ABSTRACT
Objective
To evaluate the clinical accuracy of the IONA® test for aneuploidy screening.
Methods
This was a multicenter blinded study in which plasma samples from pregnant women at increased risk of trisomy 21 underwent cell‐free DNA analysis utilizing the IONA test. For each sample, the IONA software generated a likelihood ratio and a maternal age‐adjusted probability risk score for trisomies 21, 18 and 13. All results from the IONA test were compared against accepted diagnostic karyotyping.
Results
A total of 442 maternal samples were obtained, of which 437 had test results available for analysis and assessment of clinical accuracy. The IONA test had a detection rate of 100% for trisomies 21 (n = 43; 95% CI, 87.98–100%), 18 (n = 10; 95% CI, 58.72–100%) and 13 (n = 5; 95% CI, 35.88–100%) with cut‐offs applied to likelihood ratio (cut‐off > 1 considered high risk for trisomy) and probability risk score incorporating adjustment for maternal age (cut‐off ≥ 1/150 considered high risk for trisomy). The false‐positive rate (FPR) was 0% for trisomies 18 and 13 with both analysis outputs. For trisomy 21, a FPR of 0.3% was observed for the likelihood ratio, but became 0% with adjustment for maternal age.
Conclusion
This study indicates that the IONA test is suitable for trisomy screening in a high‐risk screening population. The result‐interpretation feature of the IONA software should facilitate wider implementation, particularly in local laboratories, and should be a useful addition to the current screening methods for trisomies 21, 18 and 13. Copyright © 2015 ISUOG. Published by John Wiley & Sons Ltd.
Keywords: aneuploidy, diagnosis, fetal DNA, non‐invasive, pregnancy, screening, sequencing, trisomy
INTRODUCTION
Non‐invasive prenatal testing (NIPT), using methods of cell‐free DNA (cfDNA) analysis, provides the most accurate assessment for fetal trisomies 21, 18 and 13, compared with screening methods that combine maternal age, and ultrasound and serum biochemical markers. In a recent meta‐analysis, the weighted pooled detection rates for trisomies 21, 18 and 13 in singleton pregnancies were 99.2%, 96.3% and 91.0%, for false‐positive rates (FPRs) of 0.09%, 0.13% and 0.13%, respectively1.
Recent NIPT technologies are based predominantly on next‐generation sequencing (NGS)2, 3. This enables rapid and effective clinical testing and commonly uses either the Illumina (Illumina, Inc., San Diego, CA, USA) or Thermo Fisher Scientific (Thermo Fisher Scientific, Waltham, MA, USA) platforms. The key advantages of an ion semiconductor sequencing platform (Ion Proton™, Thermo Fisher Scientific) are the rapid sequencing speed and fast turnaround time with low upfront capital and operating costs. These are important considerations in the context of screening for aneuploidy, particularly if wider implementation is envisaged. The IONA® test (Premaitha Health plc, Manchester, UK) uses the Ion Proton sequencing platform and has a turnaround time, from the start of sample processing to a result, of 3 days. It uses bioinformatics interpretation to determine the relative number of chromosomal copies, which enables the detection of a fetal aneuploidy. Published reviews indicate that various technical issues have the potential to affect the accuracy of sequences obtained by NGS, such as frequent insertion and deletion errors and difficulties posed by homopolymer tracts of identical bases4, 5, 6, 7, 8. The algorithms developed for use in IONA software (Premaitha Health plc) are designed to overcome such issues.
The main aim of this study was to investigate the accuracy of the IONA test in the discrimination between euploid pregnancies and those affected by fetal trisomies 21, 18 and 13.
METHODS
In this multicenter blinded study, the screening accuracy of the IONA test by NGS was evaluated using plasma samples obtained from pregnant women considered to be at higher risk of fetal trisomy on conventional screening and who underwent definitive karyotyping by invasive testing. The likelihood ratio, age‐adjusted probability (risk) and sensitivity and specificity in the detection of trisomies 21, 18 and 13 were calculated.
Study population and sample collection
The study was conducted using blood samples collected from pregnant women under appropriate institutional and ethical approval. Written informed consent was obtained from all participants. Study participants were recruited between April 2008 and November 2014 from six hospital centers in England. Eligible patients were at least 18 years of age, with a singleton or twin pregnancy of at least 10 weeks' gestation and a clinical indication for an invasive procedure (screen‐positive result from conventional aneuploidy screening such as the combined test, quadruple test, fetal structural anomaly on ultrasound examination or advanced maternal age). Exclusion criteria included higher‐order multiple pregnancy (triplets or more), known mosaicism, partial trisomy or translocations, fetal demise, disappearing twin, malignancy or known aneuploidy in the pregnancy.
