Abstract
Acquisition of drug-resistant phenotypes is often associated with chemotherapy in osteosarcoma. A number of studies have demonstrated a critical role for autophagy in osteosarcoma development, therapy and drug resistance. However, the molecular mechanisms underlying the autophagy-mediated chemotherapy resistance of osteosarcoma cells remain largely unknown. In the present study, we determined the autophagy and microRNA-140 (miR-140-5p, miRBase ID: MIMAT0000431) expression induced by chemotherapeutic drugs in osteosarcoma cells. Then we determined the promotory role of miR-140-5p to the chemotherapy-induced autophagy. Our results demonstrated that miR-140-5p expression was highly induced during chemotherapy of osteosarcoma cells, and this was accompanied by up-regulated autophagy. The increased miR-140-5p expression levels up-regulated anticancer drug-induced autophagy in osteosarcoma cells and ameliorated the anticancer drug-induced cell proliferation and viability decrease. Importantly, miR-140-5p regulates this context-specific autophagy through its target, inositol 1,4,5-trisphosphate kinase 2 (IP3k2). Therefore, the results of the present study demonstrated that miR-140-5p mediated drug-resistance in osteosarcoma cells by inducing autophagy. The present study provides evidence of miRNA regulation of autophagy through modulation of IP3 signalling. The present study recognized a novel mechanism of chemoresistance in osteosarcoma cancers.
Keywords: autophagy, drug resistance, miRNA-140, osteosarcoma cells, 1, 4, 5-trisphosphate (IP3) signalling
INTRODUCTION
Osteosarcoma is the eighth most common type of cancer found in children and adolescents, accounting for 2.4% of all malignancies in paediatric patients and ∼20% of all primary bone cancers [1]. Chemotherapy is the first choice treatment for osteosarcoma, with multiple anticancer drugs, including doxorubicin (Dox), cisplatin (Cis) and high-dose methotrexate [2,3]. In the last three decades, neoadjuvant chemotherapy has increased the long-term survival rate of osteosarcoma patients from <20 to ∼80% [4–6]. However, patients that are less responsive to these drugs have a poor prognosis. In addition, the frequent acquisition of drug resistance and the occurrence of ‘secondary malignancies’ are often associated with chemotherapy and are significant obstacles to achieving favourable outcomes. Thus, it is important to identify the molecular mechanisms underlying the drug resistance of osteosarcoma cancer cells.
Autophagy is a universal process whereby cellular components and damaged organelles are sequestered within autophagosomes for lysosomal degradation. Autophagy has proven to be an essential pathway for cellular homoeostasis. In addition to removing dysfunctional proteins and organelles, autophagy provides amino acids, monosaccharides, nucleic acids and lipids during times of nutrient deprivation [7,8]. Autophagy is a key pathway for cell survival but, if protein loss becomes excessive, cell death will result. This degradative pathway has been implicated in the progression of a number of diseased states including cancer. Suppressed autophagy can result in net protein gain and neoplastic growth, and defects in autophagy have been implicated in poor outcomes for hepatocellular carcinoma [9]. To the contrary, autophagy promotes cell survival in tumours undergoing nutrient deprivation or chemotherapy. The overproduction of the autophagy protein, LC3B (microtubule-associated protein 1 light chain 3B), is associated with tumour growth and poor prognosis in aggressive pancreatic, colorectal and breast carcinoma [10–12].
During tumour development, autophagy is enhanced to promote cell survival under ischaemic conditions [13–15]. Autophagy can also enhance cell survival by removing organelles damaged by chemotherapy agents [16,13,17]. Resistance of osteosarcoma cell lines to Dox, Cis and methotrexate has been shown to be due to the induction of autophagy by the DNA-binding protein HMGB1 (high mobility group box 1) [18]. On the other hand, autophagy is one of three primary venues of cell death, which also includes apoptosis and necrosis. Many existing chemotherapy drugs act by inducing apoptosis whereas others promote autophagy-mediated cell death of neoplastic cells [19,20]. Given that autophagy can promote cell survival or cell death, its regulation is critical for the developing tumour.
There are two primary regulatory pathways of autophagy: MTOR (mechanistic target of rapamycin), a negative regulator, and PtdIns3K (class III phosphatidylinositol 3-kinase), a positive regulator. MTOR inhibits the ULK1/2 (mammalian orthologues of yeast Atg1) complex, which activates autophagy by stimulating PtdIns3K activity [21]. The MTOR inhibitor, rapamycin, induces autophagy-mediated cell death in glioma cells [22]. PtdIns3K synthesizes phosphatidylinositol 3-phosphate, which provides a docking site for ATG proteins at the sequestering membranes of the forming autophagosome [23,24]. Chemoresistance is attenuated in hepatocarcinoma cells when treated with the PtdIns3K antagonist, 3MA (3-methyladenine) [25]. Both pathways modulate the lipidation of LC3B by presumably regulating the activities of ATG4, ATG7 or ATG3. Of the four autophagins (ATG4A, ATG4B, ATG4C and ATG4D) identified, Yin and co-workers have shown that ATG4B had the highest catalytic efficiency for cleaving the C terminus of LC3B [26]. Once the C-terminal glycine of LC3B is exposed by ATG4B, ATG7 in an ATP-dependent manner activates LC3B for delivery to ATG3, which conjugates LC3B to phosphatidylethanolamine. The lipidation of LC3B anchors this protein to the forming autophagosome where it promotes membrane expansion to enlarge the autophagosome thus increasing the amplitude of autophagy [27]. The lipidated LC3B is either degraded within the autolysosome or cleaved by ATG4B and the LC3B recycled. ATG4B provides the cell with enough LC3B to amplify autophagy and recycles the lipidated LC3B to sustain autophagy [28].
MicroRNAs (miRNAs) are family of endogenous non-coding RNA molecules that comprise 22 nucleotides, which regulate gene expression [29] in organisms ranging between nematodes and humans and in a broad array of mammalian cell processes [30]. Recently, miRNAs have been associated with cell chemosensitivity or chemotherapy resistance in a variety of cancer cell types [31], including osteosarcoma [32]. miR-140 was reported to be involved in the chemoresistance of osteosarcoma cells via the suppression of histone deacetylase [4], which in turn reduced cell proliferation [32]. Furthermore, an increasing number of studies have demonstrated that miRNA molecules regulate cellular autophagy processes [33–35]. Zhu et al. [34] reported that miR-30a targets beclin 1, resulting in decreased autophagic activity. In addition, Brest et al. [35] showed that a miR-196-based alteration in the expression of immunity-related GTPase family M protein can affect the efficacy of autophagy. However, the role of miRNAs in autophagy-mediated chemotherapy resistance in osteosarcoma remains unknown.
