Abstract
The amygdala plays a pivotal role in processing anxiety and connects to large‐scale brain networks. However, intrinsic functional connectivity (iFC) between amygdala and these networks has rarely been examined in relation to anxiety, especially across the lifespan. We employed resting‐state functional MRI data from 280 healthy adults (18–83.5 yrs) to elucidate the relationship between anxiety and amygdala iFC with common cortical networks including the visual network, somatomotor network, dorsal attention network, ventral attention network, limbic network, frontoparietal network, and default network. Global and network‐specific iFC were separately computed as mean iFC of amygdala with the entire cerebral cortex and each cortical network. We detected negative correlation between global positive amygdala iFC and trait anxiety. Network‐specific associations between amygdala iFC and anxiety were also detectable. Specifically, the higher iFC strength between the left amygdala and the limbic network predicted lower state anxiety. For the trait anxiety, left amygdala anxiety–connectivity correlation was observed in both somatomotor and dorsal attention networks, whereas the right amygdala anxiety–connectivity correlation was primarily distributed in the frontoparietal and ventral attention networks. Ventral attention network exhibited significant anxiety–gender interactions on its iFC with amygdala. Together with findings from additional vertex‐wise analysis, these data clearly indicated that both low‐level sensory networks and high‐level associative networks could contribute to detectable predictions of anxiety behaviors by their iFC profiles with the amygdala. This set of systems neuroscience findings could lead to novel functional network models on neural correlates of human anxiety and provide targets for novel treatment strategies on anxiety disorders. Hum Brain Mapp 37:1178–1193, 2016. © 2015 The Authors Human Brain Mapping Published by Wiley Periodicals, Inc.
Keywords: anxiety, amygdala, functional connectivity, fMRI, connectomics
INTRODUCTION
Although anxiety is a common negative emotional state that occurs during daily life, it has a complex nature. Anxiety involves physical, affective, and cognitive changes underlain by a complex neural mechanism [Grupe and Nitschke, 2013]. An exploration of this neural mechanism is essential for understanding normal and pathological anxiety processes. Studies from both animals and humans have shown that amygdala is a central structure to this mechanism by playing a crucial role in processing anxiety and fear [Bishop, 2007; Phelps and LeDoux, 2005]. Both morphological and functional characteristics of the amygdala have been linked to anxiety‐related processes. Healthy children and adults with higher anxiety levels tend to have a larger amygdala [Baur et al., 2012; Holmes et al., 2012; Qin et al., 2014]. This relationship is altered in various anxiety disorders [Hayano et al., 2009; Milham et al., 2005]. Previous functional magnetic resonance imaging (fMRI) studies have demonstrated that amygdala activation was also modulated by anxiety level [Bishop et al., 2004; Etkin et al., 2004], and altered in abnormal anxiety conditions [Etkin and Wager, 2007; Hattingh et al., 2012].
Given that the amygdala has rich connections (both structure and function) with numerous regions involved in emotion processes, it is reasonable to examine how interactions between amygdala and other brain regions contribute to anxiety‐related processes [Roy et al., 2009; Young et al., 1994]. Many studies have detected anxiety‐related changes in amygdala structural and functional connectivity [Kim et al., 2011b]. The level of anxiety was associated with the structural integrity of connections between amygdala and prefrontal cortex (PFC) and insula [Baur et al., 2013; Clewett et al., 2014; Kim and Whalen, 2009]. In accordance with the structural findings, evidence from both resting‐state fMRI (rfMRI) and task‐based fMRI (tfMRI) studies has indicated that functional connectivity (FC) between amygdala and multiple regions could predict anxiety levels in healthy participants [Baur et al., 2013; Kim et al., 2011a; Qin et al., 2014]. This brain–behavior association can be disturbed in various anxiety disorders [Hamm et al., 2014; Roy et al., 2013; Swartz et al., 2014b]. The above studies have revealed that distributed brain regions might work together and underlie anxiety processes. Thus, these regions should be investigated as an anxiety‐related circuit rather separately.
The cognitive and physiological aspects of anxiety have been assigned to different functional brain networks [Bijsterbosch et al., 2014]. Previous studies have also observed that high trait anxiety and anxiety disorders are associated with dysfunction of several brain networks that support not only emotion but also cognition and physical behavior [Soares et al., 2013; Sylvester et al., 2012]. Considering the complex composition of anxiety and the extensive connection profile of human amygdala, investigating how functional interactions between the amygdala and different brain networks engage in anxiety processes will provide new insights into anxiety at the systems level.
RfMRI measures the functional interactions of spontaneous activity between different brain areas, namely intrinsic FC (iFC). This method is not confounded by a specific task design and is thus widely used for measuring inherent neural network patterns [van den Heuvel and Hulshoff Pol, 2010]. Yeo et al. [2011] have developed a full cortical parcellation based upon iFC data from 1,000 healthy participants. Specifically, this functional parcellation contains seven networks, namely the visual network, somatomotor network, dorsal attention network, ventral attention network, limbic network, frontoparietal control network, and default network. These networks involve both low‐level processes (e.g., perception and motor) and high‐level processes (e.g., attention, emotion, motivation, and cognition) in the human brain by representing the intrinsic functional organization of the human brain system. From this perspective, this parcellation provides a reference for examining iFC at a systems neuroscience level. According to the observations that the widely distributed amygdala connectivity and its anxiety associations across different ages, we hypothesize that the lifespan anxiety will present significant and heterogeneous relationships with iFC between amygdala and the seven cortical networks. To test this hypothesis, we adopted a cross‐sectional adult lifespan dataset of rfMRI images for detection of the common correspondence patterns between anxiety and amygdala connectivity.
MATERIALS AND METHODS
Participants
We used a publicly available dataset of the enhanced Nathan Kline Institute‐Rockland Sample (NKI‐RS), which is an ongoing, institutionally centered endeavor aimed at creating a large‐scale community sample of participants across the lifespan [Nooner et al., 2012]. In total, 418 participants from five NKI‐RS releases were included in the present study. The NKI institutional review boards approved the data collection, and all participants provided informed consent. The participants underwent multimodal MRI scans, semi‐structured diagnostic psychiatric interviews and a battery of psychiatric, cognitive, and behavioral assessments. Total 102 participants were excluded due to incomplete multimodal images (32), psychiatric conditions (51), and failure in passing the quality control process (19) (for details, see Quality Control Procedures).
Questionnaire Measures
Anxiety levels were assessed with the State and Trait Anxiety Inventory (STAI) for the adult [Spielberger et al., 1970], which is a self‐report questionnaire consisting of 40 items that measures state anxiety and trait anxiety, respectively. Among the 316 participants, there were only 280 healthy adults (18–83.5 years, 99 males) with available STAI measures. Table 1 presented the descriptive statistics of these STAI measures and other demographic information of these participants. General linear models revealed that no significant correlation of state anxiety or trait anxiety with age (state anxiety: p = 0.9383, t(276) = 0.0775; trait anxiety: p = 0.1868, t(276) = −1.3233), sex effect (state anxiety: p = 0.4917, t(276) = −0.6885; trait anxiety: p = 0.6871, t(276) = −0.4032), or the interaction between age and sex (state anxiety: p = 0.5847, t(276) = −0.5471; trait anxiety: p = 0.1788, t(276) = −1.3479).
Table 1.
