Abstract
Mesenchymal stem cells are known to exert immunomodulatory effects in inflammatory diseases. Immuneregulatory cells lead to progressive joint destruction in rheumatoid arthritis (RA). Proinflammatory cytokines, such as tumour necrosis factor α (TNF‐α) and interleukins (ILs) are the main players. Here, we studied progenitor cells from RA cartilage (RA‐CPCs) that are positive for IL‐17 receptors to determinate the effects of inflammation on their chondrogenic potenial. IL‐17A/F reduced the chondrogenic potential of these cells via the upregulation of RUNX2 protein and enhanced IL‐6 protein and MMP3 mRNA levels. Blocking antibodies against IL‐17 positively influenced their repair potential. Furthermore, treating the RA‐CPCs with the anti‐human IL‐17 antibody secukinumab or the anti‐TNF‐α antibody adalimumab reduced the proinflammatory IL‐6 protein level and positively influenced the secretion of anti‐inflammatory IL‐10 protein. Additionally, adalimumab and secukinumab in particular reduced RUNX2 protein to promote chondrogenesis. The amelioration of inflammation, particularly via IL‐17 antagonism, might be a new therapeutic approach for enhancing intrinsic cartilage repair mechanisms in RA patients.
Keywords: Cartilage Chondrogenesis ⋅ Chondrogenic progenitor cells ⋅ Rheumatoid arthritis ⋅ Interleukin 17 (IL‐17)
Introduction
Rheumatoid arthritis (RA) is the most common chronic inflammatory joint disease and leads to progressive cartilage and bone destruction. In total, 35% of RA patients are fully or partially incapable of working within 10 years of developing the disease 1. The use of novel biologicals, such as TNF‐α blockers (e.g., infliximab, adalimumab, and etanercept), has substantially improved clinical outcomes 2. However, serious side effects of these biological therapies and the nonresponse rate of up to 30–40% 3 reveal the need to develop novel treatment strategies.
RA pathogenesis consists primarily of chronic synovial inflammation with aggressive pannus tissue overgrowth of the cartilage, resulting in joint destruction. Fibroblast‐like synoviocytes (FLSs) and synovial macrophages have been shown to synthesise proinflammatory cytokines, such as TNF‐α, IL‐1β, IL‐6 4. Furthermore, CD4+ T cells accumulate within the RA synovium 5, and two primary subsets of the CD4+ T‐cell lineage (i.e., interferon‐producing T helper cell type 1 (TH1) and type 17 (TH17) cells, which are characterized by the production of IL‐17) were identified 6.
TH17 T cells produce IL‐17A, IL‐17F, and heterodimeric IL‐17A/IL‐17F. These cytokines act via IL‐17RA and IL‐17RC receptors 7. Increased IL‐17 levels were detected in the synovial fluid of patients with RA compared to patients with osteoarthritis (OA) 8. The results from studies in animals and humans led to the development of biological therapies that aim to inhibit the IL‐17 pathway via either IL‐17A monoclonal antibodies (i.e., secukinumab and ixekizumab) or blockade of the IL‐17 receptor via brodalumab 9. IL‐17 intensifies the chronic inflammatory process by inducing proinflammatory cytokines 10. Furthermore, IL‐17/TH17 may support cartilage degradation and bone resorption during RA 11. However, the molecular mechanisms of IL‐17‐induced cartilage breakdown have not been elucidated in human RA. Here, we demonstrate the influence of IL‐17 on chondrogenic progenitor cells in human RA.
