Abstract
Aims
Iron deficiency (ID) is highly prevalent in patients with heart failure (HF) worldwide regardless of haemoglobin levels. Results from therapeutic trials of intermittently dosed intravenous (i.v.) iron are promising in the ambulatory Caucasian population with HF with reduced left ventricular ejection fraction, although evidence is scarce in Asia. The Pilot Randomized Controlled Trial to Assess the Role of Intravenous Ferric Carboxymaltose in Asian Patients with Heart Failure aims to assess the effect of single‐dose i.v. ferric carboxymaltose (FCM) in a multi‐ethnic Asian population with HF and ID.
Methods and results
This is an open‐label, randomized, placebo‐controlled trial recruiting stabilized inpatients with decompensated HF (regardless of left ventricular ejection fraction), ID [defined as serum ferritin <300 ng/mL if transferrin saturation <20%] and haemoglobin ≤14 g/dL. Patients from two tertiary institutions were randomized in a 1:1 ratio to receive a single dose of either i.v. FCM (Ferinject®) 1000 mg or i.v. normal saline. The primary endpoint is the change in 6‐min walk distance at Weeks 4 and 12, and secondary endpoints are changes at Weeks 4 and 12 in (i) quality of life as measured by the Kansas City Cardiomyopathy Questionnaire and Visual Analogue Scale scores and (ii) New York Heart Association functional class.
Conclusions
Preliminary efficacy data on i.v. FCM therapy in Asian HF are expected from this pilot study to support larger‐scale multicentre therapeutic i.v. FCM trials within Asia.
Keywords: Heart failure, Intravenous iron
Introduction
Heart failure (HF) is a significant and worsening burden in Asia, with rising hospital admissions across the region in recent years.1, 2 Of more concern is that HF affects Asians at a younger age compared with their Western counterparts from the USA and Europe, with the average age of Asian patients being 66 years in the ADHERE International Asia Pacific Registry compared with a median age of 75 years in the US‐based ADHERE Registry and a mean age of 70 years in the European Heart Failure Survey II.3, 4 Despite their younger age, Asian patients were more symptomatic than Western patients. Furthermore, compared with HF patients in the USA, Asian patients had longer lengths of stay and higher in‐hospital mortality.3 These data highlight the huge public health burden and large unmet needs of HF in Asia. There is an urgent need for simple, accessible, effective therapies for patients with HF in Asia.
Iron deficiency (ID) has recently emerged as an important therapeutic target in HF. Beyond being the commonest cause of anaemia in patients with HF, ID is highly prevalent in HF even in the absence of anaemia. In fact, 50% of European patients with HF have coexisting ID regardless of anaemia,5, 6, 7 and the prevalence of ID in Asian patients with HF is even higher (61% overall, rising to 80% in those of Indian ethnicity).8 Furthermore, we recently showed in our multiethnic Asian HF population that ID was independently associated with impaired exercise capacity, worse quality of life, and increased risk of the composite endpoint of death or HF hospitalization, regardless of left ventricular ejection fraction (LVEF).8 These data are consistent with findings from Western HF populations9, 10, 11 and provide significant support for ID as a key therapeutic target in HF.
Prior to ID being identified as a therapeutic target in HF, anaemia was associated with poor outcomes in patients with HF and thought to be a main contributor in the pathophysiology of HF.12, 13 Indeed, several approaches to treat anaemia have been tested in clinical trials, including therapeutic blood transfusion and erythropoiesis‐stimulating agents (ESAs).14 However, correction of anaemia did not lead to improvement in survival or clinically meaningful change in quality of life and conversely increased the risk for thrombo‐embolic adverse events.15, 16 As such, emphasis has shifted towards addressing ID instead.