The demographic information of participants
| N | Age range (mean ± SD) | STAI‐state (mean ± SD) | STAI‐trait (mean ± SD) | |
|---|---|---|---|---|
| Males | 99 | 18.39–83.18 (42.65 ± 19.2) | 34–67 (45.51 ± 7.89) | 33–76 (49.51 ± 9.36) |
| Females | 181 | 18.15–83.36 (49.55 ± 17.19) | 35–69 (46.24 ± 9.22) | 33–79 (49.54 ± 10.34) |
MRI Data Acquisition
All MRI images were acquired with a 3.0 T scanner (TrioTim™, Siemens Medical Systems, Erlangen, Germany). Each participant underwent a set of multimodal imaging scans, including structural MRI, rfMRI, diffusion tensor imaging (DTI) and tfMRI. Details of the parametric settings for these imaging sequences can be found at the NKI‐RS website. We only analyzed data from a high‐resolution structural scan with a magnetization‐prepared rapid gradient echo sequence and rfMRI scans with multiband echo planar imaging (mbEPI) sequence. The recently developed mbEPI sequence has been demonstrated to be more accurate and robust in mapping human brain function [Moeller et al., 2010]. It enables the acquisition of fMRI data with unprecedented sampling rates for full‐brain coverage through the simultaneous acquisition of multiple slices over the same time required to obtain a single‐slice image using standard EPI [Feinberg and Yacoub, 2012]. Three rfMRI sequences were included in the protocol: (1) mbEPI645, (2) mbEPI1400, and (3) a standard EPI sequence. Our previous studies demonstrated higher test–retest reliability with the mbEPI1400 sequence and with longer scan duration in high‐resolution functional connectomics [Zuo and Xing, 2014; Zuo et al., 2013]. We thus only analyzed the mbEPI1400 rfMRI images. A b0 image with the same spatial resolution from a multiband DTI sequence was also used for individual coregistration.
MRI Data Preprocessing
Data were processed and analyzed using the Connectome Computation System (CCS: https://github.com/zuoxinian/CCS) developed by our laboratory [Xu et al., 2015]. This connectome pipeline combines the different functionalities of multiple neuroimaging packages and novel algorithms developed in‐house and is designed to provide an integrated computational platform for multimodal imaging human brain connectomics by hierarchically integrated functional modules, such as preprocessing individual images, mapping individual connectomes, group‐level connectomics, and visualization. One advantage of CCS is the reliable and natural method of preforming multimodal neuroimaging data analyses on the cortical mantle.
The major purpose of structural MRI data preprocessing is to segment bilateral amygdala structures from the gray matter tissue and reconstruct cortical surfaces from the brain tissue boundaries. These steps were primarily completed using FreeSurfer and included (1) spatial noises removal with a non‐local means filter [Zuo and Xing, 2011]; (2) brain extraction or skull stripping; (3) visual inspection and manual intervention of brain tissues by removing the residual non‐brain tissues or patching the excluded brain tissues; (4) tissue segmentation and surface reconstruction by identifying the boundary between white and gray matter; (5) subcortical segmentation by labeling each voxel to one of 40 subcortical regions based on an existing atlas containing probabilistic location information; (6) b0 image realignment by a 6‐parameter rigid transformation to the mean rfMRI image and boundary‐based registration (BBR) between individual structural image and the realigned b0 image [Greve and Fischl, 2009]; and (7) the generation of regions of interests (ROIs) for individual left and right amygdala areas in functional space by transferring structurally segmented amygdala areas to their ROIs in individual functional spaces with BBR.
The rfMRI image preprocessing method is consistent with of our previous publications [Jiang et al., 2015a; Xu et al., 2015; Yang et al., 2014a, 2014b; Zuo et al., 2013]. Specifically, individual rfMRI images were (1) removed of the first 8 volumes to reach stable scanning; (2) despiked by removing and interpolating spikes from hardware or in‐scanner head motion (in theory, this preprocessing equals motion scrubbing with interpolation, see Power et al., 2015; Carp, 2013 for details); (3) further corrected for head motion across different volumes; (4) masked by the functional brain mask generated based on both structural brain and 4D individual time series; (5) normalized to have a comparable 4D global mean intensity of 10,000 across individuals; (6) regressed out of mean signals from white matter and cerebrospinal fluid masks as well as Friston‐24 head motion parameters; (7) temporally smoothed with a band‐pass filtering; (8) detrended by removing both linear and quadratic trends in time domain; and (9) projected onto the fsaverage surface grid and down‐sampled to the fsaverge6 surface grid.
Quality Control Procedure
With the quality control procedure module in CCS [Xu et al., 2015], we performed following steps to ensure the quality of preprocessed images. Specifically, we first excluded data with obvious imaging artifacts regarding generated screenshots for visual inspection of (1) brain extraction, (2) tissue segmentation, (3) cortical surface reconstruction, (4) BBR‐based functional image registration, and (5) dynamic monitoring head motion during rfMRI scans. From a quantitative perspective, CCS also computes four metrics to guide the quality control: (1) the maximum distance of translational movement (maxTran); (2) the maximum degree of rotational movement (maxRot); (3) the mean frame‐wise displacement (meanFD) [Patriat et al., 2013; Power et al., 2012]; and (4) the minimal BBR cost (mcBBR). All participants entering the final brain‐anxiety association study must have had usable datasets from all three rfMRI sequences meeting the following criteria: (1) maxTran ≤ 3 mm, (2) maxRot ≤ 3°, (3) meanFD ≤ 0.5 mm, and (4) mcBBR < 0.6.
Amygdala iFC: From Vertices to the Seven Cerebral Networks
In individual functional image spaces, all timeseries of voxels within masks of bilateral amygdala (Fig. 1A) were averaged to generate representative timeseries of the amygdala ROIs, respectively. Each participant's amygdala time series was correlated with time series of all vertices across the cortical mantle to produce a vertex‐wise correlation map of the amygdala. Individual correlation maps were further converted into iFC maps using Fisher's r‐to‐z transform. Seven networks were established from a functional parcellation developed by a large‐scale study [Yeo et al., 2011] including visual, somatomotor, dorsal attention, ventral attention, limbic, frontoparietal control, and default networks (Fig. 1B). For each network, we calculated its iFC with bilateral amygdala areas as the mean iFC of all vertices within it. Positive and negative iFC values were separately averaged and analyzed in following statistical tests.
Figure 1.
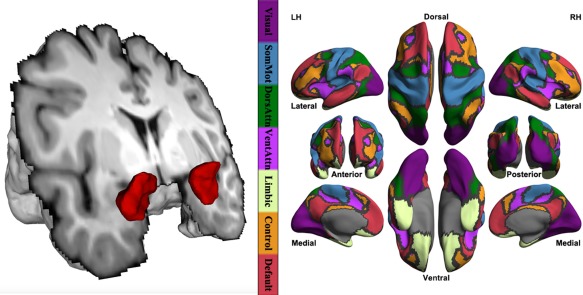
Human amygdala and seven intrinsic connectivity networks. FreeSurfer segments bilateral amygdala volumes from an individual high‐resolution structural image, which is rendered into its 3D model (the left panel). The right panel plots seven intrinsic networks established from a functional parcellation developed based upon 1,000 healthy participants including visual (Visual), somatomotor (SomMot), dorsal attention (DorsAttn), ventral attention (VentAttn), limbic (Limbic), frontoparietal control (Control), and default (Default) networks. The cortical grid model “inflated_pre” of the fsaverage6 in FreeSurfer was employed for rendering surfaces of left hemisphere (LH) and right hemisphere (RH). Gray curves indicate the boundaries between the seven networks. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Anxiety–iFC Association Studies
A set of general linear models was implemented with multiple linear regressions to test whether the anxiety measures could significantly explain amygdala iFC. We first regressed out meanFD and mcBBR from the amygdala iFC vertex‐wise. To examine the association between anxiety and the overall cortical iFC of the amygdala, we computed the global mean iFC (gmiFC) across the entire cortex and conducted a general linear model with stepwise regression, in which gmiFC is the response variable and anxiety, age, and sex are predictors. After the initial fit of a constant model, this approach examines the set of terms (predicting variables and their interactions) and adds the best term to the model if a test for adding the term has a p‐value of 0.05 or less. If no terms can be added, it examines the terms currently in the model, and removes the worst term if a test for removing it has a p‐value of 0.10 or greater. This process is repeated until no terms can be added or removed. To examine network‐specific association between anxiety and the amygdala iFC, we regressed out gmiFC from each network iFC and conducted a set of stepwise general linear models in which the response variable is the network‐specific iFC (nsiFC) and the predictors are anxiety, age, and sex. Of note, state and trait anxiety were tested separately. The final p‐values of all tests are Bonferroni‐corrected for multiple comparisons (uncorrected p < 0.00625) across the global mean and the 7 networks’ iFC of the amygdala. As complementary analyses to the network‐wise analyses, we also performed vertex‐wise association analysis using general linear model approach in FreeSurfer to probe specific regions linking to anxiety (class variable: gender; continuous variables: anxiety and age). Corrections for multiple comparisons were implemented with Random Field Theory (uncorrected p < 0.01, corrected p < 0.05/2) in SurfStat [Chung et al., 2010], which has been integrated as part of the CCS pipeline.