Results
Molecular characteristics of RA‐CPCs
In RA, the typical histopathological signs of synovial pannus tissue invading the hyaline cartilage (Fig. 1A, left), as well as surface fissures and chondrocyte clusters (Fig. 1A, upper right) were found. The chondrocytes in situ were positive for IL‐17RA (Fig. 1A, lower right) and IL‐17RC (data not shown). Explant cultures of cartilage tissue from the RA patients after 10 days released migrating cells in vitro (Fig. 1B, left), which were positive for SOX9 (Fig. 1B, middle) and RUNX2 proteins (Fig. 1B, right) in immunocytochemistry, as well as in the Western blots (Fig. 1C), indicating the chondro‐osteogenic nature of these cells. In agreement with results obtained for OA 12, the cells were positive for the mesenchymal stem cell (MSC) markers CD29, CD44, CD73, CD90, and CD105 (Fig. 1D, left) and negative for the hematopoietic markers CD34 (Fig. 1D, left) and CD45 (data not shown). Because samples from RA patients are rare due to reduced surgical interventions (drug therapies are more effective), the RA‐CPCs were hTERT immortalized. The immortalized cells exhibited characteristics similar to the cells in P1 prior to immortalization, as shown here by their CD patterns (Fig. 1D, right). The SOX9 and RUNX2 mRNA patterns and the migration and differentiation capacities of the immortalized cells were identical to those of the P1 cells (data not shown). RT‐qPCR analysis of the RA‐CPCs revealed the chondro‐osteotypic expression of SOX9, COL2A1, RUNX2, and COL1A1 mRNAs and baseline expression levels of MMP3, TIMP1, TIMP3, and IL‐6 mRNAs (Fig. 1E). Significantly more RA‐CPCs migrated against a gradient of platelet‐derived growth factor (PDGF) in comparison to controls without foetal calf serum (Fig. 1F).
Figure 1.
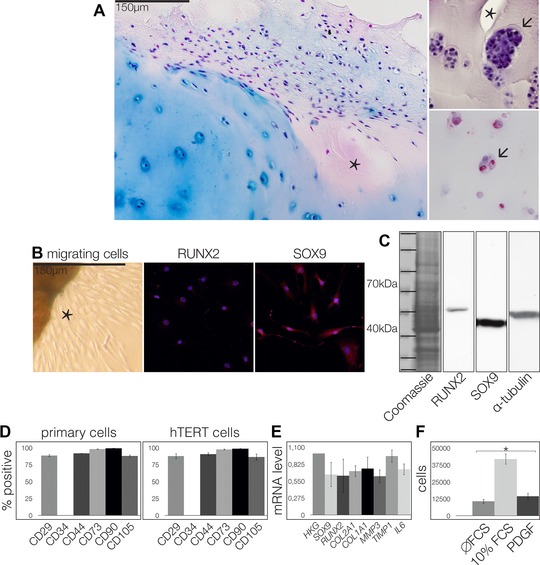
Characteristics of RA‐CPCs. (A, left) Ten samples were analyzed by immunohistochemistry. RA pannus tissue infiltrating the cartilage (asterisks). (A, upper right) Surface fissures (asterisk) and cluster formation (arrow). (A, lower right) Chondrocytes positive for IL‐17RA (arrow). Bar = 150 μm. Explant culture of RA cartilage (asterisk) exhibiting migrating cells. (B) Thousand cells were stained for RUNX2 and SOX9 and analyzed by immunocytochemistry. (A and B) Images are representative of ten independent experiments. (C) Fifty thousand cells were analyzed by Western blot. Blots are representative of three independent experiments. (D) FACS analysis of CD29, CD34, CD44, CD73, CD90, and C105 in primary and hTERT immortalized cells. (E) SOX9, RUNX2, COL2A1, COL1A1, MMP3, TIMP1, TIMP3, and IL‐6 mRNA levels were analyzed by PCR. (F) Ten thousand cells were subjected and migrated toward a gradient of PDGF, and quantified by counting. (D–F) Data are shown as mean ± SD (n = 3 samples) and are representative of three independent experiments. *p ≤ 0.05.
Differentiation potential of RA‐CPCs
After adipogenic induction, the RA‐CPCs were positive for PPARγ mRNA, and Oil Red staining also indicated that the cells were adipocytes (Fig. 2A). After osteogenic differentiation, the cells became positive for alkaline phosphatase (AP) and osteocalcin in immunocytochemistry, demonstrating an osteoblastic lineage (Fig. 2B). During chondrogenic differentiation in 3D culture with TGFβ3, the RA‐CPCs proliferated to form cell clusters, which were positive for COL2A1 and SOX9 proteins (Fig. 2C, upper panel). When subjected to 3D culture without TGFβ3, the RA‐CPCs did not proliferate and did not secrete COL2A1 or SOX9 proteins (Fig. 2C, lower panel). The RA‐CPCs treated with TGFβ3 exhibited significantly less RUNX2, COL1A1, and ADAMTS5 mRNA (Fig. 2D). These experiments were performed using three primary RA‐CPCs from three different patients and hTERT‐RA‐CPCs. The results were similar between these two groups (data not shown).