In the present study, we determined the targeting role of miR-140-5p (miRBase ID: MIMAT0000431) to inositol 1,4,5-trisphosphate kinase 2 (IP3K2), the regulation of miR-140-5p on the IP3K2-mediated cell autophagy during chemotherapy, and the suppression of miR-140-5p inhibitor in the cell proliferation of osteosarcoma cells. Thus, we identified the tumour suppressive role of miR-140-5p inhibitor in osteosarcoma cells in vitro.
MATERIALS AND METHODS
Cell culture and reagents
Human osteosarcoma cell lines (Saos-2 and MG-63) were obtained from the Cell Resource Center of the Chinese Academy of Medical Sciences. The cells were cultured in Eagle's Minimum Essential Medium (Invitrogen) or McCoy's 5A Modified Medium (Invitrogen) supplemented with 10% FBS (GIBCO), and were incubated at 37°C with 5% CO2. Antibodies against GAPDH, LC3-II and p62 were obtained from Santa Cruz Biotechnology and rapamycin was purchased from Sigma–Aldrich. The coding sequence of microtubule-associated protein 1-LC3 fusion with GFP was synthesized and cloned into pcDNA3.1(+) to construct the LC3-GFP-expressing plasmid.
Cell transfection
miR-140-5p mimic, miR-140-5p inhibitor and the corresponding control oligonucleotides (purchased from RiboBio) were transfected into cells as described previously [36]. The sequence of miR-140-5p mimics was 5′-UGAGAACUGAAUUCCAUGGGUU-3′, and miR-control was 5′-UUC UCC GAA CGU GUC ACG UTT-3′. The sequence of miR-140-5p inhibitor was 5′-AA CCC AUG GAA UUC AGU UCU CA-3′, and miR-NC was 5′-UCU ACU CUU UCU AGG AGG UUG UGA-3′. siRNAs targeting IP3K2 were obtained from RiboBio and sequences were 5′-GCU AUC AAC UGC AGA GAU U-3′. The IP3K2 siRNA and control siRNA transfections were conducted as recommended by the manufacturer.
Quantitative GFP-LC3 light microscopy autophagy assays were performed in Saos-2 cells with various treatments. Cells were grown to 80% confluency and were transfected with a GFP-LC3-expressing plasmid using Lipofectamine 2000 (Invitrogen Life Technologies). At 24 h following transfection, the cells were subjected to 0.2 μg/ml Dox (Sigma–Aldrich) or 20 μM Cis (Sigma–Aldrich) for an additional 24 h. In a separate experiment, cells were simultaneously and additionally transfected with 20 nM miR-140-5p and analysed with fluorescence microscopy. The number of punctate GFP-LC3 dots in each cell was counted and at least 100 cells were included for each group.
miRNA extraction and quantitative PCR
Total miRNA extraction was performed using a mirVana miRNA Isolation kit (Ambion). Quantification of miR-140-5p expression was conducted using the mirVana qRT-PCR miRNA Detection kit (Ambion), where U6 small nuclear RNA was used as an internal control, according to the protocol previously described [37]. The specific primer of miR-140-5p was: GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACC TAC CAT.
For mRNA detection, total RNA was extracted using TRIzol reagent (Life Technologies), according to the manufacture's instruction. The mRNA expression was determined by using the standard SYBR-Green RT-PCR kit (Takara), in accordance with the manufacturer's instructions. The specific primers were as follows: IP3K2, 5′-TTA CTC AAG GAC GCG GTC TGT GAT C-3′ (forward) and 5′-ATT GGC CCC AGC TTG CTT-3′ (reverse). GAPDH was used as an internal control with primers: 5′-AGC CTT CTC CAT GGT GGT GAA-3′ (forward) and 5′-ATC ACC ATC TTC CAG GAG CGA-3′ (reverse).
Western blot analysis
Cell extracts were prepared according to the standard protocol, and protein expression levels were detected by western blot analysis using polyclonal (rabbit) anti-LC3-II, anti-p62 or anti-GAPDH antibodies. Goat anti-mouse IgG or goat anti-rabbit IgG (Pierce Biotechnology) secondary antibodies, that were conjugated to horseradish peroxidase, were used for detection via an enhanced chemiluminescence detection system (Super Signal West Femto, Pierce Biotechnology).
Cell proliferation assay
Cell viability was expressed as the relative percentage of viable cells to control human umbilical vein endothelial cells. For the proliferation assay, following transfection with miR-140-5p mimics or miRNA control, cells were incubated with Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies). The absorbance of each well at 450 nm was detected following visual colour occurrence at 24, 48 or 72 h. Independent experiments were performed in triplicate.
Ca2+ measurements
Fura-2 fluorescence was utilized to determine intracellular Ca2+ concentrations [38]. Cells were loaded with Fura-2/AM (2 μM, Invitrogen) for 20 min at 37°C. Cells were excited alternatively at 340 and 380 nm through an objective (Fluor 40×/1.30 oil) built in an inverted phase-contrast microscope (Axiovert100, Zeiss). Emitted fluorescence intensity was recorded at 505 nm. Data were acquired using specialized computer software (Metafluor, Universal Imaging). Cytosolic Ca2+ activity was estimated from the 340 nm/380 nm ratio. Store-operated Ca2+ entry (SOCE) was determined by extracellular Ca2+ removal and subsequent Ca2+ readdition in the presence of thapsigargin (1 μM, Invitrogen). For quantification of Ca2+ entry, the slope (delta ratio/s) and peak (delta ratio) were calculated following readdition of Ca2+. Experiments were performed with Ringer solution containing: 125 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 2 mM CaCl2, 2 mM Na2HPO4, 32 mM HEPES, 5 mM glucose, pH 7.4. To reach nominally Ca2+-free conditions, experiments were performed using Ca2+-free Ringer solution containing: 125 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 2 mM Na2HPO4, 32 mM HEPES, 0.5 mM EGTA, 5 mM glucose, pH 7.4.
Statistical analysis
For GFP-LC3 dot number analysis, relative miR-140-5p expression, conversion of LC3-I to LC3-II, relative expression of p62 against GAPDH and CCK-8 measurements, the statistical evaluations are presented as the mean ± S.E. Data were analysed using the Student's t test. All data were analysed by the SPSS v16.0 (SPSS). P<0.05 was considered to indicate a statistically significant result.
