Abstract
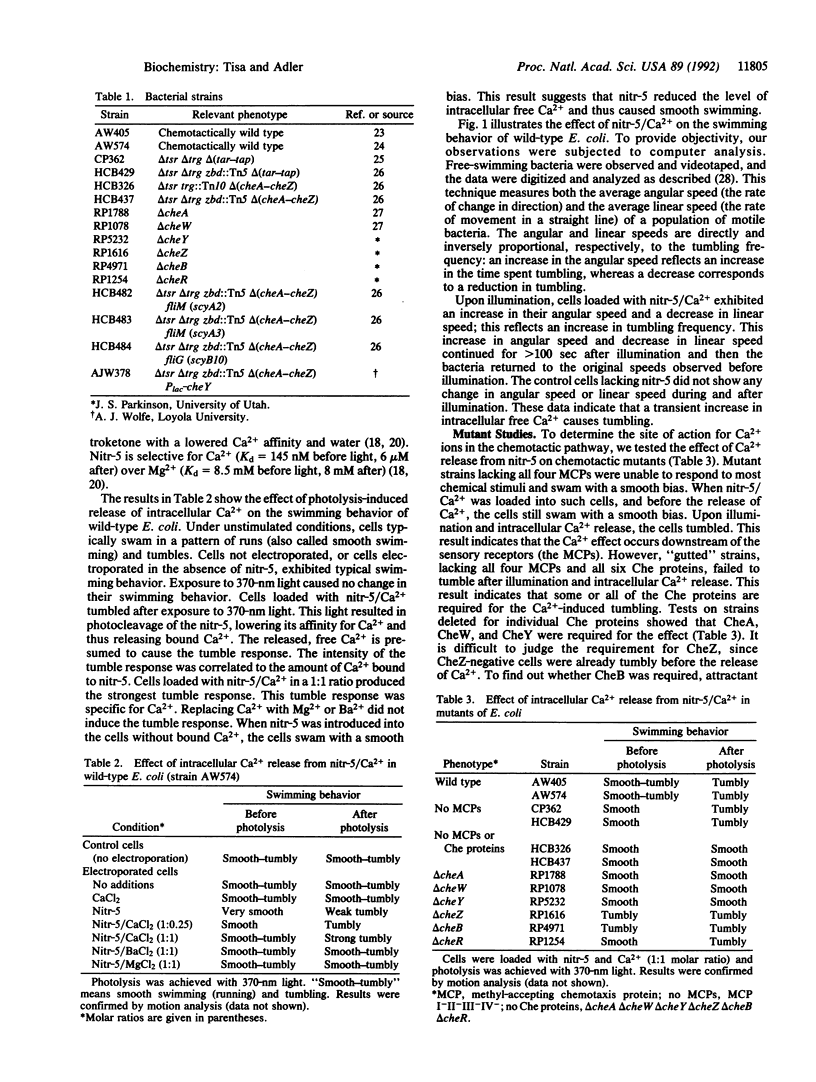
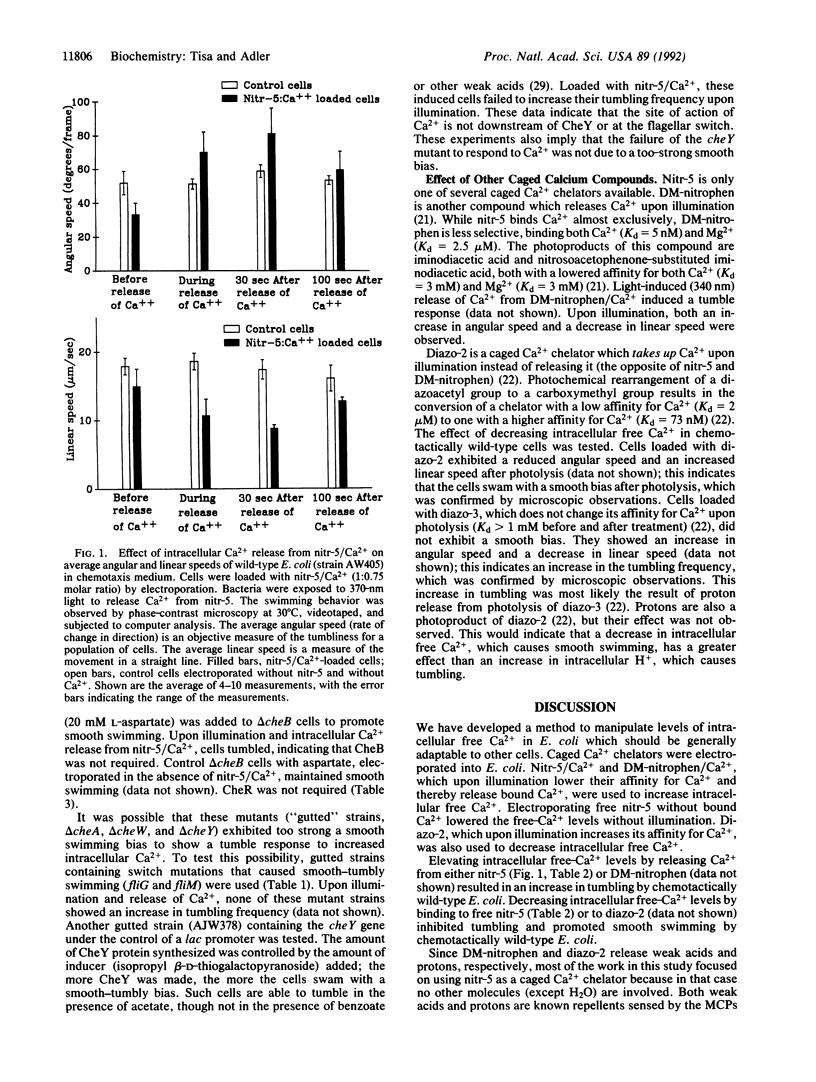
Escherichia coli regulates intracellular free Ca2+ at about 90 nM [Gangola, P. & Rosen, B. P. (1987) J. Biol. Chem. 262, 12570-12574]. To increase intracellular free Ca2+, nitr-5/Ca2+, a "caged" Ca2+ compound, was electroporated into cells and then its affinity for Ca2+ was reduced by exposure to 370-nm light. Upon release of the Ca2+ ions, the cells tumbled. Studies on mutant strains showed that the receptor proteins (methyl-accepting chemotaxis proteins, MCPs) were not required for the Ca(2+)-induced tumbling but that CheA, CheW, and CheY proteins were required. Similar results were obtained with DM-nitrophen/Ca2+, another caged calcium compound that releases Ca2+ upon illumination at 340 nm. Diazo-2, a caged Ca2+ chelator that takes up Ca2+ upon illumination at 340 nm, was used to decrease intracellular free Ca2+, and this caused smooth swimming.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourret R. B., Borkovich K. A., Simon M. I. Signal transduction pathways involving protein phosphorylation in prokaryotes. Annu Rev Biochem. 1991;60:401–441. doi: 10.1146/annurev.bi.60.070191.002153. [DOI] [PubMed] [Google Scholar]
- Conley M. P., Wolfe A. J., Blair D. F., Berg H. C. Both CheA and CheW are required for reconstitution of chemotactic signaling in Escherichia coli. J Bacteriol. 1989 Sep;171(9):5190–5193. doi: 10.1128/jb.171.9.5190-5193.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangola P., Rosen B. P. Maintenance of intracellular calcium in Escherichia coli. J Biol Chem. 1987 Sep 15;262(26):12570–12574. [PubMed] [Google Scholar]
- Hess J. F., Oosawa K., Kaplan N., Simon M. I. Phosphorylation of three proteins in the signaling pathway of bacterial chemotaxis. Cell. 1988 Apr 8;53(1):79–87. doi: 10.1016/0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H. Photochemical manipulation of divalent cation levels. Annu Rev Physiol. 1990;52:897–914. doi: 10.1146/annurev.ph.52.030190.004225. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H., Somlyo A. P. Flash photolysis of caged compounds: new tools for cellular physiology. Trends Neurosci. 1989 Feb;12(2):54–59. doi: 10.1016/0166-2236(89)90136-7. [DOI] [PubMed] [Google Scholar]