Peripheral maternal blood (20 mL) was collected into standard ethylenediaminetetraacetic acid blood collection tubes. Demographic information obtained at subject recruitment included maternal age, height, weight and gestational age. Data on pregnancy outcome in all participants were obtained from each center. The results of cfDNA testing were not made available to participants. The study samples analyzed included all trisomy 21, 18 and 13 samples available that met the eligibility criteria, plus unaffected samples that were selected to reflect the prevalence of trisomy 21 observed during sample collection in the high‐risk population (i.e. 1:9). The unaffected samples were selected using random sampling techniques from all eligible samples.
Cell‐free DNA preparation and sequencing
The procedural workflow for the IONA test was divided into the following steps. Day 1: (1) plasma separation and cfDNA extraction (3.5 h), (2) automated library construction (8 h), and (3) automated emulsion polymerase chain reaction (PCR) (library amplification and enrichment; 13.5 h, running overnight). Day 2: (4) Library DNA sequencing (5 h), and (5) automated data analysis (3 h). This results in a processing time of 35 h for up to 32 samples, in multiples of eight, for optimal throughput.
Within 8 h of blood draw, the maternal blood samples were centrifuged at 1600 g for 10 min and the plasma fraction was removed and stored at −20 °C or below. On receipt of the samples at the study laboratory they were centrifuged for a further 10 min at 16 000 g to remove any cellular material, before being frozen at −80 °C. Prior to analysis, the plasma sample was defrosted and centrifuged for 1 min at 3000 g. The resultant cfDNA was isolated, extracted and sequenced from 1.0–2.5 mL of maternal plasma, which contained both maternal and fetal cfDNA. The cfDNA was extracted using an automated DNA extraction platform (QIASymphony, Qiagen, Hilden, Germany) and used for DNA library preparation using the IONA Library Preparation Kit in combination with an automated liquid handling platform (Sciclone, Perkin Elmer, Inc., Waltham, MA, USA). The IONA run control DNA library was included in order to ascertain the validity of the sequencing run performed. The run control material consisted of a DNA library of two cell lines combined to give an input concentration of 10% trisomy 21. Semiconductor whole‐genome sequencing was performed using Ion Chef™ and Ion Proton™ systems (Thermo Fisher Scientific), according to the manufacturer's instructions, as a multiplex of eight samples in addition to the IONA run control (four sequencing runs per day were performed to achieve the 32‐sample optimal throughput).
Sample processing and analysis were performed by appropriately trained and experienced operators who were blinded to karyotype results throughout the study. The IONA test results were compared to the results from invasive testing or birth outcome.
Data analysis
Overview of process
Sample information, including anonymized patient data, was entered via the IONA software interface. A unique sample identifier (‘test instance ID’) was allocated and used to identify the passage of a sample through the system, linked to demographic and patient data entered previously. This was used to identify each sample against its corresponding barcoded adapter in the sequencing multiplex. The multiplex data were presented to the IONA software, which executed the main bioinformatics processing pipeline, generating the test report ‘run and sample validity’ information.
Data generation and bioinformatics processing
Execution of the main bioinformatics pipeline proceeded as follows. For each sequencing run of eight samples, multiplexed sequence reads were retrieved from the sequencing platform in the form of an unmapped BAM file. The multiplexed assembly of reads was initially subject to a barcode classification step, in which barcoded 5′ adapters were identified and matched against a predefined set, in order to separate the multiplex into reads against individual samples for further processing. Following an early filtering step to remove a small number of very short reads, fragments were mapped to the ‘hg19’ human genome reference using a gap‐tolerant read‐alignment module. Post‐filtering of alignment results was then carried out to remove duplicate reads arising in PCR stages of the test workflow, determined as those whose 5′ end mapped to the reference at the same position as any other read. Fragments determined to have aligned uniquely in the genome reference were then binned according to autosome origin, with the resulting counts subject to a calibration step to correct sequencing coverage bias correlated to GC base pair content. Finally, the resulting fragment count data were used as input to a set of mixture models that incorporate distributions of expected values under both trisomy‐affected and ‐unaffected hypotheses for trisomies 13, 18 and 21 tests. Each model generated a test likelihood ratio that was then used, together with maternal age‐derived prior probability of trisomy, to quantify the probability of each trisomy, taking into account both age and the corresponding DNA test result. In this way the IONA software interprets the results, so the pipeline generates a final risk result without the need for further local bioinformatics analysis.
The IONA software also performed internal validity checks. Following the generation of per‐autosome fragment counts, the ‘run validity’ check took place. This step first isolated fragments derived from sequencing the run control, and then compared the proportion of counts from these fragments that aligned against chromosome 21 using a reference range determined previously as part of a specification‐setting study. If the proportion met the reference criteria, the ‘run validity’ check passed.