RESULTS
miR-140-5p expression increases in osteosarcoma cells following treatment with chemotherapy agents
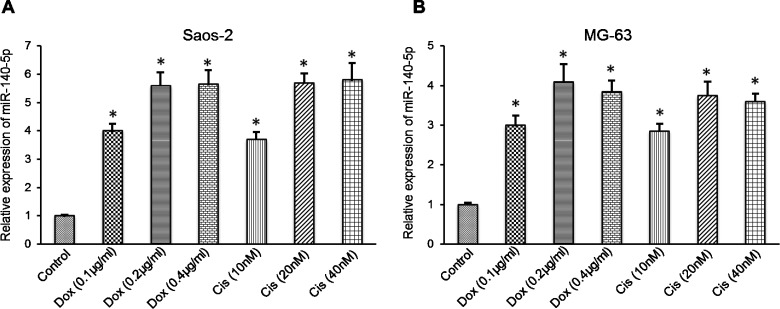
The role of miRNAs in chemotherapy-induced autophagy of cancer cells remains unknown. To screen possible miRNAs that may be important for anticancer drug-induced autophagy in osteosarcoma cells, miRNA expression levels were analysed by microarray in osteosarcoma cells following treatment with Dox. miR-140-5p was demonstrated to be the most highly-expressed miRNA. Thus, the expression level of miR-140-5p was quantified in Saos-2 and MG-63 cells following treatment with Dox or Cis. The results indicated that treatment with 0.2 μg/ml Dox or 20 μM Cis significantly up-regulated the miR-140-5p expression levels in the two cell lines. A quantitative PCR (qPCR) assay demonstrated that significantly higher expression levels of miR-140-5p were induced in Saos-2 or MG-63 cells following Dox or Cis treatment (Figures 1A and 1B). Therefore, miR-140-5p expression is induced in vitro during anticancer drug therapy in osteosarcoma cells.
Figure 1. miR-140-5p expression was up-regulated in osteosarcoma cells following treatment with chemotherapeutic drugs.
qPCR analysis showing the relative miR-140-5p expression to U6 in (A) Saos-2 and (B) MG-63 cells. All the experiments were performed in triplicate. *P<0.05 compared with control.
Inhibition of miR-140-5p promotes the anticancer drug-induced cell proliferation decrease
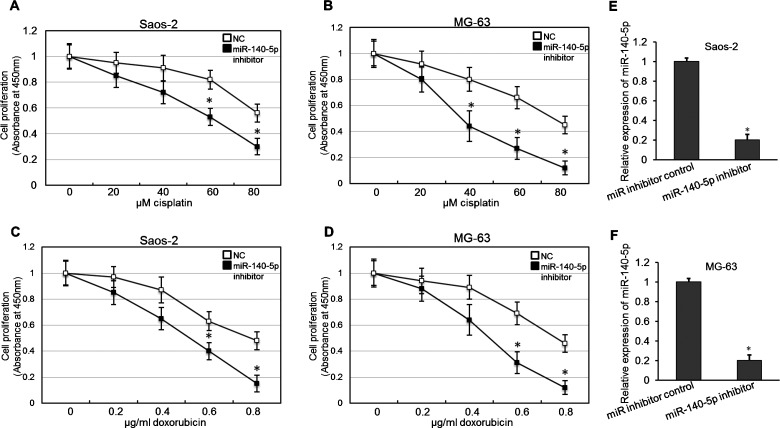
To determine the possible effect of miR-140-5p on osteosarcoma cell proliferation, the proliferation of Saos-2 or MG-63 cells that had been treated with Dox or Cis and transfected with miR-140-5p inhibitors was determined using a CCK-8 assay. As shown in Figures 2(A) and 2(B), transfection with miR-140-5p inhibitor gave rise to a marked increase in sensitivity after treatment with 60 and 80 μM Cis in the Saos-2 and MG-63 cell lines. As shown in Figures 2(C) and 2(D), miR-140-5p in transfection resulted in a dose-dependent amelioration of 0.6 and 0.8 μg/ml Dox-induced cell proliferation inhibition in the Saos-2 and MG-63 cell lines. The effect of inhibiting was determined by real-time PCR shown in Figures 2(E) and 2(F). Thus, inhibition of miR-140-5p ameliorated the anticancer drug-induced cell proliferation decrease in osteosarcoma cells.
Figure 2. Inhibition of miR-140-5p ameliorated the anticancer drug-induced cell proliferation decrease in vitro.
(A and B) Osteosarcoma cells were transfected with miR-140-5p inhibitor or a scrambled control. Forty-eight hours after transfection, Cis was added and after 24 h cell viability was determined with a CCK-8 assay. Independent experiments were performed in triplicate. *P<0.05 compared with control. (C and D) Growth curves showing the cell proliferation following Dox treatment and miR-140-5p inhibitor or miR-control transfection in osteosarcoma cells. *P<0.05 compared with control. (E and F) When transfected with miR-140-5p inhibitor or miR inhibitor control, real-time PCR was used to validate the changes of miR-140-5p expression in Soas-2 and MG-63 cells. *P<0.05.
Overexpression of miR-140-5p up-regulates anticancer drug-induced autophagy in osteosarcoma cells
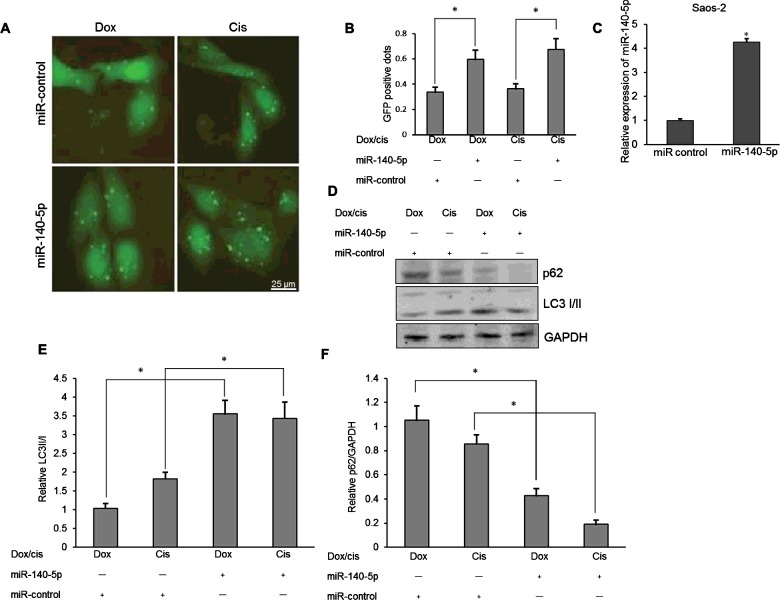
To determine the possible contribution of miR-140-5p to autophagy in drug-treated osteosarcoma cells, miR-140-5p expression was manipulated in Saos-2 cells via transfection with miR-140-5p mimics or miRNA control. The level of autophagy was determined in Saos-2 cells following miR-140-5p mimics transfection. As shown in Figures 3(A) and 3(B), there were more GFP-positive dots (LC3 punctas) in the Saos-2 cells that had been transfected with miR-140-5p mimics when compared with transfection with miRNA control (P<0.05). The effect of miR-140-5p overexpression was determined by real-time PCR shown in Figure 3(C). In addition, significantly higher conversion levels of LC3-I to LC3-II and decreased expression levels of p62 were also confirmed in the osteosarcoma cells transfected with miR-140-5p mimics (P<0.05 respectively; Figures 3D–3F). These results confirm that overexpression of miR-140 contributes to anticancer drug-induced autophagy in osteosarcoma cells.
