before the start of our study interval (2001, 2000, and 1999), and we wished to make consistent the duration of time each patient was “at risk” for amputation.
Within this cohort, we identified not only patient-level claims indicating major amputation (above-knee or below-knee) but also patient-level claims indicating the use of hemoglobin A1c testing. We stratified our main outcome measure—number of amputations per 1,000 Medicare patients—by race, and adjusted for patient demographic factors, regional health characteristics, and then performed a propensity-weighted analysis.
Cohort Creation and Stratification by Race
We utilized the Medicare Physician and Supplier file and the Medicare Denominator file between 2002 and 2012 to identify all patients with diagnosis codes indicating the presence of diabetes during that time period. Patients who appeared in the cohort across multiple years were required to have a diagnosis codes for diabetes in 2 of 3 consecutive years. We excluded patients less than 65 years of age, greater than 99 years of age, and those not enrolled in fee-for-service Medicare plans. Further information was obtained using the denominator file, which contains information about Medicare and Medicaid eligibility, as well as information about age, sex, and race. We recorded patient zip code and the hospital referral region of residence, as described by the Dart-mouth Atlas of Health Care.14
While Medicare claims allow designation of more than 5 racial groups; for clarity in these analyses, we performed comparisons across groups where disparities are commonly encountered when studying risks for amputation—black, Hispanic, and white patients. Patients categorized as white included patients designated as Asian, Native American, and those designated in Medicare claims as “other”. Traditionally, the term “Hispanic” refers to an ethnicity instead of a race. However, in the Medicare data set, only one variable exists for race and/or ethnicity and Hispanic is one of its classifications. Therefore, in our analysis, the term “Hispanic” was analyzed as a race variable just like black and white.
Hemoglobin A1c Testing and Testing Consistency
We analyzed this diabetic patient cohort for claim-based evidence of hemoglobin A1c testing in 2 ways. First, we examined whether the patients had ever undergone hemoglobin A1c testing by means of the Current Procedural Terminology code available for this laboratory test (Appendix 1). Second, due to the recommendation that patients with diabetes undergo hemoglobin A1c testing annually,15 we analyzed how consistent hemoglobin A1c testing was in patients at risk for amputation from diabetes. We determined testing consistency by measuring the number of years an individual patient received testing compared with the number of years that they were present in our cohort. While these analyses were done in a continuous fashion, we created terciles of testing consistency for presentation, using patients who were present in our cohort for at least 3 years. These categories were: low consistency (testing in 0–50% of years in our analysis), medium consistency (testing in 50–90% of the years in our analysis), and high consistency (testing in >90% of the years in our analysis).
Main Outcome Measure: Lower-Extremity Amputation
We recorded the occurrence of each major amputation at the patient level using Current Procedural Terminology codes indicative of above-knee or below-knee amputation (Appendix 1). We excluded toe and forefoot amputations. While toe and forefoot amputations have important clinical consequence, the disability incurred by an above-knee or below-knee amputation is of much greater clinical consequence to patients, and therefore, we focused on these events in this analysis. In sensitivity analyses, our results remained similar when we included toe or forefoot amputations in our analysis. We excluded traumatic amputations.
Statistical Analyses
We began by examining the annual rate of amputation among Medicare patients with diabetes between 2002 and 2012. We stratified these results by race and examined differences in amputation over time using a nonparametric test of trend. Next, we examined the annual proportion of Medicare patients who received hemoglobin A1c testing each year and measured the overall consistency of testing and its relationship to amputation rate.