RESULTS
Both state and trait anxiety scores were analyzed for mapping amygdala iFC–anxiety associations, and the most significant relationships were primarily detected for trait anxiety, either for the global cortical mean iFC or network‐specific iFC of the amygdala. We report these results in details below. The main effect was reported with the statistical significance (p‐values) of the predicting variable, anxiety.
Global Amygdala Connectivity and Anxiety
No significant relationship between state anxiety and global mean iFC (either positive or negative) was detectable in the left and right amygdala. In contrast, significant associations between trait anxiety and global mean positive iFC were detected for bilateral amygdala areas (Fig. 2, left panel: left amygdala, p < 0.0066, marginally significant, t(276) = −2.74; right panel: right amygdala, p < 0.0061, t(276) = −2.77). The general linear models selected by the stepwise regression all considered trait anxiety, age, and sex as predictors without any interactions. These outcomes indicated that at the global cortex level, higher levels of trait anxiety were related to lower iFC strengths of the amygdala across the human adulthood lifespan.
Figure 2.
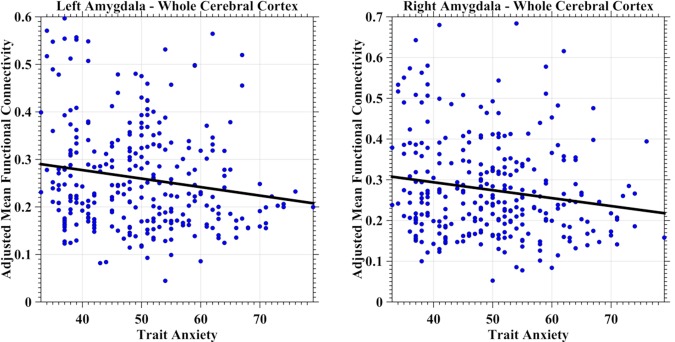
Scatter plots depict the intrinsic functional connectivity (iFC)–anxiety association at the whole cortex level for the human amygdala. The global mean positive iFC was estimated as the averaged iFC value across the cortical mantle and was further adjusted for age, head motion and registration quality by a regression model. The adjusted mean iFC values were plotted as blue scatter circles against the anxiety measures. The fitted lines were estimated and plotted as black lines for both the left amygdala (left panel) and the right amygdala (right panel). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Network‐Specific Amygdala Connectivity and Anxiety
Negative iFC between the limbic network and the left amygdala demonstrated a positive correlation with state anxiety (Fig. 3, left panel, predicting variable: state anxiety; p = 0.0051, t(278) = 2.83). The somatomotor network demonstrated a similar correlation between its negative iFC of the left amygdala but with trait anxiety (Fig. 3, right panel, predicting variable: trait anxiety; p = 0.0022, t(278) = 3.09). Dorsal attention network iFC with the left amygdala exhibited inverse relationships with trait anxiety (Fig. 4, left panel, predicting variables: trait anxiety, age; p = 0.0057, t(277) = −2.79), whereas frontoparietal control network showed the same relationship between its iFC with the right amygdala (Fig. 4, right panel, predicting variable: trait anxiety; p = 0.0017, t(278) = −3.17). The ventral attention network exhibited a much more complicate correlation patterns (Fig. 5). First, the iFC between this network and the right amygdala positively correlated with the trait anxiety (Fig. 5, left panel, predicting variables: trait anxiety, age, sex and trait–sex interaction; p = 0.0048, t(275) = 2.84). Interestingly, the negative iFC between the right amygdala and the ventral attention network also had a significant interaction effect between trait anxiety and sex (p = 0.0062, t(275) = −2.76) (Fig. 5, right panel). Weaker iFC between the ventral attention network and right amygdala correlated with higher level of the trait anxiety in females (blue solid line), whereas no significant iFC–anxiety correlation was detectable in males (red dashed line). No network‐specific anxiety correlation was observed with positive iFC of the amygdala.
Figure 3.
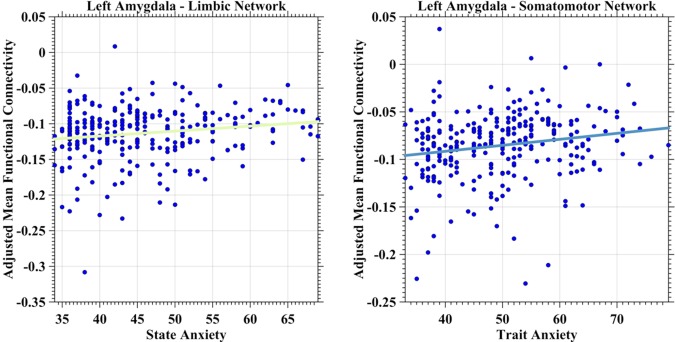
Scatter plots depict associations between anxiety and amygdala iFC with limbic and somatomotor networks. The network‐specific mean iFC of the left amygdala was estimated as the averaged iFC value across the specific network and was further adjusted for global mean iFC, age, head motion, and registration quality by a regression model. The adjusted mean iFC values were plotted as blue scatter circles against the anxiety measures. The fitted lines were estimated and plotted as lines for both limbic network (left panel) and somatomotor network (right panel) with the colors of the same networks as illustrated in Figure 1. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 4.
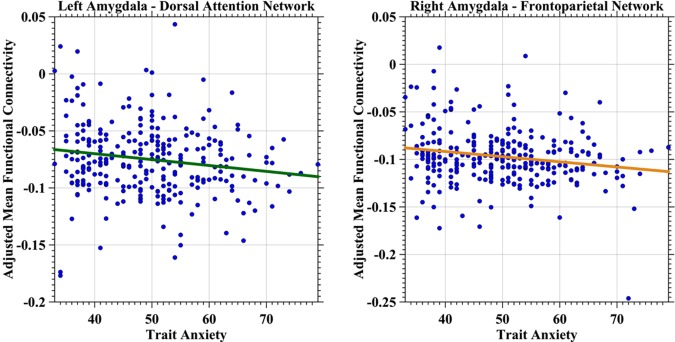
Scatter plots depict associations between trait anxiety and amygdala iFC with dorsal attention and frontoparietal networks. The network‐specific mean iFC of the amygdala was estimated as the averaged iFC value across the specific network and was further adjusted for global mean iFC, age, head motion, and registration quality by a regression model. The adjusted mean iFC values were plotted as blue scatter circles against the trait anxiety measures. The fitted lines were estimated and plotted as lines for both dorsal attention network (left panel) and frontoparietal network (right panel) with the colors of the same networks. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 5.