Figure 3. Overexpression of miR-140-5p up-regulated anticancer drug-induced autophagy in osteosarcoma cells.
(A) LC3 punctas under fluorescence microscopy in Saos-2 cells following 0.1 μg/ml Dox or 10 μM Cis treatment and miR-140-5p mimics or miR-control transfection. (B) Quantitative analysis of the punctate GFP-LC3 dots. (C) Real-time PCR was used to validate the changes of miR-140-5p expression after miR-140-5p mimics transfection. *P<0.05. (D) Western blotting of LC3-I/II and p62 in miR-140-5p mimics or miR-control-transfected Saos-2 cells. (E) Relative expression of LC3-II to LC3-I in miR-140-5p mimics or miR-control-transfected Saos-2 cells. (F) Relative expression of p62 to GAPDH in miR-140-5p mimics or miR-control-transfected Saos-2 cells. Independent experiments were performed in triplicate. *P<0.05 compared with control.
miR-140-5p targets the IP3k2 protein to regulate autophagy during osteosarcoma cell death
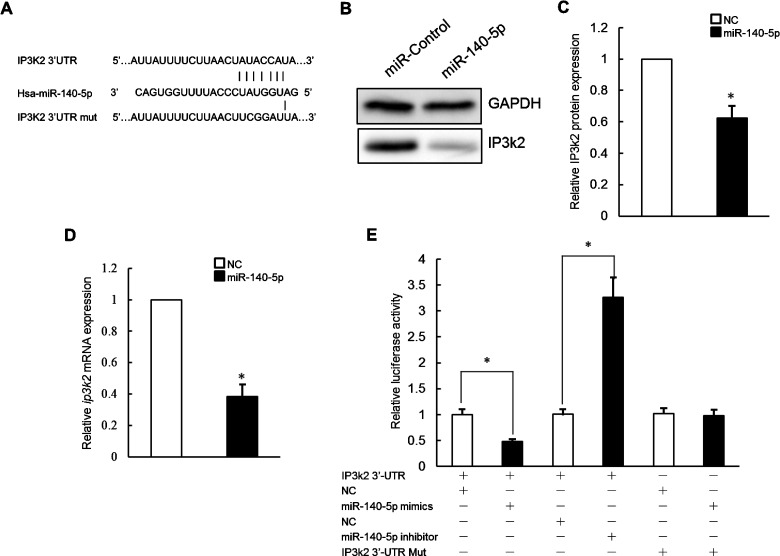
Our next goal was to identify targets of miR-140-5p that influence autophagy. We used the miRNA binding site prediction programmes Pictar and Targetscan to identify candidate miR-140-5p target genes. Of the 31 genes, IP3k2 is involved in autophagy regulation. To investigate if ip3k2 3′-UTR sequences can mediate regulation by miR-140-5p, we examined whether miR-140-5p directly reduces endogenous IP3k2 levels. Saos-2 cells were transfected with either miR-control or miR-140-5p mimics. IP3k2 protein levels were then directly assayed by western blot. Forty-eight hours after transfection, IP3k2 protein levels were decreased significantly following transfection of miR-140-5p mimics as compared with negative control (Figures 4B and 4C). In a separate experiment, ip3k2 mRNA levels were directly assayed by real time PCR after miR-140-5p mimics transfection. Forty-eight hours after transfection, ip3k2 mRNA levels were decreased significantly following transfection (Figure 4D). Taken together, these results suggested that exogenous miR-140-5p likely inhibits IP3k2 expression via mRNA destabilization.
Figure 4. miR-140-5p inhibited IP3k2 expression in osteosarcoma cells.
(A) The binding sites and the corresponding mutated sequences within the ip3k2 3′-UTR for miR-140-5p were presented. (B and C) Immunoblot analyses of the effect of transient transfection of miR-140-5p mimics on IP3k2 protein levels in Saos-2 cells. (D) Quantitative RT-PCR analyses of the effect of transient transfection of miR-140-5p mimics on ip3k2 mRNA levels in Saos-2 cells. (E) The effects of miR-140-5p mimics or miR-140-5p inhibitor on the activity of ip3k2 3′-UTR or ip3k2 3′-UTR-Mut in transiently co-transfected Saos-2 cells. The reporter activities were determined at 48 h after transfection. Data are presented as the means ± S.E.M. from n=3 replicates. *P<0.05.
miR-140-5p inhibits IP3k2 expression via predicted 3′-UTR target sites
To validate the functionality of the putative miR-140-5p/ip3k2 3′-UTR interaction, a reporter construct was prepared containing the full-length ip3k2 3′-UTR. This reporter was generated by PCR-amplifying the ip3k2 3′-UTR from human genomic DNA and inserting the amplicon downstream of a Renilla luciferase CDS. A separate firefly luciferase CDS under independent transcriptional control was also present in this construct to serve as an internal control. Co-transfection of the reporter construct along with miR-140-5p mimics in Saos-2 cells resulted in significantly reduced Renilla activity relative to co-transfection with negative control mimic or transfection of reporter construct alone (53% of negative control mimics) suggesting an inhibitory regulatory interaction between miR-140-5p and the ip3k2 3′-UTR.
To confirm that the inhibitory effect of miR-140-5p on ip3k2 3′-UTR reporter expression was mediated specifically via predicted miR-140-5p target sites located in the ip3k2 3′-UTR, mutations were introduced in the seed sequences of both target sites in the reporter construct (Figure 4A). Perfect complementarity at the seed sequence is critical for functional miRNA interactions, and mutation at this position should eliminate effective interaction between miRNA and target site. These mutant reporter constructs were then co-transfected along with miR-140-5p mimic into Saos-2 cells and reporter expression compared with wild-type reporter (Figure 4E). Mutation of target site partially eliminated the inhibitory effect of miR-140-5p mimics on reporter expression (Figure 4E). Therefore, miR-140-5p mediates its inhibitory effect on ip3k2 3′-UTR reporter expression by interacting with at least one of predicted target sites in the ip3k2 3′-UTR.