Crude results were examined across our 3 racial categories and over time. Amputation-free survival was defined as time from enrollment in the cohort to the occurrence of a censoring event (either death or major amputation). We created Cox survival models to understand associations between the risk of amputation, race, and hemoglobin A1c testing. These models were adjusted for patient demographics, such as age, disability status, Medicaid benefits, as well as comorbidities as indicated by the Charlson score and its components. We also adjusted for regional health characteristics, smoking status, obesity, intensity of vascular care, and the use of invasive diagnostic or therapeutic lower-extremity vascular procedures. Given prior studies that reveal an association between the use of vascular care and amputation for patients with peripheral arterial disease, nearly half of whom had diabetes, we examined the use of vascular procedures—both diagnostic and therapeutic, endovascular and open surgical procedures—for patients in our study cohort.16,17 Both inpatient and outpatient vascular procedures were considered.
We used inverse propensity weighting to adjust these models for the propensity for patients to receive hemoglobin A1c testing (Appendix 2). These models were used to compare the risks of amputation among 2 distinct ways. In the first group of models, we studied hemoglobin A1c testing in a binary fashion (patients who did and did not receive hemoglobin A1c testing at any one point in time during the study interval). In the second group of models, we studied the consistency of hemoglobin A1c testing, using 3 categories of testing consistency defined as low, moderate, and high.
All analyses were performed using SAS and STATA (College Station, TX). The Geisel School of Medicine’s Center for the Protection of Human Subjects granted institutional review board exemption for our study. Patient consent was not required as this was deidentified claims data.
RESULTS
Demographics
Between 2002 and 2012, we identified 11,942,840 individual patients with diabetes, who were followed for a mean of 6.6 years within our cohort. Patients were a mean of 76 years of age; 12% were African-American, 2% were Hispanic, and the remaining 86% were white. No clinically significant changes occurred in demographic characteristics over time (Table I). The rates of most comorbidities, such as congestive heart failure, dementia, and cerebrovascular disease, also changed little during the study period, although the proportion of patients receiving disability benefits at the time of entry into the cohort increased by 21%. The proportion of patients with chronic renal insufficiency also increased, from 6% of the population in 2002 to 17% of the population by 2012. Regarding objective health characteristics, the proportion of patients in the cohort with a body mass index greater than 30 (29%) or were actively smoking (20%) did not change from 2002 to 2012.
Table I.
Demographics of our cohort of Medicare patients with diabetes
| Variable | Overall | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | Absolute % change between 2002 and 2012 | Relative % change between 2002 and 2012 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||||||||