Direct iron therapy has been tested in HF: oral iron supplementation did not show clinically meaningful benefits in studies compared with ESAs in patients with HF and anaemia.17, 18 On the other hand, intravenous (i.v.) iron therapy showed efficacy in small early clinical studies.19, 20, 21, 22 The Ferinject Assessment in patients with IRon deficiency and chronic Heart Failure (FAIR‐HF) study was the landmark large, randomized, double‐blind, placebo‐controlled trial, which showed that treatment with i.v. ferric carboxymaltose (FCM) in repeated 200 mg doses amounting to an average of 1851 mg of iron per subject over a mean of 9.46 injections improved symptoms, exercise capacity, and quality of life at 6 months in ambulatory stable HF patients (LVEF ≤45%) with ID irrespective of the presence or absence of anaemia.23, 24 Most recently, the Ferric CarboxymaltOse evaluatioN on perFormance in patients with IRon deficiency in coMbination with chronic Heart Failure (CONFIRM‐HF) trial was completed. This was a multicentre randomized double‐blind placebo‐controlled trial of i.v. FCM given in two phases—one to two injections between 500 and 2000 mg in the therapy phase (dosed at baseline and Week 6) and further 500 mg injections at each of Weeks 12, 24, and 36 in the maintenance phase if ID was not corrected—in stable ambulatory HF patients with LVEF ≤45% and ID. At Week 24, there was a significant increase in the primary endpoint of 6‐min walk test (6MWT) distance by 33 ± 11 m with treatment compared with placebo (P = 0.002), independent of the presence or absence of anaemia. This improvement was sustained to 52 weeks and associated with a significant reduction in the risk of hospitalizations for worsening HF [hazard ratio (95% confidence interval): 0.39 (0.19 – 0.82), P = 0.009].25
These recent studies have provided strong rationale for the evaluation and treatment of ID in patients with HF. As a result, the most recent European Society of Cardiology Guidelines for management of HF have given a Class I recommendation for ID testing in all HF patients and recommend consideration of iron therapy in those found to be deficient.26 However, none of the clinical trials so far included Asian HF patients, in whom there may be intrinsic differences in iron‐handling mechanisms.8 Furthermore, current clinical trial evidence is limited to the ambulatory setting and patients with HF with reduced ejection fraction (HFrEF), leaving evidence gaps for the half of the HF population with preserved ejection fraction (HFpEF) as well as higher‐risk hospitalized HF patients.
We therefore designed the Pilot Randomized Controlled Trial to Assess the Role of Intravenous Ferric Carboxymaltose in Asian Patients with Heart Failure (PRACTICE‐ASIA‐HF) to assess the role of single‐dose i.v. FCM in stabilized Asian patients following hospitalization for HF (regardless of LVEF) and found to have concurrent ID.
Methods
Study design
The PRACTICE‐ASIA‐HF is an open‐label, randomized, placebo‐controlled trial conducted in two large tertiary centres in Singapore. The schematic study design is presented in Figure 1.
Figure 1.
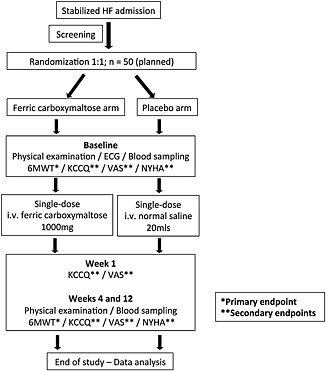
Flowchart of the Pilot Randomized Controlled Trial to Assess the Role of Intravenous Ferric Carboxymaltose in Asian Patients with Heart Failure study procedure (Abbreviations: HF, heart failure; ECG, electrocardiogram; 6MWT, 6‐min walk test; KCCQ, Kansas City Cardiomyopathy Questionnaire; VAS, visual analogue scale; NYHA, New York Heart Association; i.v., intravenous).
The study is conducted in accordance with the principles of the World Medical Association Declaration of Helsinki on ethics in medical research and International Conference on Harmonization guidelines for Good Clinical Practice, with approval by the local ethics institutional regulatory board. Informed consent was obtained for all eligible participants.
Eligibility
All patients hospitalized for acute decompensated HF (diagnosed clinically based on the European Society of Cardiology guidelines), regardless of LVEF, and with concurrent ID [defined as serum ferritin <300 ng/mL if transferrin saturation <20%] are eligible for the study. Further inclusion criteria are ability to complete the 6MWT and age 21 years and above. Subjects with haemoglobin levels above 14 g/dL are excluded because of the risk of iron overload with i.v. iron supplementation. In addition, subjects with known sensitivity to FCM, who received i.v. iron therapy or blood transfusions within 4 weeks prior to randomization, with body weight ≤35 kg or with diagnosed iron storage disorders (e.g. haemochromatosis) are excluded. Patients with serious medical conditions, either cardiac (e.g. acute coronary syndrome and acute valvular heart dysfunction) or non‐cardiac (e.g. active bacterial infection) in nature, which are deemed prohibitive to study participation or completion by the investigators, are also excluded.