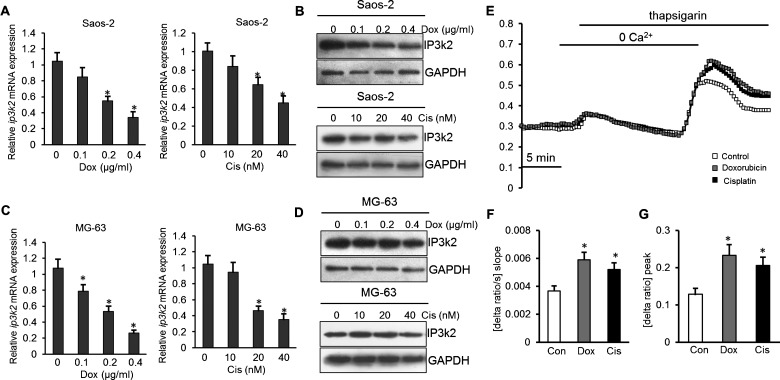
Next, we tested whether the predicted target of miR-140-5p was down-regulated in response to drug treatment. The result of real-time PCR showed that the expression level of ip3k2 mRNA was significantly lower after Dox and Cis treatment in Soas-2 and MG-63 cells (P<0.05, Figures 5A and 5C). And Western blot analysis revealed that IP3K2 protein levels were significantly lower in the drug treatment group than those control cells (Figures 5B and 5D). Furthermore, Fura-2 fluorescence was employed in order to test whether the differences in IP3K2 protein abundance were paralleled by corresponding differences in SOCE. As illustrated in Figure 5, both peak and slope of SOCE were significantly higher after Dox (0.2 μg/ml) and Cis (20 nM) pretreatment than in control cells. The Dox pretreatment increased the peak Ca2+ increase from 0.13±0.016 arbitrary units (n=6) to 0.23±0.028 arbitrary units (n=5) in Saos-2 cells. The Cis pretreatment increased the peak Ca2+ increase from 0.13±0.016 arbitrary units (n=6) to 0.21±0.023 arbitrary units (n=5) (Figures 5E–5G). These results showed that the decreased expression of IP3K2 was paralleled by corresponding differences in SOCE in response to drug treatment.
Figure 5. IP3K2 expression and Ca2+ entry were up-regulated in osteosarcoma cells following treatment with chemotherapeutic drugs.
(A and B) Analyses of IP3K2 mRNA and protein levels in Soas-2 cells after drug treatment. (C and D) Analyses of IP3K2 mRNA and protein levels in MG-63 cells after drug treatment. (E) Representative tracings of Fura-2 fluorescence ratio in fluorescence spectrometry during and after Ca2+ depletion with subsequent thapsigargin (1 μM) addition in Soas-2 cells without (white squares) and with presence of Dox (0.2 μg/ml, black squares) or Cis (20 nM, grey squares). (F and G) Arithmetic means (±S.E.M., n=5–6, each experiment 10–30 cells) of slope (C) and peak (D) increase in Fura-2 fluorescence ratio following Ca2+ readdition in the absence (white bars) and presence of drug (black or grey bars). * (P<0.05) indicate statistically significant difference from control.
Inhibiting IP3k2 protein expression promotes autophagy in response to anticancer drug treatment
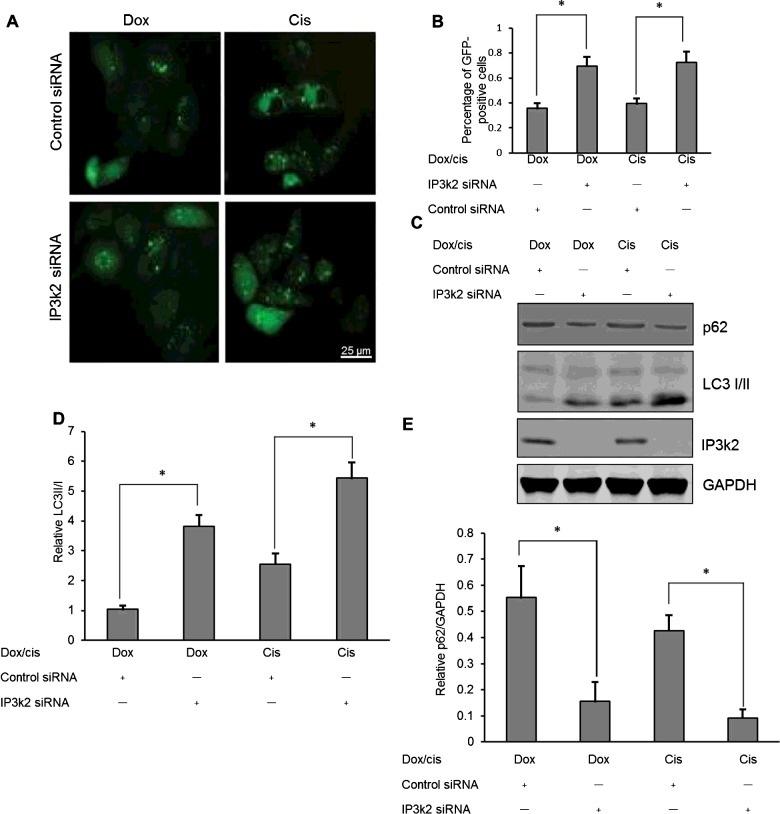
To determine the possible contribution of IP3k2 protein to autophagy in drug-treated osteosarcoma cells, IP3k2 protein expression was inhibited in Saos-2 cells via transfection with IP3k2 siRNA or control siRNA. The effect of inhibiting was determined by western blot shown in Figure 6(C). The level of autophagy was determined in Saos-2 cells following IP3k2 siRNA transfection. There were more GFP-positive dots (LC3 punctas) in the Saos-2 cells that had been transfected with IP3k2 siRNA when compared with transfection with control siRNA (Figures 6A and 6B, P<0.05). In addition, significantly higher conversion levels of LC3-I to LC3-II and decreased expression levels of p62 were also confirmed in the osteosarcoma cells transfected with IP3k2 siRNA (P<0.05, Figures 6C–6E). These results confirm that overexpression of IP3k2 contributes to anticancer drug-induced autophagy in osteosarcoma cells.