| Mean number of years present in Medicare | 6.7 | 6.0 | 6.5 | 6.8 | 7.0 | 7.2 | 7.2 | 7.1 | 7.0 | 6.7 | 6.2 | 5.7 | −0.34 | −6 |
| Age (at entry into the analysis; years) | 75.8 | 75.6 | 75.6 | 75.6 | 75.6 | 75.7 | 75.8 | 75.8 | 75.9 | 75.9 | 75.9 | 75.9 | 0.29 | 0 |
| Medicaid (%) | 19.9 | 20.4 | 20.4 | 20.2 | 20.0 | 19.7 | 19.4 | 19.3 | 19.6 | 20.0 | 20.2 | 20.1 | −0.22 | −1 |
| Receiving disability benefits (%) | 12.8 | 11.8 | 12.0 | 12.2 | 12.3 | 12.2 | 12.3 | 12.7 | 13.1 | 13.5 | 13.8 | 14.3 | 2.46 | 21 |
| Black (%) | 11.5 | 12.2 | 12.2 | 12.2 | 11.8 | 11.5 | 11.1 | 10.9 | 11.1 | 11.2 | 11.4 | 11.5 | −0.68 | −6 |
| Hispanic (%) | 2.3 | 2.4 | 2.4 | 2.4 | 2.3 | 2.2 | 2.2 | 2.2 | 2.3 | 2.3 | 2.4 | 2.4 | −0.02 | −1 |
| White (includes Asian, Native American, other; %) | 86.2 | 85.4 | 85.4 | 85.5 | 85.9 | 86.3 | 86.7 | 86.9 | 86.6 | 86.4 | 86.2 | 86.1 | 0.69 | 1 |
| Female (%) | 55.3 | 56.9 | 56.5 | 56.2 | 56.0 | 55.7 | 55.3 | 55.0 | 54.7 | 54.5 | 54.3 | 54.0 | −2.91 | −5 |
| Mean Charlson Comorbidity Score | 1.7 | 1.8 | 1.8 | 1.8 | 1.8 | 1.6 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | −0.03 | −2 |
| Dementia (%) | 5.5 | 5.3 | 5.3 | 5.4 | 5.4 | 5.4 | 5.5 | 5.6 | 5.6 | 5.6 | 5.6 | 5.4 | 0.14 | 3 |
| Congestive heart failure (%) | 18.3 | 20.5 | 20.4 | 20.0 | 19.3 | 18.8 | 18.2 | 17.5 | 17.3 | 17.1 | 16.8 | 16.5 | −4 | −20 |
| Cerebrovascular disease (%) | 8.8 | 9.6 | 9.4 | 9.1 | 8.8 | 8.7 | 8.5 | 8.7 | 8.6 | 8.7 | 8.6 | 8.6 | −1 | −10 |
| Chronic renal failure (%) | 12.0 | 6.1 | 6.5 | 6.9 | 8.2 | 11.1 | 13.3 | 13.5 | 14.5 | 15.5 | 16.4 | 17.2 | 11.08 | 183 |
| Regional health characteristics | ||||||||||||||
| Average number of reported physically unhealthy days per month | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | −0.02 | −1 |
| Percent of adults that report BMI ≥30 (%) | 28.5 | 28.7 | 28.6 | 28.6 | 28.6 | 28.5 | 28.5 | 28.4 | 28.4 | 28.4 | 28.4 | 28.4 | −0.27 | −1 |
| Percent of adults that reported currently smoking (%) | 19.8 | 20.0 | 19.9 | 19.9 | 19.9 | 19.8 | 19.8 | 19.8 | 19.7 | 19.7 | 19.7 | 19.7 | −0.27 | −1 |
| Median household income in the past 12 months (in 2010 inflation-adjusted dollars; $) | 53,561 | 51,945 | 52,323 | 52,585 | 52,884 | 53,304 | 53,759 | 54,073 | 54,165 | 54,365 | 54,464 | 54,607 | 2,662 | 5 |
| Procedural characteristics of diabetic care | ||||||||||||||
| Major (above-knee or below-knee) amputation rate (%) | 0.8 | 1.2 | 1.1 | 1.0 | 0.9 | 0.8 | 0.8 | 0.7 | 0.6 | 0.6 | 0.6 | 0.5 | −0.62 | −54 |
| HgbA1c testing use: percent of patients with diabetes with one or more test (%) | 78.0 | 71.3 | 73.9 | 75.6 | 76.4 | 77.6 | 78.4 | 79.0 | 79.8 | 80.6 | 81.2 | 81.4 | 10.12 | 14 |
| Open lower-extremity bypass procedure rate in Medicare patients with diabetes (%) | 0.3 | 0.5 | 0.4 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | −0.23 | −51 |
| Endovascular (therapeutic) lower-extremity bypass procedure rate in Medicare patients with diabetes (%) | 0.8 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 0.9 | 0.8 | 0.9 | 0.9 | 0.8 | 1.0 | 0.46 | 90 |
| Endovascular (diagnostic) lower-extremity bypass procedure rate in Medicare patients with diabetes (%) | 1.2 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.3 | 1.3 | 1.3 | 0.8 | 0.9 | −0.48 | −35 |
BMI, body mass index.