Figure 2.
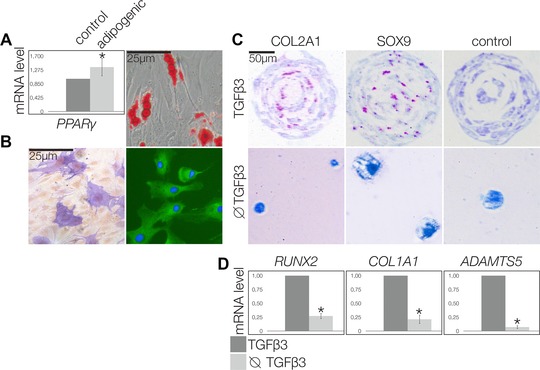
Multilineage differentiation potential of RA‐CPCs. (A) PPARγ mRNA level of 100,000 cells was measured by PRC 1000 cells stained histochemistry and analyzed by lightmicroscopy, and Oil Red positive cells (A, right) after adipogenic differentiation and AP‐positive (B, left) and osteocalcin‐positive cells (B, right) after osteogenic differentiation. (C) Chondrogenic differentiation in 3D culture with TGFβ3 promoted cluster formation among cells positive for COL2A1 (C, upper left) and SOX9 (C, upper and lower middle). The controls exhibited no reactions (C, upper right). Chondrogenic differentiation without TGFβ3 resulted in round cells that were negative for COL2A2 (C, lower left) and SOX9 (C, lower middle). (A–C) Images are representative of three independent experiments. (D) Chondrogenic differentiation of 10,000 cells with TGFβ3. RUNX2, COL1A1, and ADAMTS5 mRNA levels were measured by PCR. Graphs are shown as mean ± SD (n = 9 samples) and are representative of three independent experiments. *p ≤ 0.05.
The effects of IL‐17 on RA‐CPCs
RT‐qPCR was used to quantify the mRNA of IL‐17RA and IL‐17RC in RA‐CPCs in comparison to the housekeeping gene (Fig. 3A). The encoded protein levels were also examined via Western blots (Fig. 3B) and immunocytochemistry (Fig. 3C). RA‐CPCs were also positive for IL‐6 (Fig. 3D, upper) and IL‐10 staining revealed a pronounced perinuclear localization via immunocytochemistry (Fig. 3D, lower). IL‐17 was not detected in RA‐CPCs by RT‐qPCR or flow cytometry, neither at the mRNA nor the protein level (data not shown). When the RA‐CPCs were stimulated with IL‐17A/F protein, MMP3 mRNA increased, in contrast, blocking IL‐17 reduced MMP3 mRNA (Fig. 3E) but increased TIMP1 (Fig. 3F) and TIMP3 (Fig. 3G) mRNA expression. IL‐6 mRNA was significantly enhanced by IL‐17. Blocking IL‐17 significantly decreased IL‐6 mRNA levels (Fig. 3H). These results indicated that IL‐17 fosters the expression of proinflammatory mediators in RA‐CPCs. Therefore, we investigated the influence of biologicals, such as the TNF‐α antagonist (i.e., adalimumab) and the monoclonal anti‐IL‐17 antibody secukinumab. Treatment with adalimumab or secukinumab significantly reduced the amount of RUNX2 protein (Fig. 3K), while only secukinumab significantly reduced the amount of IL‐6 protein (Fig. 3L). Western blots of low molecular weight enriched medium from cells treated with adalimumab or secukinumab (Fig. 3M) exhibited significantly more IL‐10 protein than the controls (Fig. 3N). This finding is consistent with the perinuclear localization of IL‐10 protein that was observed via immunofluorescence staining (Fig. 3D) and indicates a higher rate of IL‐10 secretion.
Figure 3.