- Kihara M., Macnab R. M. Cytoplasmic pH mediates pH taxis and weak-acid repellent taxis of bacteria. J Bacteriol. 1981 Mar;145(3):1209–1221. doi: 10.1128/jb.145.3.1209-1221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. D., Parkinson J. S. Role of CheW protein in coupling membrane receptors to the intracellular signaling system of bacterial chemotaxis. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8703–8707. doi: 10.1073/pnas.86.22.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukat G. S., Stock A. M., Stock J. B. Divalent metal ion binding to the CheY protein and its significance to phosphotransfer in bacterial chemotaxis. Biochemistry. 1990 Jun 12;29(23):5436–5442. doi: 10.1021/bi00475a004. [DOI] [PubMed] [Google Scholar]
- Matsushita T., Hirata H., Kusaka I. Calcium channel blockers inhibit bacterial chemotaxis. FEBS Lett. 1988 Aug 29;236(2):437–440. doi: 10.1016/0014-5793(88)80072-3. [DOI] [PubMed] [Google Scholar]
- McCray J. A., Trentham D. R. Properties and uses of photoreactive caged compounds. Annu Rev Biophys Biophys Chem. 1989;18:239–270. doi: 10.1146/annurev.bb.18.060189.001323. [DOI] [PubMed] [Google Scholar]
- Mesibov R., Adler J. Chemotaxis toward amino acids in Escherichia coli. J Bacteriol. 1972 Oct;112(1):315–326. doi: 10.1128/jb.112.1.315-326.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K., Sugiura A., Momoi H., Mizuno T. Phosphotransfer signal transduction between two regulatory factors involved in the osmoregulated kdp operon in Escherichia coli. Mol Microbiol. 1992 Jul;6(13):1777–1784. doi: 10.1111/j.1365-2958.1992.tb01350.x. [DOI] [PubMed] [Google Scholar]
- Norris V., Chen M., Goldberg M., Voskuil J., McGurk G., Holland I. B. Calcium in bacteria: a solution to which problem? Mol Microbiol. 1991 Apr;5(4):775–778. doi: 10.1111/j.1365-2958.1991.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Onek L. A., Smith R. J. Calmodulin and calcium mediated regulation in prokaryotes. J Gen Microbiol. 1992 Jun;138(6):1039–1049. doi: 10.1099/00221287-138-6-1039. [DOI] [PubMed] [Google Scholar]
- Ordal G. W. Calcium ion regulates chemotactic behaviour in bacteria. Nature. 1977 Nov 3;270(5632):66–67. doi: 10.1038/270066a0. [DOI] [PubMed] [Google Scholar]