Amputation Rate
The incidence of major amputation in Medicare patients with diabetes declined by 54% during the study period, from 1.15 per 1,000 per year in 2002 to 0.53 per 1,000 per year in 2012 (rate ratio = 0.53, 95% CI = 0.51–0.54, P < 0.001). Averaged over the study period, major amputation was more common among black (1.78 per 1,000 per year) and Hispanic (1.15 per 1,000 per year) patients while the lowest in white patients (0.56 per 1,000 per year). The absolute change in amputation rates was more pronounced among black patients as seen in Figure 1. However, the rate of decline of major amputation, that is the relative change in amputation rates, was similar for each racial group: 52% for black patients, 61% for Hispanic patients, and 55% for white patients, all from 2002 to 2012.
Fig. 1.
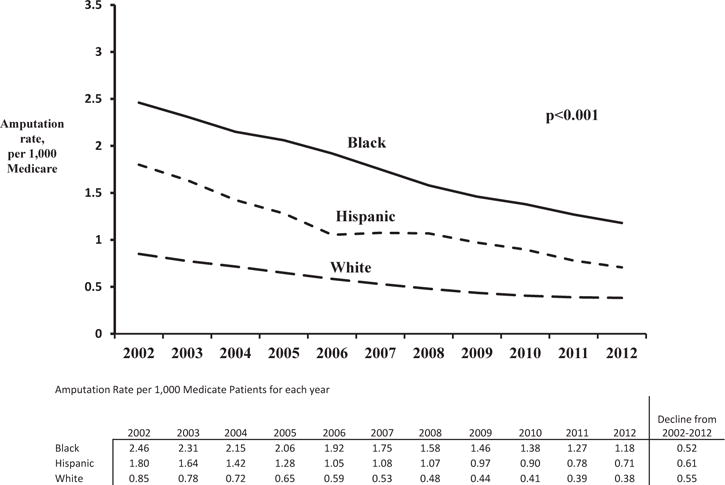
Trends in amputations among Medicare patients with diabetes, by race.
Use of Hemoglobin A1c Testing and Relationship with Amputation
The use of annual hemoglobin A1c testing increased in the cohort during the study period from 78% in 2002 to 81% in 2012 (Table I). Hemoglobin A1c testing increased similarly across racial groups rising from 73% to 81% for African American patients, from 71% to 82% for Hispanic patients, and from 81% to 87% for white patients during the study period. All these increases in hemoglobin A1c testing were statistically significant (P < 0.001).
We compared amputation rates between those patients who received hemoglobin A1c testing and those who did not receive hemoglobin A1c testing over time. We found that patients who received testing had slightly lower amputation rates, 1.11 amputations per 1,000 patients per year compared with 1.45 amputations per 1,000 patients per year (P = .01). This effect was consistent over the study period. In subanalysis by race, a similar trend of lower amputations remained among the white, black, and Hispanic patients who received hemoglobin A1c testing (Fig. 2). Furthermore, the rate of decline of amputations was similar for patients who did and did not have hemoglobin A1c testing across all 3 race categories. In multivariable cox regression models adjusting for patient characteristics, including race, we found that hemoglobin A1c testing at any point during the study period was associated with a 15% decline in amputation risk (hazard ratio, 0.85; 95% CI, 0.83–0.87; P = 0.001; Appendix 2).
Fig. 2.
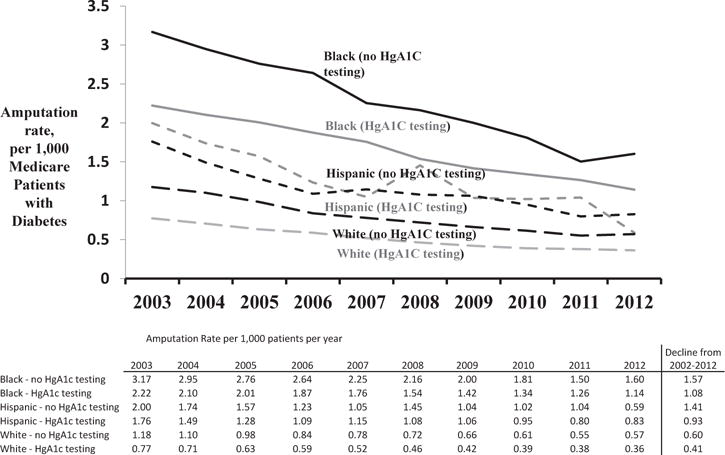
Trends in amputations among Medicare patients with diabetes, by race and HgA1c testing.