Screening and randomization
Subjects were randomized in a 1:1 ratio to FCM or placebo using a phone‐based system where investigators received a treatment code by phone from an off‐site staff not involved in the screening process. These codes were prepared in randomized blocks of four for each site by an independent statistician.
Study treatment
Active treatment
Intravenous FCM solution (Ferinject®, Vifor Pharma) contains 50 mg iron/mL. Eligible subjects received a single dose of FCM 1000 mg administered as 20 mL of an undiluted slow i.v. bolus injection over 15 min.
Placebo
An equivalent volume of i.v. normal saline 0.9% (i.e. 20 mL) was used as a placebo.
The use of oral iron supplementation was permitted in all subjects.
Assessments
Recruited subjects undergo physical examination, electrocardiogram, blood sampling, 6MWT, and quality of life assessment, using the Visual Analogue Scale (VAS) and the Kansas City Cardiomyopathy Questionnaire (KCCQ), at baseline upon stabilization of HF prior to administration of treatment. Physical examination, blood sampling, 6MWT, VAS, and the KCCQ are repeated after 4 and 12 weeks. In addition, the KCCQ is performed via phone call 1 week after study drug administration. The KCCQ has been validated for use in English, Chinese, Malay, and Tamil language versions, and licenses have been obtained for all versions used within our study.
Study endpoints
The primary endpoint is change in 6MWT distance over the 12‐week period.
The secondary endpoints are the following:
Change in quality of life as assessed by the KCCQ and VAS at Weeks 4 and 12
Change in New York Heart Association (NYHA) functional class at Weeks 4 and 12
HF readmission
Adverse effects reported during the study
Study personnel blinded to treatment allocation were responsible for questionnaire administration and conduct of 6MWT.
Statistical considerations
Sample size
Sample size calculations are based on the 6MWT distance throughout the study period following study drug administration. Assuming a mean difference of 40 m in 6MWT between treatment arms and a standard deviation of 60 m, with an autocorrelation coefficient of 0.6, then based on a repeated measures study design with one pre‐intervention and two post‐intervention measurements, a minimum sample size of 25 per group, or 50 in total, would have a 90% power to detect the hypothesized difference at two‐sided alpha of 5%.
Statistical analysis
The mixed model will be implemented to evaluate the effect of i.v. iron therapy compared with placebo on change in exercise capacity over time as assessed by the 6MWT at baseline, 4th week, and 12th week after administration of treatment. This approach takes into account possible intra‐subject correlation arising from repeated measures on exercise capacity. Similarly, the effect of i.v. FCM compared with placebo on change in KCCQ quality of life dimensions will be evaluated using the mixed model, with adjustment made for potential confounders as necessary.
A comparison of NYHA functional class at Week 12 between treatment arms will be made using the χ 2 test. The ordinal logistic regression model will be implemented to adjust for potential confounders, including baseline NYHA functional class. The rate of HF hospitalization and adverse events will be summarized using proportions and compared between groups using Fisher's exact test. In addition, time to re‐admission will be analysed with the logrank test and Cox proportional hazard regression and presented using Kaplan–Meier survival curves. All statistical analyses will be evaluated using STATA statistical software assuming a two‐sided test at the 5% level of significance. The outcomes will be analysed on the basis of intention‐to‐treat.
Discussion
To our knowledge, the PRACTICE‐ASIA‐HF trial is currently the only clinical study assessing the role of a single‐dose of i.v. iron in Asian HF patients with ID.
Compared with other interventional trials involving i.v. iron, PRACTICE‐ASIA‐HF shows several key differences as follows: (i) a simple dosing regimen of 1000 mg as a single dose; (ii) study subjects comprise an Asian population with three major ethnic backgrounds (Chinese, Malay, and Indian); and (iii) inclusion of HF patients regardless of LVEF.
A simplified dosing was utilized in PRACTICE‐ASIA‐HF for applicability in clinical practice. A single i.v. administration of 1000 mg of FCM was chosen as it is the minimum cumulative iron dose assuming baseline haemoglobin of ≥10 g/dL and body weight between 35 and 70 kg, which is the case in the majority of our Asian patients.8 Moreover, the tested protocols of multi‐dose i.v. iron administration in outpatient settings are not feasible in many countries in Asia, where resources for outpatient i.v. services are scarce. Under these circumstances, the inpatient setting is ideal for detecting and treating high‐risk HF patients with ID.