Separate validity checks were also performed for each sample. These ensured that the aligned fragment count was sufficient for the likelihood mixture model to be used, and that the fraction of cfDNA in the sample that was fetal in origin was sufficient for a result to be reported; fetal fraction was quantified using a custom bioinformatics method developed in‐house.
Statistical analysis
Sensitivity and specificity values were reported based on the predefined cut‐offs for likelihood ratio and probability. Point estimates have been provided together with 95% CIs (Clopper–Pearson binomial proportions method). For the purposes of the IONA test, a ‘trisomy test result’ is considered to be ‘positive’ if the likelihood ratio result is > 1; this threshold value follows implicitly from the conventional statistical interpretation of a likelihood ratio value. With regards to age‐adjusted probability (risk) of trisomy, a probability ≥ 1 in 150 was considered to be a screen‐positive (high‐risk) result, as used in the UK National Health Service (NHS)9.
RESULTS
In this study 442 samples were processed with the IONA test; however, five of these did not meet the validity criteria applied by the IONA software (two samples did not have sufficient DNA fragment counts and three samples did not contain sufficient fetal DNA) and were excluded from subsequent analysis. Reports were generated by the IONA software for all remaining 437 samples.
Median maternal age at testing was 35 (range, 18–55) years and median gestational age was 15 + 3 (range, 11 + 0 to 36 + 4) weeks. Median maternal weight was 66 (range, 46.0–151.5) kg and median maternal height was 165 (range, 152–189) cm.
Data from the reference method (amniocentesis, chorionic villus sampling (CVS) or birth outcome) were available for all 437 cases, and identified 43 fetuses with trisomy 21 (one twin), 10 with trisomy 18 (one twin), five with trisomy 13 and 379 that were unaffected by trisomies 13, 18 or 21 (nine twins) (Figure 1).
Figure 1.
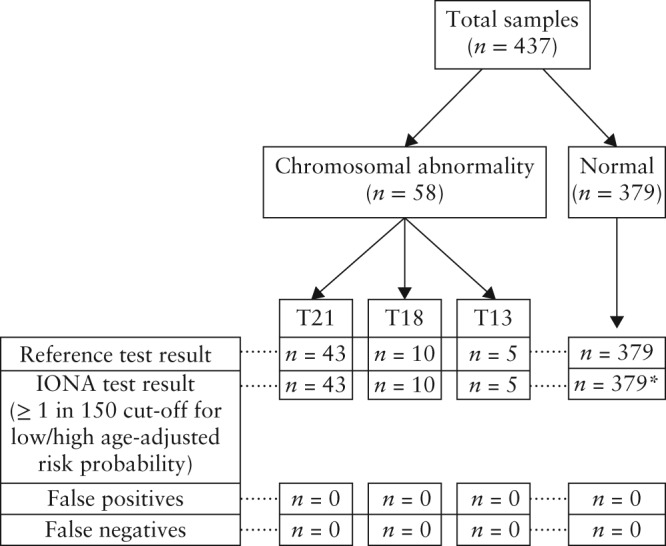
Patient flow through the study showing results of the reference test (amniocentesis, chorionic villus sampling or birth outcome) and the IONA® test. *One sample was positive for trisomy (T) 21 using just the likelihood ratio but was normal once adjusted for age‐related prior risk.
Table 1 shows the summary of cfDNA analysis results for the age‐adjusted likelihood ratio probability model for each trisomy screened for compared with the results of the reference method (amniocentesis, CVS or birth outcome). Table 2 shows the sensitivity (detection rate) and specificity measures for the likelihood ratio model and the maternal age‐adjusted probability model. Plots of the age‐adjusted probability (risk score) distribution for each trisomy are shown in Figures 2, 3 and 4, showing all valid samples analyzed.
Table 1.
Likelihood ratio and maternal age‐adjusted probability of the IONA® test, tabulated against results of the reference method (amniocentesis, chorionic villus sampling or birth outcome), for detection of trisomies 21, 18 and 13 in 437 maternal serum samples, using a probability risk cut‐off of ≥ 1 in 150
| Reference method | |||
|---|---|---|---|
| IONA test result | Unaffected | Trisomy | Total |
| Likelihood ratio | |||
| Unaffected | 378 | 0 | 378 |
| Trisomy 21 | 1 | 43 | 44 |
| Trisomy 18 | 0 | 10 | 10 |
| Trisomy 13 | 0 | 5 | 5 |
| Total | 379 | 58 | 437 |
| Age‐adjusted probability | |||
| Unaffected | 379 | 0 | 379 |
| Trisomy 21 | 0 | 43 | 43 |
| Trisomy 18 | 0 | 10 | 10 |
| Trisomy 13 | 0 | 5 | 5 |
| Total | 379 | 58 | 437 |
Table 2.