Consistency of Hemoglobin A1c Testing and Relationship with Amputation
When we examined relationships between the consistency of hemoglobin A1c testing and race, we found that black and Hispanic patients most commonly received low-quality testing, and white patients were most likely to receive the high consistency testing (Fig. 3). For example, 17% of black patients, 16% of Hispanic patients, and 12% of white patients received low consistency hemoglobin A1c testing. Conversely, while 52% of white patients received high consistency testing, fewer than half of black and Hispanic patients (46% and 43%, respectively) received this quality of diabetic care.
Fig. 3.
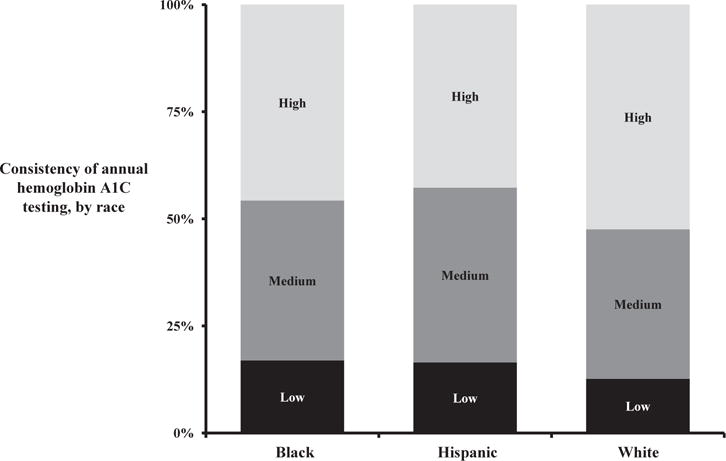
Consistency of Hemoglobin A1c testing among diabetic Medicare patients, by race.
Testing consistency was directly related to amputation for all racial groups (Fig. 4). Across racial categories, there was an inverse relationship between testing consistency and amputation rate. While the absolute rate of amputation was the highest in black and Hispanic patients, within racial groups the lowest amputation rates were seen in patients who received the most consistent testing. In multivariable analysis adjusting for patient characteristics, including race, high consistency testing was associated with a 39% decline in amputation (hazard ratio, 0.61; 95% CI, 0.59–0.62; P < 0.0001) when compared with low consistency testing (Appendix 2).
Fig. 4.
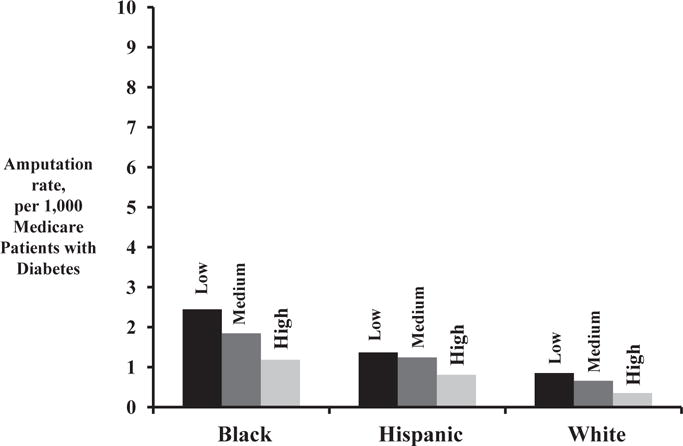
Amputation rate among diabetic Medicare patients compared by consistency in hemoglobin A1c testing, by race.