We acknowledge that in subjects with lower baseline haemoglobin or those weighing more than 70 kg, this dose may potentially result in underdosing. Nonetheless, ID tends to be a chronic process resulting from an imbalance of iron intake vs. output via iron metabolism, with the exception of more rapid losses of iron as a result of bleeding. Following iron repletion, normal body iron stores total 3–4 g. Assuming a continued net loss of 1–3 mg per day due to an ongoing underlying aetiology (e.g. insufficient iron intake), it would theoretically require at least 6 months before the clinical impact of ID would be observed, and likely even longer (up to 24 months and beyond). We elected to persist with this common dosing regime for all study subjects as it is compatible with our study duration of 12 weeks and is practically feasible as a one‐time dose in an outpatient setting.
We have previously shown that ethnic differences in Asian patients with HF contribute significantly to the prevalence of ID, where more than 80% of Indian HF patients had ID compared with 58% in Chinese HF patients. Current evidence suggests ethnic‐specific practices as contributing factors, in particular, black tea consumption and vegetarianism that are common amongst Indians. Thankachan et al. demonstrated up to a 67% decrease in iron absorption following the consumption of two cups of black tea taken with a rice meal.27 The role of diet as a contributor to ID certainly requires additional scrutiny within the population of Asian HF patients.
More recently, with advances in genome‐wide association studies, the transmembrane protease, serine 6 gene (TMPRSS6) has been identified as an important regulator of iron homeostasis in the body. The minor allele of a single nucleotide polymorphism affecting TMPRSS6 in Caucasians leads to decreased levels of serum iron, transferrin saturation, haemoglobin, and mean corpuscular volume. Conversely, this allele was found to be the major allele in Asians, suggesting a genetic predisposition to ID in this population.28, 29, 30 These fascinating differences between Caucasian and Asian populations deserve further investigation in order to shed light on the contribution of genetics to ID in Asian HF patients.
Conclusions
The PRACTICE‐ASIA‐HF is the first randomized controlled interventional trial in stabilized Asian patients with HF and ID. A single large dose of i.v. FCM is compared with placebo in improvement of 6MWT distance, quality of life, and NYHA functional class. Data obtained from this pilot study are expected to provide the framework for the efficacy of i.v. iron in the large population of Asian HF patients with ID, as well as shed light on iron replacement as a potential therapeutic option for those with HFpEF. Results from the PRACTICE‐ASIA‐HF will support the conduct of a larger‐scale trial of i.v. iron replacement incorporating dietary and genetic factors contributing to ID.
Conflict of interest
C.S.L. has received research support from Boston Scientific, Medtronic, and Vifor Pharma, and has consulted for Bayer and Novartis. F.A.H. and T.C. are employees of Vifor Pharma.
Funding
This work was supported by the National Medical Research Council, Singapore [Centre Grant: R‐172‐003‐219‐511 and Clinician Scientist Award to C.S.L.] and an unrestricted research grant from Vifor Pharma.
Acknowledgements
We would like to acknowledge the research coordinators, nurses and ward staff members who contributed to this paper, including Ms Marissa Lim, as well as Drs Loh Seet Yoong and Lim Shir Lynn for their tireless efforts in patient recruitment for this study.
Yeo, T. J. , Yeo, P. S. D. , Hadi, F. A. , Cushway, T. , Lee, K. Y. , Tai, B. C. , and Lam, C. S. P. (2016) Rationale and design of a pilot randomized controlled trial to assess the role of intravenous ferric carboxymaltose in Asian patients with heart failure (PRACTICE‐ASIA‐HF). ESC Heart Failure, 3: 71–76. doi: 10.1002/ehf2.12075.
References
- 1. Sakata Y, Shimokawa H. Epidemiology of heart failure in Asia. Circulation J Off J Japanese Circulation Soc 2013; 77: 2209–2217. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organisation . Global atlas on cardiovascular disease prevention and control, 2011.
- 3. Atherton JJ, Hayward CS, Wan Ahmad WA, Kwok B, Jorge J, Hernandez AF, Liang L, Kociol RD, Krum H. Patient characteristics from a regional multicenter database of acute decompensated heart failure in Asia Pacific (ADHERE International‐Asia Pacific). J Card Fail 2012; 18: 82–88. [DOI] [PubMed] [Google Scholar]
- 4. Lam CS, Anand I, Zhang S, Shimizu W, Narasimhan C, Park SW, Yu CM, Ngarmukos T, Omar R, Reyes EB, Siswanto B, Ling LH, Richards AM. Asian sudden cardiac death in heart failure (ASIAN‐HF) registry. Eur J Heart Fail 2013; 15: 928–936. [DOI] [PubMed] [Google Scholar]
- 5. Huch RS, Roland. Iron deficiency and iron deficiency anemia. Thieme Medical Publishers, 2006.