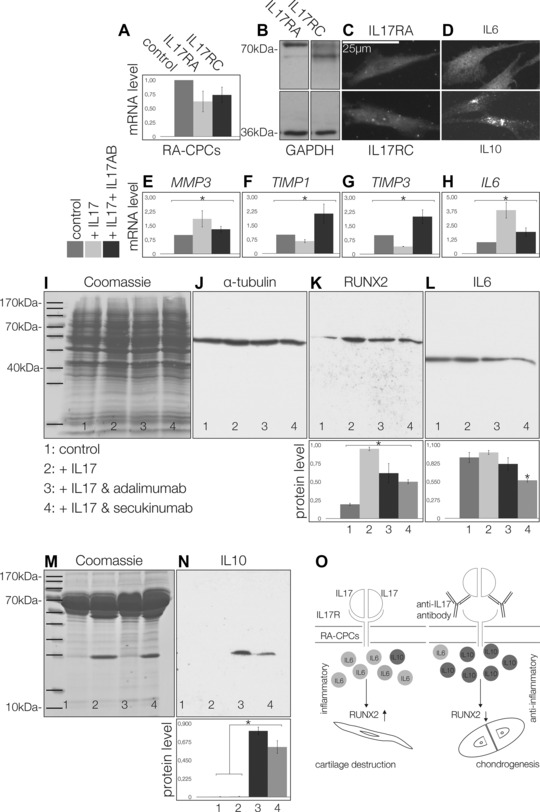
The effects of IL‐17 on RA‐CPCs: expression of IL‐17RA and IL‐17RC receptor mRNA (A), Western blots (B), and immuncytochemistry (C) of the IL‐17RA and IL‐17RC proteins. RA‐CPCs are also positive for IL‐6 (D, upper), as well as IL‐10 (D, lower). MMP3 mRNA was significantly upregulated by IL‐17 (E). TIMP1 (F) and TIMP3 (G) were significantly downregulated. However, IL‐6 was significantly upregulated by IL‐17 (H). In contrast, blocking IL‐17 (IL‐17AB) produced the opposite effects (E–H). Western blot analysis (I, J), secukinumab significantly reduced the amount of RUNX2 (K), as well as the amount of IL‐6 (L). Western blots of low molecular weight enriched medium from RA‐CPCs treated with adalimumab or secukinumab (M) exhibited significantly more IL‐10 than the controls (N). (O) Role of IL‐17 on RA‐CPCs, with IL‐17 promoting IL‐6, while antagonizing IL‐17 promotes IL‐10 and chondrogenesis. All figures are representative of at least three different experiments (n = 3). Significant differences (*p ≤ 0.05); error bars denote the means ± SD.
Discussion
RA‐CPCs are progenitor cells influenced via IL‐17
Several researchers have demonstrated the presence of progenitor cells in both healthy and OA cartilage samples 12, 13, 14. Here, we report that osteochondrogenic progenitor cells, which we named RA‐CPCs, reside in the cartilage tissue from patients with RA. The characterization of these cells revealed several similarities to the previously described OA‐CPCs, as they are migratory, multipotent, and MSC marker‐positive.
Further analyses of the RA‐CPCs revealed two main differences compared to OA‐CPCs. First, the RA‐CPCs expressed IL‐17 receptors and IL‐17 upregulated IL‐6. An IL‐17‐induced increase in IL‐6 has previously been demonstrated in chondrocytes and FLS in RA 15 and plays an important role in T helper cell differentiation toward TH17 T cells 16. Furthermore, in agreement with previous studies of chondrocytes and FLS 17, MMP3 mRNA synthesis was significantly enhanced in RA‐CPCs after IL‐17 exposure.
The chondrogenic potential of RA‐CPCs
Second, the RA‐CPCs exhibited a reduced chondrogenic potential compared to OA‐CPCs. Blocking IL‐17 restored the chondrogenic potential of these cells, as well as reduced MMP3 mRNA and IL‐6 protein synthesis. This IL‐17‐induced upregulation of proinflammatory cytokines and matrix metalloprotease, as well as the reduction of the chondrogenic potential in RA‐CPCs, highlights the importance of cytokines and inflammatory mediators for the regenerative capacity of progenitor cells 18. A similar inhibition of chondrogenic differentiation via IL‐17 has been described for human MSCs 19. Due to the beneficial effects of blocking IL‐17, we studied secukinumab, a human anti‐IL‐17A monoclonal antibody showing promising results in phase I and II clinical studies 20.
Through the upregulation of secreted, not intracellular IL‐10 protein from RA‐CPCs, secukinumab may support the anti‐inflammatory and chondroprotective effects of IL‐10 in RA 21. In agreement with previous results of TNF‐α blockade 22, IL‐10 upregulation was also demonstrated for adalimumab. Most important, secukinumab revealed a significant downregulation of the osteogenic transcription factor RUNX2 in RA‐CPCs thereby promoting chondrogenesis. Overall, secukinumab may enhance the intrinsic repair mechanisms of articular cartilage in RA by reducing RUNX2 expression and enhancing the production of the anti‐inflammatory cytokine IL‐10.