Sensitivity and specificity of likelihood ratio and maternal age‐adjusted probability of the IONA® test for detection of trisomies 21, 18 and 13 using amniocentesis, chorionic villus sampling or birth outcome as the reference method
| Likelihood ratio > 1 | Maternal age‐adjusted probability ≥ 1:150 | |||
|---|---|---|---|---|
| Trisomy | Sensitivity (% (95% CI))* | Specificity (% (95% CI))* | Sensitivity (% (95% CI))* | Specificity (% (95% CI))* |
| Trisomy 21 | 100.00 (87.98–100.00) | 99.75 (98.59–99.99) | 100.00 (87.98–100.00) | 100.00 (98.60–100.00) |
| Trisomy 18 | 100.00 (58.72–100.00) | 100.00 (98.71–100.00) | 100.00 (58.72–100.00) | 100.00 (98.71–100.00) |
| Trisomy 13 | 100.00 (35.88–100.00) | 100.00 (98.72–100.00) | 100.00 (35.88–100.00) | 100.00 (98.72–100.00) |
Any sample not affected by trisomy 21, 18 or 13 was considered unaffected.
Figure 2.
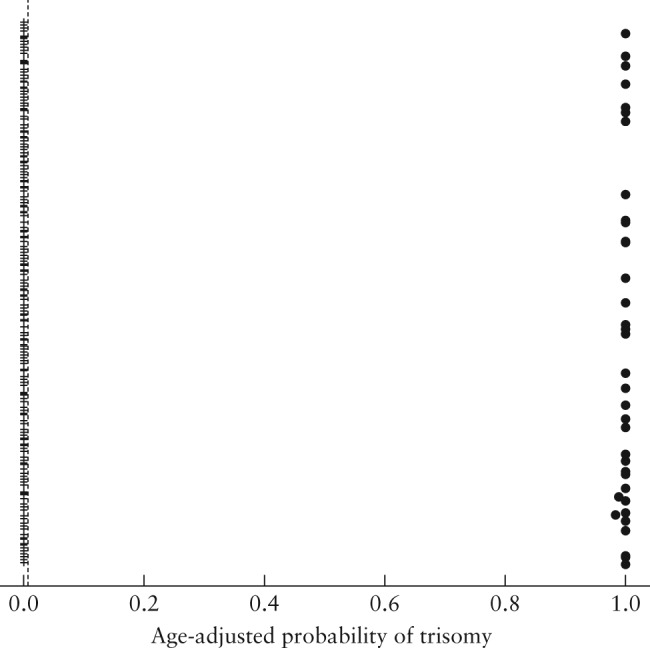
Maternal age‐adjusted probability (risk score) for trisomy 21, computed at blood‐draw date, in 437 maternal serum samples that were tested using the IONA® test.  , unaffected sample;
, unaffected sample;  , trisomy‐21 sample;
, trisomy‐21 sample;  , cut‐off (1 in 150).
, cut‐off (1 in 150).
Figure 3.
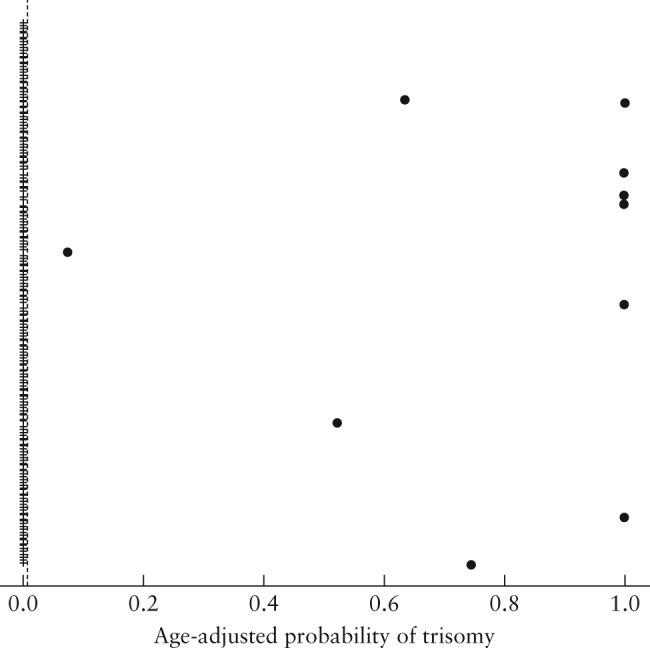
Maternal age‐adjusted probability (risk score) for trisomy 18, computed at blood‐draw date, in 437 maternal serum samples that were tested using the IONA® test.  , unaffected sample;
, unaffected sample;  , trisomy‐18 sample;
, trisomy‐18 sample;  , cut‐off (1 in 150).