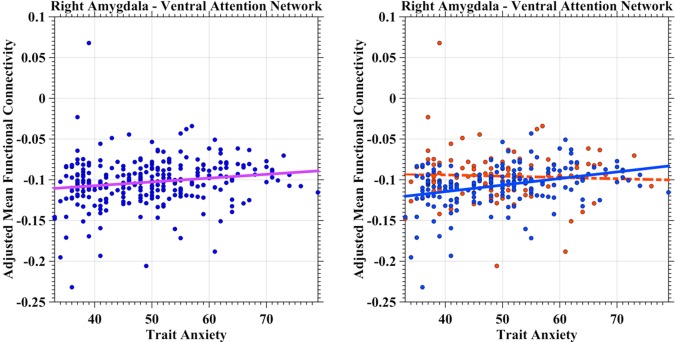
Scatter plots depict associations between trait anxiety and amygdala iFC with ventral attention network. The adjusted mean iFC values were plotted as blue scatter circles against the anxiety measures. The fitted lines were estimated and plotted as lines with the same color of the network in Figure 1 (left panel). The gender–anxiety interaction was illustrated in the right panel, indicating a significant positive correlation in females (blue circles and line) while no significant correlation in males (red circles and line). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Both Figures 3 and 4 indicated potential interactions between trait anxiety and network‐specific iFC with the human amygdala. Specifically, for the left amygdala, its network connectivity showed distinct anxiety correlational profiles between somatomotor network (r = 0.1820) and dorsal attention network (r = −0.1659), and this difference is statistically significant tested by the Fisher Z‐test (Z = 4.14, p = 3.8e‐5). A similar anxiety–connectivity interaction was also significantly detectable for the right amygdala (Z = 4.31, p = 1.9e‐5) but for different networks: ventral attention network (r = 0.1756) and frontoparietal network (r = −0.1866). The positive versus negative valence of the network connectivity was not different between the left amygdala and the right amygdala (Z = −0.1376, p > 0.2).
Vertex‐Wise Amygdala Connectivity and Anxiety
At vertex level, a set of regions exhibited anxiety‐related changes across the cortical mantle (Fig. 6 and Tables 2 and 3) regarding their iFC with amygdala. An area adjacent to left lateral occipital, parietal, and superior temporal cortex showed positive correlation between its ipsilateral amygdala iFC and anxiety (state: Fig. 6A and Table 2; trait: Fig. 6B and Table 3). Of note, the individual differences in trait anxiety were also demonstrated similar variation pattern with ipsilateral iFC between this area and amygdala in right hemisphere (Table 3). Beyond this region, the right posterior central area had stronger iFC of the left amygdala correlated with higher level of trait anxiety whereas an inverse correlational profile existed for the left dorsal lateral prefrontal cortex (Fig. 6B).
Figure 6.
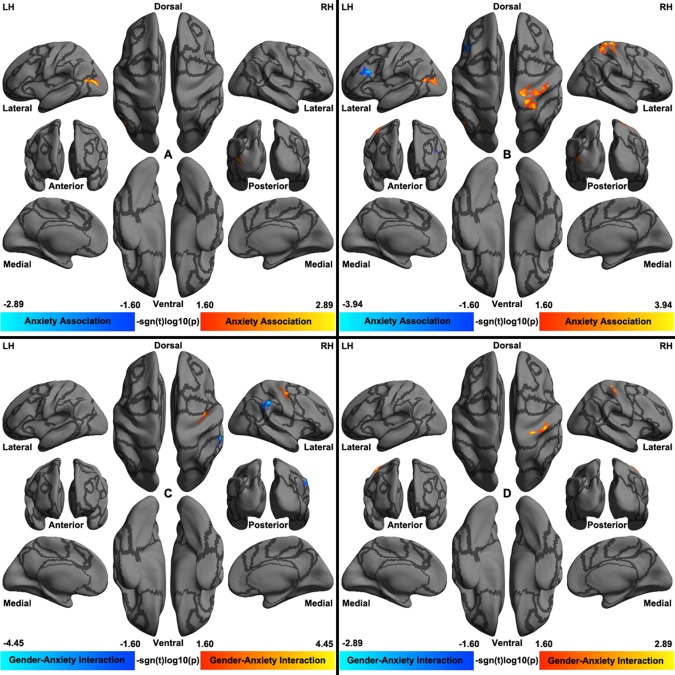
Cortical surfaces render for vertex‐wise association on anxiety‐iFC of amygdala. Both main effects of anxiety on the left amygdala iFC (A and B) and their interactions with gender (C and D) are visualized. Panels A and C are rendered for state anxiety whereas panels B and D are rendered for trait anxiety. The cortical grid model “inflated_pre” of the fsaverage6 in FreeSurfer was employed for rendering surfaces of left hemisphere (LH) and right hemisphere (RH). Gray curves indicate the boundaries between the seven cortical networks. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Brain regions showing significant state anxiety effects on iFC
| Region | Amygdala | Significance (peak) | Size (mm2) | X, Y, Z |
|---|---|---|---|---|
| Positive correlation | ||||
| L middle occipital sulcus | Left | 2.088 | 550 | −41, −78, 10 |
| Positive interaction | ||||
| R central sulcus | Left | 1.693 | 19 | 40.2, −8.8, 39.8 |
| Negative interaction | ||||
| R sulcus intermedius primus (of Jensen) | Left | −2.187 | 142 | 53.3, −42, 28.9 |
Table 3.
Brain regions showing significant trait anxiety effects on iFC
| Region | Amygdala | Significance (peak) | Size (mm2) | X, Y, Z |
|---|---|---|---|---|
| Positive correlation | ||||
| L middle occipital gyrus | Left | 2.468 | 130 | −43.9, −72.9, 17.3 |
| R postcentral gyrus | Left | 5.975 | 17 | 29.2, −27.1, 65.2 |
| R superior temporal sulcus | Right | 1.793 | 573 | 37.9, −59.5, 15 |
| Negative correlation | ||||
| L inferior frontal sulcus | Left | −2.136 | 13 | −37.2, 24.7, 21.4 |
| Positive interaction | ||||
| R postcentral gyrus | Left | 2.269 | 238 | 41.2, −22.7, 56.3 |
Gender–anxiety interactions were significantly detectable across the lifespan samples (state: Fig. 6C and Table 2; trait: Fig. 6D and Table 3). Specifically, in the right inferior parietal sulcus, state anxiety was negatively correlated with the left amygdala iFC in males (r(98) = −0.3226, p = 0.0011) but a marginal positive correlation in females (r(180) = 0.1371, p = 0.0658). In contrast, in the right posterior central sulcus, state anxiety was positively correlated with the left amygdala iFC in males (r(98) = 0.3171, p = 0.0014) while only a marginal negative correlation was observed in females (r(180) = −0.1295, p = 0.0822). The trait anxiety showed a similar correlational pattern amygdala iFC with the right posterior central gyrus in males (r(98) = 0.3207, p = 0.0012) but no significant correlation in females (r(180) = −0.0238, p = 0.7508).
Sex Differences and Age‐Related Changes of Amygdala Connectivity
Sex differences in network‐specific iFC with the amygdala were observed within multiple brain networks. Specifically, ventral attention network exhibited higher strength of positive iFC with bilateral amygdala areas in males (left amygdala: p < 0.0001, t(278) = 3.97; right amygdala: p < 0.0005, t(278) = 3.57) but higher strength of negative iFC with the right amygdala in females (p = 0.0012, t(275) = 3.28). Females also had stronger iFC between the left amygdala and the frontoparietal control network (p = 0.006, t(278) = 2.77).
Age‐related changes of network‐wise amygdala iFC were also detectable across the lifespan. The global mean positive iFC of the left amygdala exhibited a linearly decreased lifespan trajectory (marginally significant: p < 0.0084, t(276) = −2.66). Network‐specific iFC demonstrated linearly detectable changes with age across several brain networks during the human lifespan. Positive iFC between bilateral amygdala areas and dorsal attention network increased with age (left amygdala: p = 0.0021, t(278) = 3.10; right amygdala: p = 0.0022, t(278) = 3.09). In contrast, the strength of negative iFC between the right amygdala and the ventral attention network decreased with age (p = 0.0034, t(275) = 2.95).