DISCUSSION
Leg amputation is a devastating complication for patients with diabetes,1 and this event often limits an elderly patient’s ability to ambulate and live independently.18 Furthermore, amputation typically occurs among the most disadvantaged patients with the poorest access to care, especially African American and Hispanic patients.19 Our analysis offers 2 encouraging findings for patients with diabetes at risk for amputation. First, amputation rates have fallen by more than 50% in recent years, and these improvements have extended essentially equally across racial groups. Second, our results begin to explain the processes of care associated with fewer amputations. Hemoglobin A1c testing—especially when provided consistently over time—appears to be associated with a lower chance of limb loss for diabetic patients, and this benefit appears to extend to patients in all racial groups.
Amputation is a complication of diabetes that has long been tied to racial disparities in care, and several reports have described racial disparities in amputation risk.2,5,10–12,19 Black and Hispanic patients, especially those in rural and impoverished regions, commonly have the highest risks of amputation. The extent of this disparity in amputation risk has been reported to be as high as 10-fold.20 A combination of patient, structural, and ecologic factors have been cited—persistent hypertension and obesity,21 poor care delivery in remote settings,22 and poor engagement in health care systems.23–25 Moreover, these risk factors often are seen in care settings wherein patients at the highest risk for amputation are often those least likely to receive preventive care.3 While medical reports and lay media have recognized this problem for several years, solutions that would specifically limit amputation have been difficult to identify.
However, as overall care for patients with diabetes has improved in recent years, and these improvements have included a decline in amputation. In absolute terms, amputation rates for white, Hispanic, and black Medicare patients are now less than half of what they were a decade ago, and these declines occurred equally across all racial groups. Endovascular procedure rates have dramatically risen during the similar time frame2 and could be associated with the drop in amputation rates; however, the delineation of why amputation rates are declining is beyond the scope of this work. Rather, while the reduction in amputation rates is laudable, it should not outweigh the fact that disparities in amputation risk still remain. While amputation risk is lower overall, the rate of limb loss from diabetes remains the highest among minority patients, more than twice as high in black patients, and nearly twice as high in Hispanic patients. Strategies to reduce the risk of amputation among minority patients with diabetes remain necessary, and new approaches will be necessary to meet these challenges.
Our findings suggest where strategies toward limiting amputation could focus next. The consistent application of an inexpensive, routine process of diabetic care—hemoglobin A1c testing—was associated with a lower rate of amputation, and this benefit extended equally to patients, irrespective of race. This suggests that initiatives focused on improving the consistency of hemoglobin A1c testing could offer potential benefits, especially if these initiatives were focused on high-risk populations.
Current measures of quality for providers who care for patients with diabetes could be revised to help to advance toward this goal. At present, the longitudinal nature of hemoglobin A1c testing is not incorporated into quality metrics, and only annual rates are reported in most settings.15,26,27 Our findings suggest that new metrics for diabetic care should consider not only quality in the short term but also the long-term effectiveness of care for this chronic disease. The direct association between higher testing consistency and lower amputation rates—an outcome of significant importance to patients—makes this an important opportunity. While the longitudinal nature of a “consistency in hemoglobin A1c testing” metric may be difficult to collect, new information technology will likely make longitudinal quality measures easier to design and measure in the future. Policy implications aside that there would be obvious benefits in the clarity offered to patients by this initiative. Information such as “partnering with your health care team for consistent hemoglobin A1c testing will lower that chance you will lose your leg to diabetes by more than half” has obvious clarity and impact. This may help patients and physicians achieve better adherence and health care engagement in the treatment of this challenging chronic disease.