, cut‐off (1 in 150).
Figure 4.
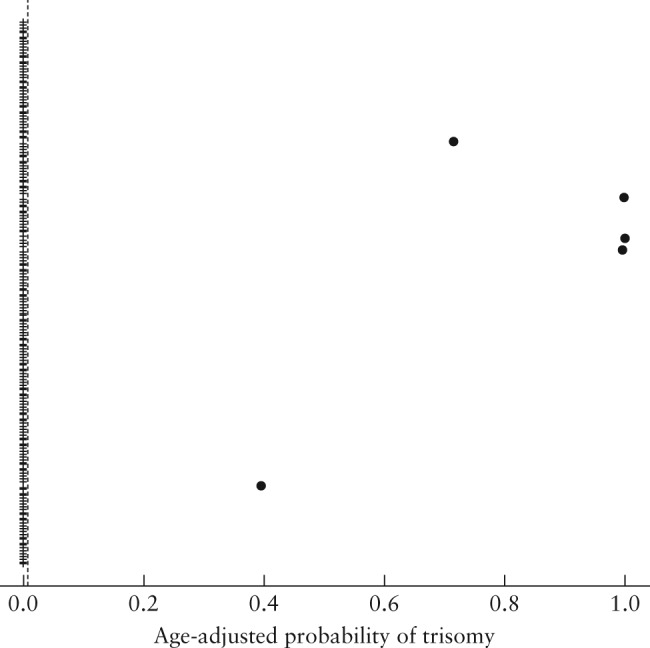
Maternal age‐adjusted probability (risk score) for trisomy 13, computed at blood‐draw date, in 437 maternal serum samples that were tested using the IONA® test.  , unaffected sample;
, unaffected sample;  , trisomy‐13 sample;
, trisomy‐13 sample;  , cut‐off (1 in 150).
, cut‐off (1 in 150).
Initial analysis, which produces the likelihood ratio, detected one result for a trisomy 21 that was discordant with that of the reference method. However, following adjustment for maternal age‐related prior risk, the probability risk score was concordant with the reference‐method result, at the UK screening cut‐off of ≥ 1 in 150. Similar results were observed at low/high risk cut‐offs of 1 in 50 and 1 in 250. At a cut‐off of 1 in 500, one false‐positive case of trisomy 21 was observed, demonstrating that the higher the cut‐off used the more likely a false‐positive result will occur.
A detection rate of 100% was demonstrated for trisomies 21 (n = 43; 95% CI, 87.98–100%), 18 (n = 10; 95% CI 58.72 to 100%) and 13 (n = 5; 95% CI, 35.88–100%) for both analysis outputs. The specificity for the likelihood ratio calculations were 99.75% (95% CI, 98.59–99.99%) for trisomy 21, 100% (95% CI, 98.71–100%) for trisomy 18 and 100% (95% CI, 98.72–100%) for trisomy 13, resulting in an FPR of 0.3%, 0% and 0%, respectively. Once the results were adjusted to give the final age‐adjusted probability (risk score), the specificity was 100% (with respective 95% CI, 98.60–100%, 98.71–100% and 98.72–100%) with a 0% FPR for all three trisomies. The failure rate was 1.1%, comprising low fetal fraction in 0.7% and low counts in 0.4%. There were no false positives or false negatives for trisomies 21, 18 and 13 identified by the IONA test when compared to the outcome by the reference method of amniocentesis, CVS or birth outcome.
DISCUSSION
We have demonstrated that the screening performance of the IONA test for the detection of trisomies 21, 18 and 13 is similar to that of other cfDNA screening techniques for these common aneuploidies1.
The typical turnaround time for the IONA test is 3 days, from plasma sample collection to report generation. This short turnaround time may reduce the levels of parental anxiety and allow decision‐making in relation to pregnancy options and management.
This study adds to a growing body of literature regarding the use of semiconductor sequencing NGS in cfDNA prenatal aneuploidy screening7, 8. The key advantages of this technology include lower upfront and operating costs as well as a reduced turnaround time, which are important considerations in the developing high‐throughput screening programs. Liao et al. recently described a detection rate of more than 98% for trisomies 21, 18 and 13 using the Ion Proton semiconductor sequencer8, whilst a feasibility study by Jeon et al. reported 100% positive and negative predictive values for both trisomies 21 and 187. Our study extends the number of samples analyzed using semiconductor sequencing and demonstrates that when this approach is coupled with a defined work flow and the error‐tolerant algorithms incorporated in the IONA software the result is an accurate cfDNA methodology appropriate for trisomy screening.