DISCUSSION
We investigated how interindividual differences in anxiety were related to interindividual variability of amygdala iFC by examining their correlations based on a human brain functional parcellation in a large sample of 280 healthy participants across the adult lifespan. In particular, higher levels of trait not state anxiety were reflected in lower global amygdala cortical connectivity strength. Regarding the network‐specific connectivity, the higher connectivity strength between the left amygdala and the limbic network could partly predict lower state anxiety levels. Trait anxiety exhibited more extensively network‐specific connectivity associations across both low‐level primary sensory and high‐level associative brain systems. Interestingly, these correlational profiles demonstrated different network distribution for the left and right amygdala structures. The left amygdala anxiety–connectivity correlation was observed in both the somatomotor and dorsal attention networks, whereas the right amygdala anxiety–connectivity correlation was mainly distributed in the frontoparietal (also called cognitive control) and ventral attention networks. Age and gender also affected the connectivity profiles of the amygdala with functional brain networks. Intriguingly, as an important part of the brain control system, ventral attention network exhibited significant contributions to age‐related changes, sex differences, trait anxiety associations, as well as anxiety–sex interactions, indicating its key role of amygdala‐based anxiety modulation at the systems level. The neurobiological implications of these observations for understanding anxiety processes in both healthy brains and brain disorders across the human lifespan are discussed below.
The amygdala has extensive structural and functional connectivity with the cerebral cortex [Roy et al., 2009; Young et al., 1994], through which it plays a key role in the functioning of various emotion‐related processes. Previous task‐based fMRI studies have revealed widely distributed anxiety‐specific activations across the entire cortical mantle [Bishop, 2007; Sylvester et al., 2012]. However, few reports have investigated how functional connectivity between the amygdala and the whole cortex is related to anxiety. To the best of our knowledge, our results revealed for the first time that this overall amygdala–cortical intrinsic connectivity was higher in individuals with lower trait anxiety, which implies that greater functional integration between the amygdala and the cerebral cortex can better modulate trait anxiety, indicating a global‐level intrinsic architecture of trait anxiety in the human brain.
This anxiety‐related functional architecture was also observed at the systems level by examining network‐specific connectivity with amygdala in term of its correlations with anxiety. Specifically, individual differences in state anxiety were correlated with individual amygdala connectivity with the limbic network. This network is primarily responsible for reward‐related processing to achieve reward and avoid negative outcomes [Koziol et al., 2014]. Of note, as part of the limbic system, the amygdala involves processing threatening stimuli and functionally connects to the limbic network regions [Bishop, 2007; Hahn et al., 2011; Mattavelli et al., 2014]. The MRI procedure can actually function as a threatening environment, might elicit increases in state anxiety [Baur et al., 2013; Muehlhan et al., 2011]. The neural basis for the current anxious state might be associated with the functional interaction between the amygdala and the limbic network. Our results suggested that the stronger negative connectivity between the left amygdala and the limbic network could better inhibit increases in state anxiety, resulting in lower state anxiety scores. These neural correlations of state anxiety were also observed in social anxiety disorders [Hahn et al., 2011].
Trait anxiety was extensively associated with amygdala connectivity with multiple brain networks. These network‐specific connectivity profiles were all negative, representing functional segregation between the amygdala and these networks [Gee et al., 2011]. Regarding connectivity with the left amygdala, the somatomotor network and dorsal attention network were both correlated with trait anxiety but in an opposite direction. Stronger amygdala connectivity with the somatomotor network was related to lower trait anxiety. The somatomotor network is involved in processing motor behaviors, among which increased muscle tension and trembling represent one anxiety‐related behavioral manifestation [Roth, 2005]. Abnormal sensory motor‐related reactions to social stimuli (e.g., gaze avoidance) have been suggested to be linked to increased gray volume in premotor cortex in social anxiety disorders compared with healthy controls [Irle et al., 2014]. Functional activations of both amygdalae and primary motor cortex were stronger under anxiety conditions; this observation has been interpreted as modulation of threat avoidance [Andreatta et al., 2015]. Taken together, changes in both the structure and function of the somatomotor network and the amygdala were related to anxiety‐related processing of motor reactions. Intrinsic amygdala–somatomotor network connectivity may underlie the motor‐behavior manifestation of trait anxiety. However, due to lack of the direct measures of behaviors, such a conclusion is speculative and should be directly tested in future work.
In contrast, weaker left amygdala connectivity with the dorsal attention network predicted lower trait anxiety. The dorsal attention network, comprising the frontal eye field and intraparietal sulcus, is believed to be involved in top–down attention orienting [Fox et al., 2006; Koziol et al., 2014]. According to the attention control theory, anxiety can impair attention control by automatically allocating more attention to threat‐related stimuli, thus weakening the function of the goal‐directed attention system [Eysenck et al., 2007]. The left amygdala has been suggested to play a key role in threat‐related attention changes and reduction in social anxiety levels [Britton et al., 2015]. Troiani et al. [2014] observed enhanced functional connectivity between the left amygdala and regions in the dorsal attention network for the contrast between fearful faces and houses, even though participants were unaware of these stimuli. This observation of increased functional connectivity between amygdala and the dorsal attention network has also been reported after cues (attention orienting) for angry faces [Mohanty et al., 2009]. These findings suggested that enhanced inputs from the amygdala to the dorsal attention network facilitated the detection of upcoming salient stimuli. We detected an anticorrelation between the amygdala connectivity with this network and the trait anxiety, which may indicate that the neural modulation (i.e., the negative connectivity as functional segregation) of the left amygdala on the dorsal attention network is stronger in participants with high anxiety, likely eliciting more attention bias. This interpretation should be read with caution until direct evidence provided in attention‐related task and fMRI procedure.
Two other networks of the brain control system—the ventral attention and frontoparietal control networks—exhibited different correlational profiles of trait anxiety regarding their connectivity with the right amygdala. Similar distinctions have been proposed in a previous review suggesting that high‐level anxiety and anxiety disorders were associated with enhanced ventral attention network functioning and reduced frontoparietal control network functioning [Sylvester et al., 2012]. We observed that stronger connectivity between the right amygdala and the control network was associated with higher trait anxiety. This control network serves maintaining and regulation of mental health [Cole et al., 2014], and alternations of the cognitive control network have been observed in anxiety disorders and individuals with high trait anxiety accompanying impaired cognitive control in task performances [Bishop, 2009; Sylvester et al., 2012]. Intranetwork functional connectivity increased with cognitive anxiety for this network [Bijsterbosch et al., 2014]. This network has been proposed to play a protective role of regulating anxiety‐related processes via direct or indirect feedback control on the amygdala [Cole et al., 2014]. As an important node of the control network, dorsal lateral prefrontal cortex (DLPFC) has been reported to exert top–down control over the amygdala in previous studies of emotional regulations, with increases in DLPFC activation but decreases in amygdala activation [Ochsner et al., 2002]. As previously noted, functional connectivity between the amygdala and the control network was increased in both adults [Etkin et al., 2009] and adolescents [Roy et al., 2013] with general anxiety disorders. This functional interaction might reflect a compensatory neural adaptation in which the cognitive control system engaged in regulating excessive anxiety. Taken together, we assumed that individuals with high‐trait anxiety lack efficient communication between the frontoparietal network and right amygdala in response to excessive anxiety.