Our study has several limitations. First, testing the value of testing—especially in a longitudinal sense—will require not just evidence that hemoglobin A1c testing has been performed but the actual testing results. Current efforts in the High Value Health Collaborative28 aim to use “enriched” claim-based data sets that have the actual values rather than just the use of testing for just this purpose and will provide useful insights on the feasibility of this goal. Second, the main “preventive measure” that we studied was hemoglobin A1c testing. While our study adjusts for many medical and surgical aspects of care, we readily recognize that vascular, podiatric and wound care, lipid testing and statin therapy, blood pressure control, and the management of obesity is all other preventive measures that need optimal approaches to ensure the best results. Hemoglobin A1c testing may simply represent a proxy for access to these services, and future efforts to better understand the role of preventive measures in limiting amputation certainly are necessary. Third, secular changes in the manner by which comorbidities are reported may have changed in recent years and would influence risk adjustment over time. However, the manner in which these changes have occurred are not likely to differ by race and would seem unlikely to explain a 50% absolute decline in amputation risk. At last, the potential for data entry error in claims databases, such as the Medicare data set, is inherent in such an analysis, however, should be offset by the large number of patients studied.
CONCLUSIONS
In summary, amputation rates have fallen by more than 50% for patients with diabetes in recent years, and these improvements have occurred evenly among racial groups at the highest risk for limb loss. Furthermore, lower amputation risk was associated with consistent hemoglobin A1c monitoring over time for white, black, and Hispanic patients. Future efforts to limit amputation across diabetics of all race strata should consider quality metrics that incentivize longitudinal approaches in ensuring high-quality diabetic care.
Supplementary Material
Acknowledgments
P.P.G. was supported by funding from the National Heart, Lung and Blood Institute with a Career Development Award (1K08HL05676–01).
Footnotes
Presented as a poster at the Society for Vascular Surgery Annual Meeting, 2015 as well as a podium presentation during the Poster Group Winner Run-Off Session.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.avsg.2016.03.035.
References
- 1.Gregg EW, Williams DE, Geiss L. Changes in diabetes-related complications in the United States. N Engl J Med. 2014;371:286–7. doi: 10.1056/NEJMc1406009. [DOI] [PubMed] [Google Scholar]
- 2.Goodney PP, Tarulli M, Faerber AE, et al. Fifteen-year trends in lower limb amputation, revascularization, and preventive measures among Medicare patients. JAMA Surg. 2015;150:84–6. doi: 10.1001/jamasurg.2014.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodney PP, McClurg A, Spangler EL, et al. Preventive measures for patients at risk for amputation from diabetes and peripheral arterial disease. Diabetes Care. 2014;37:e139–40. doi: 10.2337/dc14-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.McKinlay J, Marceau L. US public health and the 21st century: diabetes mellitus. Lancet. 2000;356:757–61. doi: 10.1016/S0140-6736(00)02641-6. [DOI] [PubMed] [Google Scholar]
- 6.Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet. 2012;379:2252–61. doi: 10.1016/S0140-6736(12)60480-2. [DOI] [PubMed] [Google Scholar]
- 7.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–28. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 8.Epstein AJ, Polsky D, Yang F, et al. Coronary revascularization trends in the United States, 2001–2008. JAMA. 2011;305:1769–76. doi: 10.1001/jama.2011.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elam MB, Hunninghake DB, Davis KB, et al. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: A randomized trial. Arterial Disease Multiple Intervention Trial. JAMA. 2000;284:1263–70. doi: 10.1001/jama.284.10.1263. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg JB, Goodney PP, Cronenwett JL, et al. The effect of risk and race on lower extremity amputations among Medicare diabetic patients. J Vasc Surg. 2012;56:1663–8. doi: 10.1016/j.jvs.2012.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen LL, Hevelone N, Rogers SO, et al. Disparity in outcomes of surgical revascularization for limb salvage: race and gender are synergistic determinants of vein graft failure and limb loss. Circulation. 2009;119:123–30. doi: 10.1161/CIRCULATIONAHA.108.810341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones WS, Patel MR, Dai D, et al. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from U.S. Medicare 2000–2008. J Am Coll Cardiol. 2012;60:2230–6. doi: 10.1016/j.jacc.2012.08.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bierman AS, Lurie N, Collins KS, et al. Addressing racial and ethnic barriers to effective health care: the need for better data. Health Aff (Millwood) 2002;21:91–102. doi: 10.1377/hlthaff.21.3.91. [DOI] [PubMed] [Google Scholar]
- 14.Cronenwett JL, Birkmeyer JD. The Dartmouth Atlas of Vascular Health Care. Cardiovasc Surg. 2000;8:409–10. [PubMed] [Google Scholar]
- 15.The National Committe for Quality Assurance. 2012 Available from: http://www.ncqa.org.