In contrast to studies reported previously7, 8, we report likelihood ratio and age‐adjusted screening results rather than a simple Z‐score. This approach has a number of advantages: the likelihood ratio is computed from a mixture model that takes into account distributions of sequence‐derived indicator values for both the unaffected and trisomy‐affected subpopulations. This value is, therefore, more informative in the overall statistical context of the test than a Z‐score, which is generally referenced only to a single assumed normal distribution of unaffected sequence values. Additionally, the use of a mixture model framework affords flexibility in including additional factors derived from the sequencing workflow, which improve the fit of the model to empirical distributions by adapting to workflow conditions and thereby improving the accuracy of the result.
Since the model operates in a similar statistical framework to that of biomarker‐based screening tests, the likelihood ratio result is directly compatible with analysis methods and models already well established in the screening community. This then allows Bayesian statistical methods to be employed to combine the cfDNA‐specific result with any prior information available on trisomy risk, such as age‐related risk or first‐trimester combined screening, in order to give a more accurate overall patient‐specific risk estimate10. Although a cut‐off of ≥ 1/150 was applied for high/low risk as used by the UK NHS9, other cut‐offs can be applied using the IONA software, to account for country or clinician preference.
Strengths and limitations of the study
In addition to the generation of a more patient‐specific risk estimate, other strengths of the study include the large number of trisomy 21 samples, robust blinding to fetal karyotype during sample processing and analysis, high ascertainment of pregnancy outcome and the reporting and break down of no‐result (‘failure’) rate.
It could be argued that one of the main limitations of this study is that all women recruited were at increased risk of aneuploidy. However, it is recognized that the accuracy of cfDNA analysis relies on the precision of the assay and the fetal fraction rather than on prevalence of aneuploidy in the study population11, 12. As the reported performance of NIPT in aneuploidy screening is similar between studies reporting on high‐risk and low‐risk populations2, 11, 13, 14, 15, 16, we believe that the results of this study, in terms of the screening performance of the IONA test, are also relevant to a population at low risk of aneuploidy.
Although the IONA test predicted accurately the aneuploidy status in the 11 twin pregnancies studied, the overall number of twin pregnancies in this study was small. The literature on NIPT in twin pregnancy remains limited, with only six studies published so far17, 18, 19, 20, 21, 22. There is a need for further evaluation of NIPT in twins as multiple pregnancies carry a higher risk of chromosomal anomalies with a higher FPR when using existing screening tests for aneuploidy and therefore a greater likelihood of being offered invasive testing with a higher risk of procedure‐related complications of invasive testing23, 24.
The design of this study intentionally excluded any known mosaicism or vanishing twin to enable direct evaluation of screening performance of the IONA test for detection of fetal aneuploidy and to make our study comparable to similar published studies25, 26, 27. It would be entirely appropriate to ensure future studies are on unselected samples without such exclusions.
The IONA test does not screen for sex‐chromosome aneuploidy, a practice that remains controversial due to ethical concerns, the mild phenotype in the majority of cases, high reported failure rate, low positive predictive value and high FPR, and the high rate of mosaicism in affected pregnancies28, 29, 30.
In conclusion, this study demonstrates that the IONA test can accurately predict the presence of trisomies 21, 18 and 13. We have demonstrated the principle of using a similar statistical framework to biomarker‐based screening tests by reporting the likelihood ratio, which allows the cfDNA‐specific result to be combined with prior information on trisomy risk, such as maternal age, in order to give a patient‐specific risk estimate. The IONA test system provides a simple, relatively inexpensive, complete CE‐marked in‐vitro diagnostic system for trisomies 21, 18 and 13 that includes automated result interpretation that facilitates implementation in local laboratories. The relatively short turnaround time has the potential to reduce the stress of prospective parents awaiting results.
ACKNOWLEDGMENTS
We thank Kypros Nicolaides and Liona Poon for supporting this study with the provision of extra samples from the King's College London sample collection.
Disclosure
M.F., R.H., R.M. and W.D. are employees of Premaitha Health plc; the other authors are Principal Investigators for the protocol under which samples were collected.
The copyright line for this article was changed on 22 April 2016 after original online publication.