In summary, RA‐CPCs are modulated by IL‐17. Antagonizing IL‐17 activity can enhance the anti‐inflammatory IL‐10 secretion and restore the chondrogenic potential of RA‐CPCs (Fig. 3O). IL‐17 blockade might be a new therapeutic option for enhancing the intrinsic repair mechanisms of cartilage in patients with RA.
Materials and methods
Patients
Cartilage specimens were obtained from ten patients with RA (four males, six females, mean age 67.1, 20% IgM‐RF+, DAS28 CRP 4.72 (where DAS is disease activity score), Number (Nr) of swollen joints 3.8, disease duration 13.6 years, with 10 on DMARD and 6 also on steroids) and 15 age‐ and sex‐matched patients with OA (seven males, eight females, mean age 64.2, IgM‐RF−) after knee arthroplasty. The Institutional Review Board at the University Medical Center at Goettingen University approved the study (No. 5/12/2009). Written informed consent was obtained, and the patients with RA met the criteria established by the American College of Rheumatology 23. The clinical disease activity was assessed based on clinical and laboratory findings according to the DAS28 CRP 24. Histological evaluation was performed as previously described 12.
Cell culture
Cartilage specimens from the intermediate zone were prepared without touching the synovial or pannus tissue. Explant cultures were generated 12. Cells from ten different patients were investigated. Immortalization of RA‐CPCs was performed as described in 25.
Antibodies
Anti‐type II collagen (CIIC1, Developmental Studies Hybridoma Bank), anti‐SOX9 (H‐90, sc‐2095, 1:1000), anti‐RUNX2 (M‐70, sc‐10758, 1:1000), anti‐IL‐10 (#E‐10, sc‐8438, 1:500), and anti‐IL‐6 (E‐4, sc‐28343, 1:500) were all from SC Biotechnology. Anti‐osteocalcin (#BT‐6015‐15, Biotrend, 1:300); anti‐IL‐4 (#PAB16160, Abnova, 1:500); and anti‐IL‐17RA (#MAB177, 1:400), IL‐17RC (#AF2269, 1:200), and anti‐IL‐17 (#MAB317, R&D Systems) were used. Phycoerythrin (PE) coupled anti‐CD29 (#555443, 1:500) and anti‐CD73 (#562817, 1:200), and uncoupled anti‐CD44 (#561858, 1:200), anti‐CD45 (#561640, 1:200), anti‐CD34 (#345802, 1:200), and anti‐CD105‐FITC (#561443, 1:500) were all from BD Pharmingen. Anti‐CD90‐PE (#328110, Biolegend, 1:200), anti‐GAPDH (3E12, ThermoScientific, 1:1000), and anti‐α‐tubulin (T6199, 1:2000, Sigma‐Aldrich) antibodies were used.
FACS analysis
The cells were processed as previously described 5, 12, and the analysis was performed in triplicate.
Immunohisto‐(cyto)‐chemistry and Western blots
The ten samples were stained for IL‐17RA and IL‐17RC HiDefDetectionR AP mouse/rabbit system (Cell Marque). Immunofluorescence and immunoblotting were performed and quantified using the ImageJ64 program 12. Three individual blots were stained for each antibody.
Cell differentiation and migration assay
Osteogenic and adipogenic differentiation were achieved using TGFβ3 (10 ng/mL, lot #MSA3612112, R&D Systems). AP and Oil Red staining, chondrogenic differentiation, and the in vitro migration assay were performed as described 12.
IL‐17A/F stimulation of RA‐CPCs
The RA‐CPCs were stimulated with 50 ng/mL of human recombinant IL‐17A/F (#5837‐IL‐010, R&D Systems) for 48 h. Blocking experiments were performed with 100 μg/mL of the IL‐17 antibody.
Effects of adalimumab and secukinumab on RA‐CPCs
IL‐17‐treated RA‐CPCs were cultured and exposed to 10 μg/mL of adalimumab (Humira®), or 10 μg/mL of secukinumab in 2D culture. Secukinumab was kindly provided by Novartis Pharma AG.