- 16.Goodney PP, Travis LL, Nallamothu BK, et al. Variation in the use of lower extremity vascular procedures for critical limb ischemia. Circ Cardiovasc Qual Outcomes. 2012;5:94–102. doi: 10.1161/CIRCOUTCOMES.111.962233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodney PP, Holman K, Henke PK, et al. Regional intensity of vascular care and lower extremity amputation rates. J Vasc Surg. 2013;57:1471–9. 80 e1–3. doi: 10.1016/j.jvs.2012.11.068. discussion 9–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suckow BD, Goodney PP, Cambria RA, et al. Predicting functional status following amputation after lower extremity bypass. Ann Vasc Surg. 2012;26:67–78. doi: 10.1016/j.avsg.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Dartmouth Atlas of HealthCare. Dartmouth College Press.
- 20.Stevens CD, Schriger DL, Raffetto B, et al. Geographic clustering of diabetic lower-extremity amputations in low-income regions of California. Health Aff (Millwood) 2014;33:1383–90. doi: 10.1377/hlthaff.2014.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCall DT, Sauaia A, Hamman RF, et al. Are low-income elderly patients at risk for poor diabetes care? Diabetes Care. 2004;27:1060–5. doi: 10.2337/diacare.27.5.1060. [DOI] [PubMed] [Google Scholar]
- 22.Dansky KH, Dirani R. The use of health care services by people with diabetes in rural areas. J Rural Health. 1998;14:129–37. doi: 10.1111/j.1748-0361.1998.tb00614.x. [DOI] [PubMed] [Google Scholar]
- 23.Porterfield DS, Kinsinger L. Quality of care for uninsured patients with diabetes in a rural area. Diabetes Care. 2002;25:319–23. doi: 10.2337/diacare.25.2.319. [DOI] [PubMed] [Google Scholar]
- 24.Peek ME, Gorawara-Bhat R, Quinn MT, et al. Patient trust in physicians and shared decision-making among African-Americans with diabetes. Health Commun. 2013;28:616–23. doi: 10.1080/10410236.2012.710873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chin MH, Drum ML, Jin L, et al. Variation in treatment preferences and care goals among older patients with diabetes and their physicians. Med Care. 2008;46:275–86. doi: 10.1097/MLR.0b013e318158af40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jencks SF, Huff ED, Cuerdon T. Change in the quality of care delivered to Medicare beneficiaries, 1998–1999 to 2000–2001. JAMA. 2003;289:305–12. doi: 10.1001/jama.289.3.305. [DOI] [PubMed] [Google Scholar]
- 27.http://www.ncqa.org/PublicationsProducts/OtherProducts/QualityProfiles/FocusonDiabetes/ManagingDiabetesComplications.aspx. Managing Diabetes Complications: Recommendations from the National Committee on Quality Assurance (NCQA). http://www.ncqa.org/PublicationsProducts/OtherProducts/QualityProfiles/FocusonDiabetes/ManagingDiabetesComplications.aspx; Available from: http://www.ncqa.org/PublicationsProducts/OtherProducts/QualityProfiles/FocusonDiabetes/ManagingDiabetesComplications.aspx.
- 28.Tomek IM, Sabel AL, Froimson MI, et al. A collaborative of leading health systems finds wide variations in total knee replacement delivery and takes steps to improve value. Health Aff (Millwood) 2012;31:1329–38. doi: 10.1377/hlthaff.2011.0935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