The ventral attention network exhibited significant functional connectivity effects on trait anxiety and anxiety–sex interactions, indicating its key role in modulating trait anxiety via its connectivity with the right amygdala at a systems neuroscience level. This network comprises the supramarginal gyrus, frontal operculum, temporoparietal junction, anterior cingulate cortex, and anterior insula [Fox et al., 2006; Koziol et al., 2014], and is largely overlapped with the salience network in response to salience stimuli and its functional connectivity correlated with anxiety [Seeley et al., 2007]. The attention control theory suggests that anxiety also impacts stimulus‐driven attention by increasing automatic processes of threat‐related stimuli [Eysenck et al., 2007]. Behavioral studies demonstrated that increased bias to threat‐related stimuli in high anxious individuals [Mathews et al., 1997]. Supporting the stimulus‐driven attention processes, the ventral attention network exhibited aberrant activation during attention tasks in individuals with high anxiety or anxiety disorders [Monk et al., 2006; Telzer et al., 2008]. On the other hand, the amygdala could modulate attention processes by orientating attention to threatening or salient stimuli [Britton et al., 2011]. Previous studies have demonstrated that the functional and structural connectivity between the left amygdala and the anterior insula is correlated with state and trait anxiety, respectively [Baur et al., 2013]. These two regions are both involved in the detection of salient events and serve as a crucial role in salience attention processing [Blackford et al., 2010; Menon and Uddin, 2010]. The negative functional connectivity between amygdala and ventral lateral prefrontal cortex, a key node of ventral attention network, was reduced in general anxiety disorders relative to healthy controls, consistent with untighten connectivity between the amygdala and the ventral attention network [Monk et al., 2008]. We found strength decrease of the negative connectivity between right amygdala and the ventral attention network in individuals with high anxiety. This association may underlie the intrinsic functional architecture of the neural mechanism of enhanced stimulus‐driven attention or salience attention in individuals with high levels of trait anxiety.
Intriguingly, we observed an interaction between trait anxiety and sex for the ventral attention network regarding its intrinsic functional connectivity with the right amygdala. Specifically, the above association between ventral attention network connectivity with the amygdala and trait anxiety was only detectable in females. The prevalence and vulnerability of anxiety or anxiety disorders are higher in females than males, implying gender‐specific neural mechanisms [McLean and Anderson, 2009]. The interaction between sex and trait anxiety was previously observed for amygdala activation to fearful faces, indicating different sensitivity in responses to threatening stimuli for men and women [Dickie and Armony, 2008]. Fractional anisotropy of uncinate fasciculus, the white matter fiber connecting the amygdala and prefrontal cortex, was positively correlated with trait anxiety in males but not in females [Montag et al., 2012]. The authors speculated that this fiber was only involved in social threat processing in males. Both the amygdala and the ventral lateral prefrontal cortex covaried with task performance in the context of emotion distraction in females not males, which may indicate that women and men employed the emotion system and executive system in distinct manners [Iordan et al., 2013]. In this study, functional connectivity between the amygdala and the ventral attention network was different across males and females, and its relationship with trait anxiety was also discrepant. This difference most likely reflects different sensitivity and resilience to threat‐related stimuli during salience attention processes for men and women, implying an important role of the connection between amygdala and the ventral attention network in gender‐specific modulation of anxiety‐related attention processes.
The present findings showed that left and right amygdala separately coupled with different networks related to anxiety levels. Previous meta‐analysis studies found more activation in the left amygdala than the right amygdala in emotion processes [Baas et al., 2004; Sergerie et al., 2008], favoring a left dominance. This is also supported by findings that trait anxiety was predicted by structural connectivity and volume of left amygdala [Baur et al., 2012]. However, previous study revealed that functional connectivity patterns between bilateral amygdala and the whole brain were similar [Roy et al., 2009]. Our vertex‐wise analysis also demonstrated such an anxiety correlational profile dominated by the left amygdala. Further investigations are warranted to directly examine the lateralization of the anxiety‐associated amygdala connectivity with the specific brain network.
Similar to the network‐wise findings, vertex‐wise anxiety‐iFC association analysis revealed that higher level of the trait anxiety was associated with stronger iFC between the left amygdala and the sensorimotor network (the right posterior central gyrus). Beyond this consistent finding, this region also showed distinct correlational profile of trait anxiety in males and females, which was not detected by the network‐wise method. The left dorsal lateral prefrontal cortex (frontoparietal network) associated its weaker iFC with the left amygdala to higher level of trait anxiety. With vertex‐wise method, much more gender–anxiety interactions were detected for both state and trait anxiety within the right posterior central cortex (sensorimotor network) and the right inferior parietal cortex (ventral attention network). In summary, as demonstrated by our work, the network‐wise and vertex‐wise approaches together lead to shared or distinct findings, indicating that these two analytic strategies are complementary due to their nature of different spatial scales.
Our findings on associations between anxiety and the cortical connectivity of amygdala were derived from a large sample of healthy participants across an adult lifespan. The age‐related changes of these functional connectivity profiles were separated from the connectivity–anxiety associations by modeling age as a covariate of interest. Outcomes of these analyses revealed a linearly decreasing lifespan trajectory of overall amygdala cortical connectivity and a linearly increasing lifespan trajectory of the attention network connectivity with the amygdala. Although previous studies have assessed amygdala functional connectivity across samples of different age ranges [Gabard‐Durnam et al., 2014; Gee et al., 2014, 2013; Swartz et al., 2014a; Winecoff et al., 2011], the present work represents the first study of amygdala connectivity across the lifespan. Anxiety has been related to amygdala functional connectivity in early childhood [Qin et al., 2014]. The analytic strategy used here enabled the identification of common anxiety–connectivity associations across all different stages in the adult lifespan. Beyond the scope of this work, a comprehensive mapping of the lifespan trajectory of the amygdala connectivity profiles will be performed in our future work.
Several limitations should be considered in interpreting our findings. Reliable and accurate segmentation of the human amygdala is a challenging topic. The manual tracing is the gold standard, although this method can be highly time‐consuming for a large sample. We thus employed an automatic segmentation procedure in FreeSurfer, which has been demonstrated to have high test–retest reliability [Jovicich et al., 2009] and be promising for functional connectivity studies [Bickart et al., 2012]. Global signal regression at the individual level has been a controversial methodology for a long time and was recently highlighted with use avoidance in functional connectomics [Saad et al., 2012, 2013]. Accordingly, we achieved the global brain standardization via regression of the global mean connectivity at the group level to enable examinations of both global mean cortical connectivity and network‐specific connectivity of the human amygdala. This mean‐based regression method can avoid introducing artifactual relationships with the global mean [Jiang et al., 2015b; Yan et al., 2013]. Finally, the amygdala is consisted of several sub‐regions that interact with regions derived from different brain networks [Bickart et al., 2012; Roy et al., 2009]. Given the consideration of accuracy of further segmenting the amygdala in such a small size, we take it as a whole organization to analysis. Meanwhile, this field faces a challenge of getting an existing age‐dependent parcellation of the human amygdala, which is fundamental for the current work on the lifespan data. We might advance the use of high‐solution imaging sequence and individualized amygdala parcellation using highly sampled retest data [Zuo et al., 2014] to address this issue in the future.
CONCLUSIONS
Intrinsic functional connectivity between amygdala and multiple cortical networks exhibit anxiety‐related changes across the human adult lifespan. High‐level trait anxiety is predicted by low‐level global amygdala connectivity. State and trait anxiety are differentiable according to their amygdala functional connectivity profiles whereas the trait anxiety showed more extensive relationships of the amygdala connectivity across the brain networks. Somatomotor and dorsal attention networks demonstrated anxiety‐related changes of connectivity with the left amygdala, whereas the frontoparietal control and ventral attention networks had such changes of connectivity with the right amygdala. These findings confirmed that both low‐level sensory networks and high‐level associative networks contribute to individual differences in anxiety behaviors by their connectivity with the human amygdala.
ACKNOWLEDGMENTS
The funding agents had no further role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All authors declared no conflict of interest.