RT‐qPCR
The primers used in this study were described previously 12 and can be found online at http://www.miosge.med.uni‐goettingen.de/de/?id=17. PPIA served as a housekeeping gene.
Statistics
Statistical Product and Service Solutions (SPSS) software version 13.0 was used. The data were tested, and the representative data are shown as the means and standard deviations of at least three different patient samples, unless stated otherwise. After testing the normality of the distribution and the homogeneity of the variances, we performed ANOVAs and post‐hoc pairwise comparisons of the mean values. The Pearson correlation coefficients were calculated to examine the relationships between parameters. A p‐value of <0.05 was considered significant (asterisks).
Conflict of interest
The authors declare that they have no conflict of interest nor did they receive financial support other than from the German Research Foundation.
Abbreviations
- AP
alkaline phosphatase
- FLSs
fibroblast‐like synoviocytes
- MSC
mesenchymal stem cell
- OA
osteoarthritis
- RA
rheumatoid arthritis
- RA‐CPCs
progenitor cells from RA cartilage
Acknowledgments
S.B. and N.M. designed the study, made substantial contributions to the data analysis and interpretation and drafted the manuscript. B.M. performed the knee arthroplasties. B.S. and S.T. performed the data acquisition and analysis and revised the manuscript. All authors read and approved the final manuscript. This work was supported by a grant from the German Research Foundation in the Priority Programme “Osteoimmunology – Immunobone” 1468, DFG, BL 1122/1‐1.
References
- 1. Young, A. , Dixey, J. , Cox, N. , Davies, P. , Devlin, J. , Emery, P. , Gallivan, S. et al, How does functional disability in early rheumatoid arthritis (RA) affect patients and their lives? Results of 5 years of follow‐up in 732 patients from the Early RA Study (ERAS). Rheumatology 2000. 39: 603–611. [DOI] [PubMed] [Google Scholar]
- 2. Finckh, A. , Liang, M. H. , van Herckenrode, C. M. and de Pablo, P. , Long‐term impact of early treatment on radiographic progression in rheumatoid arthritis: a meta‐analysis. Arthritis Rheum. 2006. 55: 864–872. [DOI] [PubMed] [Google Scholar]
- 3. Aaltonen, K. J. , Virkki, L. M. , Malmivaara, A. , Konttinen, Y. T. , Nordstrom, D. C. and Blom, M. , Systematic review and meta‐analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PLoS One 2012. 7: e30275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McInnes, I. B. and Schett, G. , The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011. 365: 2205–2219. [DOI] [PubMed] [Google Scholar]
- 5. Blaschke, S. , Middel, P. , Dorner, B. G. , Blaschke, V. , Hummel, K. M. , Kroczek, R. A. , Reich, K. et al, Expression of activation‐induced, T cell‐derived, and chemokine‐related cytokine/lymphotactin and its functional role in rheumatoid arthritis. Arthritis Rheum. 2003. 48: 1858–1872. [DOI] [PubMed] [Google Scholar]
- 6. Benedetti, G. and Miossec, P. , Interleukin 17 contributes to the chronicity of inflammatory diseases such as rheumatoid arthritis. Eur. J. Immunol. 2014. 44:339–347. [DOI] [PubMed] [Google Scholar]
- 7. Gaffen, S. L. , Structure and signalling in the IL‐17 receptor family. Nat. Rev. Immunol. 2009. 9: 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kotake, S. , Udagawa, N. , Takahashi, N. , Matsuzaki, K. , Itoh, K. , Ishiyama, S. , Saito, S. et al, IL‐17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 1999. 103: 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burmester, G. R. , Feist, E. and Dorner, T. , Emerging cell and cytokine targets in rheumatoid arthritis. Nat. Rev. Rheumatol. 2014. 10: 77–88. [DOI] [PubMed] [Google Scholar]