REFERENCES
- 1. Gil MM, Quezada MS, Revello R, Akolekar R, Nicolaides KH. Analysis of cell‐free DNA in maternal blood in screening for fetal aneuploidies: updated meta‐analysis. Ultrasound Obstet Gynecol 2015; 45: 249–266. [DOI] [PubMed] [Google Scholar]
- 2. Bianchi DW, Parker RL, Wentworth J, Madankumar R, Saffer C, Das AF, Craig JA, Chudova DI, Devers PL, Jones KW, Oliver K, Rava RP, Sehnert AJ; CARE Study Group . DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med 2014; 370: 799–808. [DOI] [PubMed] [Google Scholar]
- 3. Nicolaides KH, Syngelaki A, Gil M, Atanasova V, Markova D. Validation study of maternal blood cell‐free DNA testing by targeted sequencing of single‐nucleotide polymorphisms at chromosomes 13, 18, 21, X, and Y. Prenat Diagn 2013; 33: 575–579. [DOI] [PubMed] [Google Scholar]
- 4. Loman NJ, Misra RV, Dallman TJ, Constantinidou C, Gharbia SE, Wain J, Pallen MJ. Performance comparison of benchtop high‐throughput sequencing platforms. Nat Biotechnol 2012; 30: 434–439. [DOI] [PubMed] [Google Scholar]
- 5. Quail MA, Smith M, Coupland P, Otto TD, Harris SR, Connor TR, Bertoni A, Swerdlow HP, Gu Y. A tale of three next generation sequencing platforms: comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genomics 2012; 13: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu L, Li Y, Li S, Hu N, He Y, Pong R, Lin D, Lu L, Law M. Comparison of next‐generation sequencing systems. J Biomed Biotech 2012; 2012: 251364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jeon YJ, Zhou Y, Li Y, Guo Q, Chen J, Quan S, Zhang A, Zheng H, Zhu X, Lin J, Xu H, Wu A, Park SG, Kim BC, Joo HJ, Chen H, Bhak J. The feasibility study of non‐invasive fetal trisomy 18 and 21 detection with semiconductor sequencing platform. PLoS One 2014; 9: e110240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liao C, Yin AH, Peng CF, Fu F, Yang JX, Li R, Chen YY, Luo DH, Zhang YL, Ou YM, Li J, Wu J, Mai MQ, Hou R, Wu F, Luo H, Li DZ, Liu HL, Zhang XZ, Zhang K. Non‐invasive prenatal diagnosis of common aneuploidies by semiconductor sequencing. Proc Natl Acad Sci U S A 2014; 111: 7415–7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Health Service (NHS). http://patient.info/doctor/prenatal‐screening‐for‐downs‐syndrome [Accessed 23 November 2015].
- 10. Wright D, Wright A, Nicolaides Kypros H. A unified approach to risk assessment for fetal aneuploidies. Ultrasound Obstet Gynecol 2015; 45: 48–54. [DOI] [PubMed] [Google Scholar]
- 11. Nicolaides KH, Syngelaki A, Ashoor G, Birdir C, Touzet G. Non‐invasive prenatal testing for fetal trisomies in a routinely screened first‐trimester population. Am J Obstet Gynecol 2012; 207: 374.e1–374.e6. [DOI] [PubMed] [Google Scholar]
- 12. Ashoor G, Syngelaki A, Wagner M, Birdir C, Nicolaides KH. Chromosome‐selective sequencing of maternal plasma cell‐free DNA for first‐trimester detection of trisomy 21 and trisomy 18. Am J Obstet Gynecol 2012; 206: 322.e1–322.e5. [DOI] [PubMed] [Google Scholar]
- 13. Song Y, Liu C, Qi H, Zhang Y, Bian X, Liu J. Non‐invasive prenatal testing of fetal aneuploidies by massively parallel sequencing in a prospective Chinese population. Prenat Diagn 2013; 33: 700–706. [DOI] [PubMed] [Google Scholar]
- 14. Comas C, Echevarria M, Rodriguez MA, Prats P, Rodriguez I, Serra B. Initial experience with non‐invasive prenatal testing of cell‐free DNA for major chromosomal anomalies in a clinical setting. J Matern Fetal Neonatal Med 2014; 12: 1–6. [DOI] [PubMed] [Google Scholar]
- 15. Quezada MS, Gil MM, Francisco C, Oròsz G, Nicolaides KH. Screening for trisomies 21, 18 and 13 by cell‐free DNA analysis of maternal blood at 10–11 weeks' gestation and the combined test at 11–13 weeks. Ultrasound Obstet Gynecol 2015; 45: 36–41. [DOI] [PubMed] [Google Scholar]
- 16. Zhang H, Gao Y, Jiang F, Meili F, Yuan Y, Guo Y, Zhu Z, Lin M, Liu Q, Tian Z, Zhang H, Chen F, Lau KL, Zhao L, Yi X, Yin Y, Wang W. Non‐invasive prenatal testing for trisomies 21, 18 and 13: clinical experience from 146 958 pregnancies. Ultrasound Obstet Gynecol 2015; 45: 530–538. [DOI] [PubMed] [Google Scholar]