Figure 6. IP3k2 regulates autophagy in anticancer drug treatment.
(A) LC3 punctas under fluorescence microscopy in Saos-2 cells following 0.1 μg/ml Dox or 10 μM Cis treatment and IP3k2 siRNA or control siRNA transfection. (B) Quantitative analysis of the punctate GFP-LC3 dots. (C) Western blotting of LC3-I/II and p62 in IP3k2 siRNA or control siRNA-transfected Saos-2 cells. (D) Relative expression of LC3-II to LC3-I in IP3k2 siRNA or control siRNA-transfected Saos-2 cells. (E) Relative expression of p62 to GAPDH in IP3k2 siRNA or control siRNA-transfected Saos-2 cells. Independent experiments were performed in triplicate. *P<0.05 compared with control.
DISCUSSION
The molecular mechanism of the sensitivity or resistance of cancers to chemotherapy is complex, involving multiple processes such as drug transport, drug metabolism, DNA repair, apoptosis and autophagy. Traditionally, DNA, mRNA and proteins have been most focused on as the targets and modulators of therapy. Therefore, mutations, copy number changes and epigenetic variables at the DNA level and expression changes at the mRNA and protein levels have been widely studied to probe mechanisms that determine the pharmacologic response [39–41]. Up-regulated autophagy has been found in various cancer cells facing therapeutic stress and contributes to the chemotherapy resistance [42,43]. Autophagy blocking in cancer cells is emerging as a novel approach to enhance the sensitivity of chemotherapy in cancers [44,45].
Tight control of autophagy is essential for normal or tumour cells to survive, and recent advances in this field have begun to unveil the molecular mechanisms underlying autophagy regulation [46]. A connection between inositol-1,4,5-triphosphate (IP3) and autophagosome formation has been also proposed [47,48]. IP3 is fundamental for Ca2+ homoeostasis since coupling its receptor (IP3R) functions as an actual gateway for every Ca2+ pulse originated from the ER [49]. Increases in [Ca2+]c mediate autophagy in mammalian cells [50] and compounds acting via this pathway (e.g. vitamin D, ATP and ionomycin) are able to promote it quite efficiently. Ca2+-mediated autophagy seems to principally occur via Ca2+/calmodulin-dependent kinase-β (CaMKKβ)-dependent activation of AMPK thus leading to an efficient inhibition of mTORC1. Increases in the [Ca2+]c can also activate death associated protein kinases and calpain proteases (both Ca2+-dependent enzymes) that have been linked to the regulation of the autophagic process [51]. In this study, we found that inhibiting IP3k2 up-regulates anticancer drug-induced but not basal level of autophagy, to promote tumour cell survival in exposure to anticancer drugs in osteosarcoma cancers. To the best of our knowledge, this is the first time to implicate the IP3-kinase is involved in control of autophagy in osteosarcoma cells.
Notably, miRNAs can regulate a multitude of targets and biological networks in autophagy [52,53]. A previous study indicated clear roles of miRNAs in autophagy induction, autophagic vesicle nucleation, autophagic vesicle elongation and vesicle fusion to lysosomes [52]. The present study confirmed that during treatment with Dox or Cis in osteosarcoma cells, miR-140-5p expression was strongly induced. The increased miR-140-5p expression facilitated tumour cell proliferation via up-regulating autophagy, thus, facilitated the resistance of osteosarcoma cells to Dox or Cis. In conclusion, the present study has demonstrated that anticancer drug treatment up-regulates miR-140-5p expression in osteosarcoma cells. Overexpression of miR-140-5p induces the activation of autophagy, which promotes tumour cell survival and chemoresistance. These observations reveal a novel role for miR-140-5p in chemotherapy resistance during the treatment of osteosarcoma.
The role of autophagy in the tumour cell's sensitivity or resistance to chemotherapy is complex. In osteosarcoma, as shown in other tumours, autophagy plays a dual role either by promoting cell survival and tumour cell resistance to chemotherapy or by acting as one of the mechanisms responsible for chemotherapy-induced cell death. Better understanding of the molecular pathways that govern the process of autophagy will allow identification of a mode to modulate these pathways in order to enhance the activity of chemotherapy. The present study has demonstrated that anticancer drug treatment up-regulates miR-140-5p expression in osteosarcoma cells. Overexpression of miR-140-5p induces the activation of autophagy, which promotes tumour cell survival and chemoresistance. These observations reveal a novel role for miR-140-5p in chemotherapy resistance during the treatment of osteosarcoma.
In summary, our study shows that miR-140-5p targeted IP3k2 and inhibited the IP3k2-mediated autophagy in osteosarcoma cells during the chemotherapy, and sensitized the osteosarcoma cells to anticancer drugs by inhibiting cell proliferation. These findings identified the novel tumour stimulative role of miR-140-5p in IP3k2-mediated autophagic chemotherapy resistance during the treatment of osteosarcoma.
Abbreviations
- CCK-8
Cell Counting Kit-8
- Cis
cisplatin
- Dox
doxorubicin
- IP3k2
inositol 1,4,5-trisphosphate kinase 2
- MTOR
mechanistic target of rapamycin
- PtdIns3K
class III phosphatidylinositol 3-kinase
- qPCR
quantitative PCR
- SOCE
store-operated Ca2+ entry
AUTHOR CONTRIBUTION
Renxiong Wei, acquisition of data and drafting of the manuscript; Gang Cao, Zhouming Deng and Jiajia Su, acquisition and analysis of data; Lin Cai, study concept and design, critical revision of the manuscript for important intellectual content and study supervision.
FUNDING
This work is supported by the Key Project of Natural Science Foundation of Hubei Province [grant number 2014CFA063].