REFERENCES
- Andreatta M, Glotzbach‐Schoon E, Muhlberger A, Schulz SM, Wiemer J, Pauli P (2015): Initial and sustained brain responses to contextual conditioned anxiety in humans. Cortex 63:352–363. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS (2004): Lateralization of amygdala activation: A systematic review of functional neuroimaging studies. Brain Res Brain Res Rev 45:96–103. [DOI] [PubMed] [Google Scholar]
- Baur V, Hanggi J, Jancke L (2012): Volumetric associations between uncinate fasciculus, amygdala, and trait anxiety. BMC Neurosci 13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur V, Hanggi J, Langer N, Jancke L (2013): Resting‐state functional and structural connectivity within an insula‐amygdala route specifically index state and trait anxiety. Biol Psychiatry 73:85–92. [DOI] [PubMed] [Google Scholar]
- Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC (2012): Intrinsic amygdala‐cortical functional connectivity predicts social network size in humans. J Neurosci 32:14729–14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijsterbosch J, Smith S, Forster S, John OP, Bishop SJ (2014): Resting state correlates of subdimensions of anxious affect. J Cogn Neurosci 26:914–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ (2007): Neurocognitive mechanisms of anxiety: An integrative account. Trends Cogn Sci 11:307–316. [DOI] [PubMed] [Google Scholar]
- Bishop SJ (2009): Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci 12:92–98. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD (2004): State anxiety modulation of the amygdala response to unattended threat‐related stimuli. J Neurosci 24:10364–10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Buckholtz JW, Avery SN, Zald DH (2010): A unique role for the human amygdala in novelty detection. Neuroimage 50:1188–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS (2011): Development of anxiety: The role of threat appraisal and fear learning. Depress Anxiety 28:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Suway JG, Clementi MA, Fox NA, Pine DS, Bar‐Haim Y (2015): Neural changes with attention bias modification for anxiety: A randomized trial. Soc Cogn Affect Neurosci 10:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J (2013): Optimizing the order of operations for movement scrubbing: Comment on Power et al. Neuroimage 76:436–438. [DOI] [PubMed] [Google Scholar]
- Chung MK, Worsley KJ, Nacewicz BM, Dalton KM, Davidson RJ (2010): General multivariate linear modeling of surface shapes using SurfStat. Neuroimage 53:491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett D, Bachman S, Mather M (2014): Age‐related reduced prefrontal‐amygdala structural connectivity is associated with lower trait anxiety. Neuropsychology 28:631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Repovs G, Anticevic A (2014): The frontoparietal control system: A central role in mental health. Neuroscientist 20:652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie EW, Armony JL (2008): Amygdala responses to unattended fearful faces: Interaction between sex and trait anxiety. Psychiatry Res 162:51–57. [DOI] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J (2004): Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron 44:1043–1055. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD (2009): Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry 66:1361–1372. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD (2007): Functional neuroimaging of anxiety: A meta‐analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164:1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG (2007): Anxiety and cognitive performance: Attentional control theory. Emotion 7:336–353. [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Yacoub E. 2012. The rapid development of high speed, resolution and precision in fMRI. NeuroImage: 720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME (2006): Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 103:10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard‐Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Hare T, Tottenham N (2014): The development of human amygdala functional connectivity at rest from 4 to 23 years: A cross‐sectional study. Neuroimage 95:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Biswal BB, Kelly C, Stark DE, Margulies DS, Shehzad Z, Uddin LQ, Klein DF, Banich MT, Castellanos FX, Milham MP (2011): Low frequency fluctuations reveal integrated and segregated processing among the cerebral hemispheres. Neuroimage 54:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard‐Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Flannery J, Lumian DS, Fareri DS, Caldera C, Tottenham N (2014): Maternal buffering of human amygdala‐prefrontal circuitry during childhood but not during adolescence. Psychol Sci 25:2067–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N (2013): A developmental shift from positive to negative connectivity in human amygdala‐prefrontal circuitry. J Neurosci 33:4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B (2009): Accurate and robust brain image alignment using boundary‐based registration. Neuroimage 48:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB (2013): Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nat Rev Neurosci 14:488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, Kasper S, Lanzenberger R (2011): Reduced resting‐state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage 56:881–889. [DOI] [PubMed] [Google Scholar]
- Hamm LL, Jacobs RH, Johnson MW, Fitzgerald DA, Fitzgerald KD, Langenecker SA, Monk CS, Phan KL (2014): Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biol Mood Anxiety Disord 4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattingh CJ, Ipser J, Tromp SA, Syal S, Lochner C, Brooks SJ, Stein DJ (2012): Functional magnetic resonance imaging during emotion recognition in social anxiety disorder: An activation likelihood meta‐analysis. Front Hum Neurosci 6:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano F, Nakamura M, Asami T, Uehara K, Yoshida T, Roppongi T, Otsuka T, Inoue T, Hirayasu Y (2009): Smaller amygdala is associated with anxiety in patients with panic disorder. Psychiatry Clin Neurosci 63:266–276. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, Buckner RL (2012): Individual differences in amygdala‐medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. J Neurosci 32:18087–18100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordan AD, Dolcos S, Denkova E, Dolcos F (2013): Sex differences in the response to emotional distraction: An event‐related fMRI investigation. Cogn Affect Behav Neurosci 13:116–134. [DOI] [PubMed] [Google Scholar]
- Irle E, Barke A, Lange C, Ruhleder M (2014): Parietal abnormalities are related to avoidance in social anxiety disorder: A study using voxel‐based morphometry and manual volumetry. Psychiatry Res 224:175–183. [DOI] [PubMed] [Google Scholar]
- Jiang L, Xu T, He Y, Hou XH, Wang J, Cao XY, Wei GX, Yang Z, He Y, Zuo XN (2015a): Toward neurobiological characterization of functional homogeneity in the human cortex: Regional variation, morphological association and functional covariance network organization. Brain Struct Funct 220:2485–2507. [DOI] [PubMed] [Google Scholar]
- Jiang L, Xu Y, Zhu XT, Yang Z, Li HJ, Zuo XN (2015b): Local‐to‐remote cortical connectivity in early‐ and adulthood‐onset schizophrenia. Transl Psychiatry 5:e566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, Pacheco J, Albert M, Killiany R, Blacker D, P Maguire, D Rosas, N Makris, R Gollub, A Dale, BC Dickerson, B Fischl (2009): MRI‐derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage 46:177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ (2011a): Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex 21:1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ (2011b): The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behav Brain Res 223:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ (2009): The structural integrity of an amygdala‐prefrontal pathway predicts trait anxiety. J Neurosci 29:11614–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziol LF, Barker LA, Joyce AW, Hrin S (2014): Structure and function of large‐scale brain systems. Appl Neuropsychol Child 3:236–244. [DOI] [PubMed] [Google Scholar]
- Mathews A, Mackintosh B, Fulcher EP (1997): Cognitive biases in anxiety and attention to threat. Trends Cogn Sci 1:340–345. [DOI] [PubMed] [Google Scholar]
- Mattavelli G, Sormaz M, Flack T, Asghar AU, Fan S, Frey J, Manssuer L, Usten D, Young AW, Andrews TJ (2014): Neural responses to facial expressions support the role of the amygdala in processing threat. Soc Cogn Affect Neurosci 9:1684–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CP, Anderson ER (2009): Brave men and timid women? A review of the gender differences in fear and anxiety. Clin Psychol Rev 29:496–505. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Nugent AC, Drevets WC, Dickstein DP, Leibenluft E, Ernst M, Charney D, Pine DS (2005): Selective reduction in amygdala volume in pediatric anxiety disorders: A voxel‐based morphometry investigation. Biol Psychiatry 57:961–966. [DOI] [PubMed] [Google Scholar]