- Park C., Hazelbauer G. L. Mutations specifically affecting ligand interaction of the Trg chemosensory transducer. J Bacteriol. 1986 Jul;167(1):101–109. doi: 10.1128/jb.167.1.101-109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampersaud A., Utsumi R., Delgado J., Forst S. A., Inouye M. Ca2(+)-enhanced phosphorylation of a chimeric protein kinase involved with bacterial signal transduction. J Biol Chem. 1991 Apr 25;266(12):7633–7637. [PubMed] [Google Scholar]
- Repaske D. R., Adler J. Change in intracellular pH of Escherichia coli mediates the chemotactic response to certain attractants and repellents. J Bacteriol. 1981 Mar;145(3):1196–1208. doi: 10.1128/jb.145.3.1196-1208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager B. M., Sekelsky J. J., Matsumura P., Adler J. Use of a computer to assay motility in bacteria. Anal Biochem. 1988 Sep;173(2):271–277. doi: 10.1016/0003-2697(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Snyder M. A., Stock J. B., Koshland D. E., Jr Role of membrane potential and calcium in chemotactic sensing by bacteria. J Mol Biol. 1981 Jun 25;149(2):241–257. doi: 10.1016/0022-2836(81)90300-4. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Lukat G. S., Stock A. M. Bacterial chemotaxis and the molecular logic of intracellular signal transduction networks. Annu Rev Biophys Biophys Chem. 1991;20:109–136. doi: 10.1146/annurev.bb.20.060191.000545. [DOI] [PubMed] [Google Scholar]
- Tso W. W., Adler J. Negative chemotaxis in Escherichia coli. J Bacteriol. 1974 May;118(2):560–576. doi: 10.1128/jb.118.2.560-576.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A. J., Conley M. P., Berg H. C. Acetyladenylate plays a role in controlling the direction of flagellar rotation. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6711–6715. doi: 10.1073/pnas.85.18.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A. J., Conley M. P., Kramer T. J., Berg H. C. Reconstitution of signaling in bacterial chemotaxis. J Bacteriol. 1987 May;169(5):1878–1885. doi: 10.1128/jb.169.5.1878-1885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack B. J., Gilmore D. F., White D. Calcium requirement for gliding motility in myxobacteria. J Bacteriol. 1989 Nov;171(11):6093–6096. doi: 10.1128/jb.171.11.6093-6096.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie D., Stock A., Wong C. Y., Stock J. Sensory transduction in bacterial chemotaxis involves phosphotransfer between Che proteins. Biochem Biophys Res Commun. 1988 Mar 15;151(2):891–896. doi: 10.1016/s0006-291x(88)80365-6. [DOI] [PubMed] [Google Scholar]