References
- 1.Ottaviani G., Jaffe N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9. [DOI] [PubMed] [Google Scholar]
- 2.Wittig J.C., Bickels J., Priebat D., Jelinek J., Kellar-Graney K., Shmookler B., Malawer M.M. Osteosarcoma: a multidisciplinary approach to diagnosis and treatment. Am. Fam. Physician. 2002;65:1123–1132. [PubMed] [Google Scholar]
- 3.Rosen G., Caparros B., Groshen S., Nirenberg A., Cacavio A., Marcove R.C., Lane J.M., Huvos A.G. Primary osteogenic sarcoma of the femur: a model for the use of preoperative chemotherapy in high risk malignant tumors. Cancer Invest. 1984;2:181–192. doi: 10.3109/07357908409104370. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z.J., Chee C.E., Huang S., Sinicrope F.A. The role of autophagy in cancer: therapeutic implications. Mol. Cancer Ther. 2011;10:1533–1541. doi: 10.1158/1535-7163.MCT-11-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Provisor A.J., Ettinger L.J., Nachman J.B., Krailo M.D., Makley J.T., Yunis E.J., Huvos A.G., Betcher D.L., Baum E.S., Kisker C.T., Miser J.S. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children's Cancer Group. J. Clin. Oncol. 1997;15:76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- 6.Goorin A.M., Schwartzentruber D.J., Devidas M., Gebhardt M.C., Ayala A.G., Harris M.B., Helman L.J., Grier H.E., Link M.P. Pediatric Oncology Group. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J. Clin. Oncol. 2003;21:1574–1580. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- 7.Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M., Tanaka K., Cuervo A.M., Czaja M.J. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortimore G.E., Poso A.R., Lardeux B.R. Mechanism and regulation of protein degradation in liver. Diabetes Metab. Rev. 1989;5:49–70. doi: 10.1002/dmr.5610050105. [DOI] [PubMed] [Google Scholar]
- 9.Ding Z.B., Shi Y.H., Zhou J., Qiu S.J., Xu Y., Dai Z., Shi G.M., Wang X.Y., Ke A.W., Wu B., Fan J. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res. 2008;68:9167–9175. doi: 10.1158/0008-5472.CAN-08-1573. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H., Yang M., Zhao J., Wang J., Zhang Y., Zhang Q. High expression of LC3B is associated with progression and poor outcome in triple-negative breast cancer. Med. Oncol. 2013;30:475. doi: 10.1007/s12032-013-0475-1. [DOI] [PubMed] [Google Scholar]
- 11.Yoshioka A., Miyata H., Doki Y., Yamasaki M., Sohma I., Gotoh K., Takiguchi S., Fujiwara Y., Uchiyama Y., Monden M. LC3, an autophagosome marker, is highly expressed in gastrointestinal cancers. Int. J. Oncol. 2008;33:461–468. [PubMed] [Google Scholar]
- 12.Fujii S., Mitsunaga S., Yamazaki M., Hasebe T., Ishii G., Kojima M., Kinoshita T., Ueno T., Esumi H., Ochiai A. Autophagy is activated in pancreatic cancer cells and correlates with poor patient outcome. Cancer Sci. 2008;99:1813–1819. doi: 10.1111/j.1349-7006.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathew R., Kongara S., Beaudoin B., Karp C.M., Bray K., Degenhardt K., Chen G., Jin S., White E. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21:1367–1381. doi: 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karantza-Wadsworth V., Patel S., Kravchuk O., Chen G., Mathew R., Jin S., White E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degenhardt K., Mathew R., Beaudoin B., Bray K., Anderson D., Chen G., Mukherjee C., Shi Y., Gélinas C., Fan Y., et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meijer A.J., Codogno P. Signalling and autophagy regulation in health, aging and disease. Mol. Aspects Med. 2006;27:411–425. doi: 10.1016/j.mam.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Amaravadi R. Autophagy can contribute to cell death when combining targeted therapy. Cancer Biol. Ther. 2009;8:130–133. doi: 10.4161/cbt.8.21.10416. [DOI] [PubMed] [Google Scholar]
- 18.Huang J., Ni J., Liu K., Yu Y., Xie M., Kang R., Vernon P., Cao L., Tang D. HMGB1 promotes drug resistance in osteosarcoma. Cancer Res. 2012;72:230–238. doi: 10.1158/0008-5472.CAN-11-2001. [DOI] [PubMed] [Google Scholar]
- 19.Kung C.P., Budina A., Balaburski G., Bergenstock M.K., Murphy M. Autophagy in tumor suppression and cancer therapy. Crit. Rev. Eukaryot. Gene Expr. 2011;21:71–100. doi: 10.1615/CritRevEukarGeneExpr.v21.i1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoyer-Hansen M., Jaattela M. Autophagy: an emerging target for cancer therapy. Autophagy. 2008;4:574–580. doi: 10.4161/auto.5921. [DOI] [PubMed] [Google Scholar]
- 21.Alers S., Loffler A.S., Wesselborg S., Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol. Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwamaru A., Kondo Y., Iwado E., Aoki H., Fujiwara K., Yokoyama T., Mills G.B., Kondo S. Silencing mammalian target of rapamycin signaling by small interfering RNA enhances rapamycin-induced autophagy in malignant glioma cells. Oncogene. 2007;26:1840–1851. doi: 10.1038/sj.onc.1209992. [DOI] [PubMed] [Google Scholar]
- 23.Proikas-Cezanne T., Waddell S., Gaugel A., Frickey T., Lupas A., Nordheim A. WIPI-1alpha (WIPI49), a member of the novel 7-bladed WIPI protein family, is aberrantly expressed in human cancer and is linked to starvation-induced autophagy. Oncogene. 2004;23:9314–9325. doi: 10.1038/sj.onc.1208331. [DOI] [PubMed] [Google Scholar]
- 24.Jeffries T.R., Dove S.K., Michell R.H., Parker P.J. PtdIns-specific MPR pathway association of a novel WD40 repeat protein, WIPI49. Mol. Biol. Cell. 2004;15:2652–2663. doi: 10.1091/mbc.E03-10-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song J., Qu Z., Guo X., Zhao Q., Zhao X., Gao L., Sun K., Shen F., Wu M., Wei L. Hypoxia-induced autophagy contributes to the chemoresistance of hepatocellular carcinoma cells. Autophagy. 2009;5:1131–1144. doi: 10.4161/auto.5.8.9996. [DOI] [PubMed] [Google Scholar]
- 26.Li M., Hou Y., Wang J., Chen X., Shao Z.M., Yin X.M. Kinetics comparisons of mammalian Atg4 homologues indicate selective preferences toward diverse Atg8 substrates. J. Biol. Chem. 2011;286:7327–7338. doi: 10.1074/jbc.M110.199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakatogawa H., Ichimura Y., Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007;130:165–178. doi: 10.1016/j.cell.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Tanida I., Sou Y.S., Ezaki J., Minematsu-Ikeguchi N., Ueno T., Kominami E. HsAtg4B/HsApg4B/autophagin-1 cleaves the carboxyl termini of three human Atg8 homologues and delipidates microtubule-associated protein light chain 3- and GABAA receptor-associated protein-phospholipid conjugates. J. Biol. Chem. 2004;279:36268–36276. doi: 10.1074/jbc.M401461200. [DOI] [PubMed] [Google Scholar]