- 17. Canick JA, Kloza EM, Lambert‐Messerlian GM, Haddow JE, Ehrich M, van den Boom D, Bombard AT, Deciu C, Palomaki GE. DNA sequencing of maternal plasma to identify Down syndrome and other trisomies in multiple gestations. Prenat Diagn 2012; 32: 730–734. [DOI] [PubMed] [Google Scholar]
- 18. Lau TK, Jiang F, Chan MK, Zhang H, Lo PSS, Wang W. Non‐invasive prenatal screening of fetal Down syndrome by maternal plasma DNA sequencing in twin pregnancies. J Matern Fetal Neonatal Med 2013; 26: 434–437. [DOI] [PubMed] [Google Scholar]
- 19. Huang X, Zheng J, Chen M, Zhao Y, Zhang C, Liu L, Xie W, Shi S, Wei Y, Lei D, Xu C, Wu Q, Guo X, Shi X, Zhou Y, Liu Q, Gao Y, Jiang F, Zhang H, Su F, Ge H, Li X, Pan X, Chen S, Chen F, Fang Q, Jiang H, Lau TK, Wang W. Non‐invasive prenatal testing of trisomies 21 and 18 by massively parallel sequencing of maternal plasma DNA in twin pregnancies. Prenat Diagn 2014; 34: 335–340. [DOI] [PubMed] [Google Scholar]
- 20. Gil MM, Quezada MS, Bregant B, Syngelaki A, Nicolaides KH. Cell‐free DNA analysis for trisomy risk assessment in first‐trimester twin pregnancies. Fetal Diagn Ther 2014; 35: 204–211. [DOI] [PubMed] [Google Scholar]
- 21. Grömminger S, Yagmur E, Erkan S, Nagy S, Schöck U, Bonnet J, Smerdka P, Ehrich M, Wegner RD, Wera Hofmann W, Stumm M. Fetal aneuploidy detection by cell‐free DNA sequencing for multiple pregnancies and quality issues with vanishing twins. J Clin Med 2014; 3: 679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bevilacqua E, Gil MM, Nicolaides KH, Ordoñez E, Cirigliano V, Dierickx H, Willems PJ, Jani JC. Performance of screening for aneuploidies by cell free DNA analysis of maternal blood in twin pregnancies. Ultrasound Obstet Gynecol 2015; 45: 61–66. [DOI] [PubMed] [Google Scholar]
- 23. National Collaborating Centre for Women's and Children's Health . Multiple pregnancy. The management of twin and triplet pregnancies in the antenatal period. NICE Clinical Guidelines, No. 129. RCOG Press: London, 2011. [PubMed]
- 24. Sparks AB, Oliphant A, Song K. Non‐invasive chromosomal evaluation (NICE) study: results of a multi‐center prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol 2012; 207: 137.e1–137.e8. [DOI] [PubMed] [Google Scholar]
- 25. Bianchi DW, Platt LD, Goldberg JD, Abuhamad AZ, Sehnert AJ, Rava RP. Genome‐wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol 2012; 119: 890–901. [DOI] [PubMed] [Google Scholar]
- 26. Pergament E, Cuckle H, Zimmermann B, Banjevic M, Sigurjonsson S, Ryan A, Hall MP, Dodd M, Lacroute P, Stosic M, Chopra N, Hunkapiller N, Prosen DE, McAdoo S, Demko Z, Siddiqui A, Hill M, Rabinowitz M. Single‐nucleotide polymorphism‐based non‐invasive prenatal screening in a high‐risk and low‐risk cohort. Obstet Gynecol 2014; 124: 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palomaki GE, Deciu C, Kloza EM, Lambert‐Messerlian GM, Haddow JE, Neveux LM, Ehrich M, van den Boom D, Bombard AT, Grody WW, Nelson SF, Canick JA. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: an international collaborative study. Genet Med 2012; 14: 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tartaglia NR, Howell S, Sutherland A, Wilson R, Wilson L. A review of trisomy X (47,XXX). Orphanet J Rare Dis 2010; 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hook EB, Warburton D. Turner syndrome revisited: review of new data supports the hypothesis that all viable 45X cases are cryptic mosaics with a rescue cell line, implying an origin by mitotic loss. Hum Genet 2014; 133: 417–424. [DOI] [PubMed] [Google Scholar]
- 30. Wang Y, Chen Y, Tian F, Zhang J, Song Z, Wu Y, Han X, Hu W, Ma D, Cram D, Cheng W. Maternal mosaicism is a significant contributor to discordant sex chromosomal aneuploidies associated with non‐invasive prenatal testing. Clin Chem 2014; 60: 251–259. [DOI] [PubMed] [Google Scholar]