- 10. Kim, H. R. , Cho, M. L. , Kim, K. W. , Juhn, J. Y. , Hwang, S. Y. , Yoon, C. H. , Park, S. H. et al, Up‐regulation of IL‐23p19 expression in rheumatoid arthritis synovial fibroblasts by IL‐17 through PI3‐kinase‐, NF‐kappaB‐ and p38 MAPK‐dependent signalling pathways. Rheumatology 2007. 46: 57–64. [DOI] [PubMed] [Google Scholar]
- 11. Lubberts, E. , Joosten, L. A. , van de Loo, F. A. , van den Gersselaar, L. A. and van den Berg, W. B. , Reduction of interleukin‐17‐induced inhibition of chondrocyte proteoglycan synthesis in intact murine articular cartilage by interleukin‐4. Arthritis Rheum. 2000. 43: 1300–1306. [DOI] [PubMed] [Google Scholar]
- 12. Koelling, S. , Kruegel, J. , Irmer, M. , Path, J. R. , Sadowski, B. , Miro, X. and Miosge, N. , Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell 2009. 4: 324–335. [DOI] [PubMed] [Google Scholar]
- 13. Alsalameh, S. , Amin, R. , Gemba, T. and Lotz, M. , Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004. 50: 1522–1532. [DOI] [PubMed] [Google Scholar]
- 14. Dowthwaite, G. P. , Bishop, J. C. , Redman, S. N. , Khan, I. M. , Rooney, P. , Evans, D. J. , Haughton, L. et al, The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 2004. 117: 889–897. [DOI] [PubMed] [Google Scholar]
- 15. Georganas, C. , Liu, H. , Perlman, H. , Hoffmann, A. , Thimmapaya, B. and Pope, R. M. , Regulation of IL‐6 and IL‐8 expression in rheumatoid arthritis synovial fibroblasts: the dominant role for NF‐kappa B but not C/EBP beta or c‐Jun. J. Immunol. 2000. 165: 7199–7206. [DOI] [PubMed] [Google Scholar]
- 16. Kimura, A. and Kishimoto, T. , IL‐6: regulator of Treg/Th17 balance. Eur. J. Immunol. 2010. 40: 1830–1835. [DOI] [PubMed] [Google Scholar]
- 17. Shalom‐Barak, T. , Quach, J. and Lotz, M. , Interleukin‐17‐induced gene expression in articular chondrocytes is associated with activation of mitogen‐activated protein kinases and NF‐kappaB. J. Biol. Chem. 1998. 273: 27467–27473. [DOI] [PubMed] [Google Scholar]
- 18. Bernardo, M. E. and Fibbe, W. E. , Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 2013. 13: 392–402. [DOI] [PubMed] [Google Scholar]
- 19. Kondo, M. , Yamaoka, K. , Sonomoto, K. , Fukuyo, S. , Oshita, K. , Okada, Y. and Tanaka, Y. , IL‐17 inhibits chondrogenic differentiation of human mesenchymal stem cells. PLoS One 2013. 8: e79463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Genovese, M. C. , Durez, P. , Richards, H. B. , Supronik, J. , Dokoupilova, E. , Mazurov, V. , Aelion, J. A. et al, Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose‐finding, double‐blind, randomised, placebo controlled study. Ann. Rheum. Dis. 2013. 72: 863–869. [DOI] [PubMed] [Google Scholar]
- 21. van Roon, J. A. , van Roy, J. L. , Gmelig‐Meyling, F. H. , Lafeber, F. P. and Bijlsma, J. W. , Prevention and reversal of cartilage degradation in rheumatoid arthritis by interleukin‐10 and interleukin‐4. Arthritis Rheum. 1996. 39: 829–835. [DOI] [PubMed] [Google Scholar]
- 22. Baldwin, H. M. , Ito‐Ihara, T. , Isaacs, J. D. and Hilkens, C. M. , Tumour necrosis factor alpha blockade impairs dendritic cell survival and function in rheumatoid arthritis. Ann. Rheum. Dis. 2010. 69: 1200–1207. [DOI] [PubMed] [Google Scholar]
- 23. Arnett, F. C. , Edworthy, S. M. , Bloch, D. A. , McShane, D. J. , Fries, J. F. , Cooper, N. S. , Healey, L. A. et al, The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988. 31: 315–324. [DOI] [PubMed] [Google Scholar]
- 24. van der Heijde, D. M. , van 't Hof, M. A. , van Riel, P. L. , Theunisse, L. A. , Lubberts, E. W. , van Leeuwen, M. A. , van Rijswijk, M. H. et al, Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann. Rheum. Dis. 1990. 49: 916–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schminke, B. , Vom Orde, F. , Gruber, R. , Schliephake, H. , Burgers, R. , and Miosge, N. , The pathology of bone tissue during peri‐implantitis. J. Dent. Res. 2015. 94: 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]