- 29.Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–676. doi: 10.1016/S0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 30.Brennecke J., Hipfner D.R., Stark A., Russell R.B., Cohen S.M. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/S0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 31.Zou Z., Wu L., Ding H., Wang Y., Zhang Y., Chen X., Zhang C.Y., Zhang Q., Zen K. MicroRNA-30a sensitizes tumor cells to cis-platinum via suppressing beclin 1-mediated autophagy. J. Biol. Chem. 2012;287:4148–4156. doi: 10.1074/jbc.M111.307405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song B., Wang Y., Xi Y., Kudo K., Bruheim S., Botchkina G.I., Gavin E., Wan Y., Formentini A., Kornmann M., et al. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene. 2009;28:4065–4074. doi: 10.1038/onc.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukhopadhyay P., Pacher P., Das D.K. MicroRNA signatures of resveratrol in the ischemic heart. Ann. N.Y. Acad. Sci. 2011;1215:109–116. doi: 10.1111/j.1749-6632.2010.05866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu H., Wu H., Liu X., Li B., Chen Y., Ren X., Liu C.G., Yang J.M. Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells. Autophagy. 2009;5:816–823. doi: 10.4161/auto.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brest P., Lapaquette P., Souidi M., Lebrigand K., Cesaro A., Vouret-Craviari V., Mari B., Barbry P., Mosnier J.F., Hébuterne X., et al. A synonymous variant in IRGM alters a binding site for miR-196 and causes deregulation of IRGM-dependent xenophagy in Crohn's disease. Nat. Genet. 2011;43:242–245. doi: 10.1038/ng.762. [DOI] [PubMed] [Google Scholar]
- 36.Shi D.B., Wang Y.W., Xing A.Y., Gao J.W., Zhang H., Guo X.Y., Gao P. C/EBPalpha-induced miR-100 expression suppresses tumor metastasis and growth by targeting ZBTB7A in gastric cancer. Cancer Lett. 2015;369:376–385. doi: 10.1016/j.canlet.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 37.Kim S.W., Li Z., Moore P.S., Monaghan A.P., Chang Y., Nichols M., John B. A sensitive non-radioactive northern blot method to detect small RNAs. Nucleic Acids Res. 2010;38:e98. doi: 10.1093/nar/gkp1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhavsar S.K., Schmidt S., Bobbala D., Nurbaeva M.K., Hosseinzadeh Z., Merches K., Fajol A., Wilmes J., Lang F. AMPKalpha1-sensitivity of Orai1 and Ca(2+) entry in T-lymphocytes. Cell. Physiol. Biochem. 2013;32:687–698. doi: 10.1159/000354472. [DOI] [PubMed] [Google Scholar]
- 39.Zugazagoitia J., Guedes C., Ponce S., Ferrer I., Molina-Pinelo S., Paz-Ares L. Current challenges in cancer treatment. Clin. Ther. 2016;38:1551–1566. doi: 10.1016/j.clinthera.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 40.Kachalaki S., Ebrahimi M., Mohamed Khosroshahi L., Mohammadinejad S., Baradaran B. Cancer chemoresistance; biochemical and molecular aspects: a brief overview. Eur. J. Pharm. Sci. 2016;89:20–30. doi: 10.1016/j.ejps.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 41.Gavande N.S., VanderVere-Carozza P.S., Hinshaw H.D., Jalal S.I., Sears C.R., Pawelczak K.S., Turchi J.J. DNA repair targeted therapy: the past or future of cancer treatment? Pharmacol. Ther. 2016;160:65–83. doi: 10.1016/j.pharmthera.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Z., Zhou L., Chen Z., Nice E.C., Huang C. Stress management by autophagy: implications for chemoresistance. Int. J. Cancer. 2016;139:23–32. doi: 10.1002/ijc.29990. [DOI] [PubMed] [Google Scholar]
- 43.Kumar A., Singh U.K., Chaudhary A. Targeting autophagy to overcome drug resistance in cancer therapy. Future Med. Chem. 2015;7:1535–1542. doi: 10.4155/fmc.15.88. [DOI] [PubMed] [Google Scholar]
- 44.Zeng X., Zhao H., Li Y., Fan J., Sun Y., Wang S., Wang Z., Song P., Ju D. Targeting Hedgehog signaling pathway and autophagy overcomes drug resistance of BCR-ABL-positive chronic myeloid leukemia. Autophagy. 2015;11:355–372. doi: 10.4161/15548627.2014.994368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai Y., Grant S. BCL2L11/Bim as a dual-agent regulating autophagy and apoptosis in drug resistance. Autophagy. 2015;11:416–418. doi: 10.1080/15548627.2014.998892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He C., Klionsky D.J. Regulation mechanisms and signaling pathways of autophagy. Ann. Rev. Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Criollo A., Vicencio J.M., Tasdemir E., Maiuri M.C., Lavandero S., Kroemer G. The inositol trisphosphate receptor in the control of autophagy. Autophagy. 2007;3:350–353. doi: 10.4161/auto.4077. [DOI] [PubMed] [Google Scholar]
- 48.Criollo A., Maiuri M.C., Tasdemir E., Vitale I., Fiebig A.A., Andrews D., Molgó J., Díaz J., Lavandero S., Harper F., et al. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ. 2007;14:1029–1039. doi: 10.1038/sj.cdd.4402099. [DOI] [PubMed] [Google Scholar]
- 49.Patterson R.L., Boehning D., Snyder S.H. Inositol 1,4,5-trisphosphate receptors as signal integrators. Ann. Rev. Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 50.Fujita Y., Xu A., Xie L., Arunachalam L., Chou T.C., Jiang T., Chiew S.K., Kourtesis J., Wang L., Gaisano H.Y., Sugita S. Ca2+-dependent activator protein for secretion 1 is critical for constitutive and regulated exocytosis but not for loading of transmitters into dense core vesicles. J. Biol. Chem. 2007;282:21392–21403. doi: 10.1074/jbc.M703699200. [DOI] [PubMed] [Google Scholar]
- 51.Demarchi F., Bertoli C., Copetti T., Tanida I., Brancolini C., Eskelinen E.L., Schneider C. Calpain is required for macroautophagy in mammalian cells. J. Cell Biol. 2006;175:595–605. doi: 10.1083/jcb.200601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frankel L.B., Lund A.H. MicroRNA regulation of autophagy. Carcinogenesis. 2012;33:2018–2025. doi: 10.1093/carcin/bgs266. [DOI] [PubMed] [Google Scholar]
- 53.Fu L.L., Wen X., Bao J.K., Liu B. MicroRNA-modulated autophagic signaling networks in cancer. Int. J. Biochem. Cell Biol. 2012;44:733–736. doi: 10.1016/j.biocel.2012.02.004. [DOI] [PubMed] [Google Scholar]