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Ugurbil K (2010): Multiband multislice GE‐EPI at 7 tesla, with 16‐fold acceleration using partial parallel imaging with application to high spatial and temporal whole‐brain fMRI. Magn Reson Med 63:1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A, Egner T, Monti JM, Mesulam MM (2009): Search for a threatening target triggers limbic guidance of spatial attention. J Neurosci 29:10563–10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Blair RJ, Chen G, Charney DS, Ernst M, Pine DS (2006): Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry 163:1091–1097. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Chen G, McClure‐Tone EB, Ernst M, Pine DS (2008): Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry 65:568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C, Reuter M, Weber B, Markett S, Schoene‐Bake JC (2012): Individual differences in trait anxiety are associated with white matter tract integrity in the left temporal lobe in healthy males but not females. Neuroscience 217:77–83. [DOI] [PubMed] [Google Scholar]
- Muehlhan M, Lueken U, Wittchen HU, Kirschbaum C (2011): The scanner as a stressor: Evidence from subjective and neuroendocrine stress parameters in the time course of a functional magnetic resonance imaging session. Int J Psychophysiol 79:118–126. [DOI] [PubMed] [Google Scholar]
- Nooner KB, Colcombe SJ, Tobe RH, Mennes M, Benedict MM, Moreno AL, Panek LJ, Brown S, Zavitz ST, Li Q, S Sikka, D Gutman, S Bangaru, RT Schlachter, SM Kamiel, AR Anwar, CM Hinz, MS Kaplan, AB Rachlin, S Adelsberg, B Cheung, R Khanuja, C Yan, CC Craddock, V Calhoun, W Courtney, M King, D Wood, CL Cox, AM Kelly, A Di Martino, E Petkova, PT Reiss, N Duan, D Thomsen, B Biswal, B Coffey, MJ Hoptman, DC Javitt, N Pomara, JJ Sidtis, HS Koplewicz, FX Castellanos, BL Leventhal, MP Milham (2012): The NKI‐Rockland sample: A model for accelerating the pace of discovery science in psychiatry. Front Neurosci 6:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD (2002): Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14:1215–1229. [DOI] [PubMed] [Google Scholar]
- Patriat R, Molloy EK, Meier TB, Kirk GR, Nair VA, Meyerand ME, Prabhakaran V, Birn RM (2013): The effect of resting condition on resting‐state fMRI reliability and consistency: A comparison between resting with eyes open, closed, and fixated. Neuroimage 78:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE (2005): Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 48:175–187. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE (2015): Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage 105:536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Young CB, Duan X, Chen T, Supekar K, Menon V (2014): Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol Psychiatry 75:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth WT. (2005): Physiological markers for anxiety: Panic disorder and phobias. Int J Psychophysiol 58(2‐3):190–198. [DOI] [PubMed] [Google Scholar]
- Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C, Benson B, Castellanos FX, Milham MP, Pine DS, M Ernst (2013): Intrinsic functional connectivity of amygdala‐based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry 52:290–299 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP (2009): Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45:614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW (2012): Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connect 2:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Reynolds RC, Jo HJ, Gotts SJ, Chen G, Martin A, Cox RW (2013): Correcting brain‐wide correlation differences in resting‐state FMRI. Brain Connect 3:339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL (2008): The role of the amygdala in emotional processing: A quantitative meta‐analysis of functional neuroimaging studies. Neurosci Biobehav Rev 32:811–830. [DOI] [PubMed] [Google Scholar]
- Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques P, Marques F, Palha JA, Cerqueira JJ, Sousa N (2013): Stress Impact on Resting State Brain Networks. PLoS One 8:e66500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. (1970): Manual for the State‐Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; pp 1–23. [Google Scholar]
- Swartz JR, Carrasco M, Wiggins JL, Thomason ME, Monk CS (2014a): Age‐related changes in the structure and function of prefrontal cortex‐amygdala circuitry in children and adolescents: A multi‐modal imaging approach. Neuroimage 86:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Phan KL, Angstadt M, Fitzgerald KD, Monk CS. (2014b): Dynamic changes in amygdala activation and functional connectivity in children and adolescents with anxiety disorders. Dev Psychopathol 26(Pt 2):1305–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, Zorumski CF, Lenze EJ (2012): Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci 35:527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Mogg K, Bradley BP, Mai X, Ernst M, Pine DS, Monk CS (2008): Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biol Psychol 79:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiani V, Price ET, Schultz RT (2014): Unseen fearful faces promote amygdala guidance of attention. Soc Cogn Affect Neurosci 9:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE (2010): Exploring the brain network: A review on resting‐state fMRI functional connectivity. Eur Neuropsychopharmacol 20:519–534. [DOI] [PubMed] [Google Scholar]
- Winecoff A, Labar KS, Madden DJ, Cabeza R, Huettel SA (2011): Cognitive and neural contributors to emotion regulation in aging. Soc Cogn Affect Neurosci 6:165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Yang Z, Jiang LL, Xing XX, Zuo XN (2015): A connectome computation system for discovery science of brain. Sci Bulletin 60:86–95. [Google Scholar]
- Yan CG, Craddock RC, Zuo XN, Zang YF, Milham MP (2013): Standardizing the intrinsic brain: Towards robust measurement of inter‐individual variation in 1000 functional connectomes. Neuroimage 80:246–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Chang C, Xu T, Jiang LL, Handwerker DA, Castellanos FX, Milham MP, Bandettini PA, Zuo XN (2014a): Connectivity trajectory across lifespan differentiates the precuneus from the default network. Neuroimage 89:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Xu Y, Xu T, Hoy CW, Handwerker DA, Chen G, Northoff G, Zuo XN, Bandettini PA (2014b): Brain network informed subject community detection in early‐onset schizophrenia. Sci Rep 4:5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, B Fischl, H Liu, RL Buckner (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MP, Scannell JW, Burns GA, Blakemore C (1994): Analysis of connectivity: Neural systems in the cerebral cortex. Rev Neurosci 5:227–250. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Xing XX (2011): Effects of non‐local diffusion on structural MRI preprocessing and default network mapping: Statistical comparisons with isotropic/anisotropic diffusion. PLoS One 6:e26703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Xing XX (2014): Test‐retest reliabilities of resting‐state FMRI measurements in human brain functional connectomics: A systems neuroscience perspective. Neurosci Biobehav Rev 45:100–118. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Xu T, Jiang L, Yang Z, Cao XY, He Y, Zang YF, Castellanos FX, Milham MP (2013): Toward reliable characterization of functional homogeneity in the human brain: Preprocessing, scan duration, imaging resolution and computational space. Neuroimage 65:374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Anderson JS, Bellec P, Birn RM, Biswal BB, Blautzik J, Breitner JC, Buckner RL, Calhoun VD, Castellanos FX, A Chen, B Chen, J Chen, X Chen, SJ Colcombe, W Courtney, RC Craddock, A Di Martino, HM Dong, X Fu, Q Gong, KJ Gorgolewski, Y Han, Y He, Y He, E Ho, A Holmes, XH Hou, J Huckins, T Jiang, Y Jiang, W Kelley, C Kelly, M King, SM LaConte, JE Lainhart, X Lei, HJ Li, K Li, K Li, Q Lin, D Liu, J Liu, X Liu, Y Liu, G Lu, J Lu, B Luna, J Luo, D Lurie, Y Mao, DS Margulies, AR Mayer, T Meindl, ME Meyerand, W Nan, JA Nielsen, D O'Connor, D Paulsen, V Prabhakaran, Z Qi, J Qiu, C Shao, Z Shehzad, W Tang, A Villringer, H Wang, K Wang, D Wei, GX Wei, XC Weng, X Wu, T Xu, N Yang, Z Yang, YF Zang, L Zhang, Q Zhang, Z Zhang, Z Zhang, K Zhao, Z Zhen, Y Zhou, XT Zhu, MP Milham (2014): An open science resource for establishing reliability and reproducibility in functional connectomics. Sci Data 1:140049. [DOI] [PMC free article] [PubMed] [Google Scholar]