- 6. Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013; 34: 816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang WH, Yeo PS. Epidemiology of anemia in heart failure. Heart Fail Clin 2010; 6: 271–278. [DOI] [PubMed] [Google Scholar]
- 8. Yeo TJ, Yeo PS, Ching‐Chiew Wong R, Ong HY, Leong KT, Jaufeerally F, Sim D, Santhanakrishnan R, Lim SL, M MYC, Chai P, Low AF, Ling LH, Ng TP, Richards AM, Lam CS. Iron deficiency in a multi‐ethnic Asian population with and without heart failure: prevalence, clinical correlates, functional significance and prognosis. Eur J Heart Fail 2014; 16: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 9. Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165: 575–582 e3. [DOI] [PubMed] [Google Scholar]
- 10. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin‐Nadzieja L, von Haehling S, Doehner W, Banasiak W, Polonski L, Filippatos G, Anker SD, Ponikowski P. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17: 899–906. [DOI] [PubMed] [Google Scholar]
- 11. Comin‐Colet J, Enjuanes C, Gonzalez G, Torrens A, Cladellas M, Merono O, Ribas N, Ruiz S, Gomez M, Verdu JM, Bruguera J. Iron deficiency is a key determinant of health‐related quality of life in patients with chronic heart failure regardless of anaemia status. Eur J Heart Fail 2013; 15: 1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anand IS. Anemia and chronic heart failure: implications and treatment options. J Am Coll Cardiol 2008; 52: 501–511. [DOI] [PubMed] [Google Scholar]
- 13. Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, van der Meer P. Anemia and mortality in heart failure patients a systematic review and meta‐analysis. J Am Coll Cardiol 2008; 52: 818–827. [DOI] [PubMed] [Google Scholar]
- 14. Kansagara D, Dyer E, Englander H, Fu R, Freeman M, Kagen D. Treatment of anemia in patients with heart disease: a systematic review. Ann Intern Med 2013; 159: 746–757. [DOI] [PubMed] [Google Scholar]
- 15. Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R, Maggioni AP, McMurray JJ, O'Connor C, Pfeffer MA, Solomon SD, Sun Y, Tendera M, van Veldhuisen DJ. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 2013; 368: 1210–1219. [DOI] [PubMed] [Google Scholar]
- 16. Kao DP, Kreso E, Fonarow GC, Krantz MJ. Characteristics and outcomes among heart failure patients with anemia and renal insufficiency with and without blood transfusions (public discharge data from California 2000–2006). Am J Cardiol 2011; 107: 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vullaganti S, Goldsmith J, Teruya S, Alvarez J, Helmke S, Maurer MS. Cardiovascular effects of hemoglobin response in patients receiving epoetin alfa and oral iron in heart failure with a preserved ejection fraction. J geriatric Cardiology : JGC 2014; 11: 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parissis JT, Kourea K, Panou F et al Effects of darbepoetin alpha on right and left ventricular systolic and diastolic function in anemic patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am Heart J 2008; 155: 751.e1–757. [DOI] [PubMed] [Google Scholar]
- 19. Bolger AP, Bartlett FR, Penston HS, O'Leary J, Pollock N, Kaprielian R, Chapman CM. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol 2006; 48: 1225–1227. [DOI] [PubMed] [Google Scholar]
- 20. Toblli JE, Lombrana A, Duarte P, Di Gennaro F. Intravenous iron reduces NT‐pro‐brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol 2007; 50: 1657–1665. [DOI] [PubMed] [Google Scholar]
- 21. Okonko DO, Grzeslo A, Witkowski T, Mandal AKJ, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole‐Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency: FERRIC‐HF: A randomized, controlled, observer‐blinded trial. J Am Coll Cardiol 2008; 51: 103–112. [DOI] [PubMed] [Google Scholar]
- 22. Usmanov RI, Zueva EB, Silverberg DS, Shaked M. Intravenous iron without erythropoietin for the treatment of iron deficiency anemia in patients with moderate to severe congestive heart failure and chronic kidney insufficiency. J Nephrol 2008; 21: 236–242. [PubMed] [Google Scholar]
- 23. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole‐Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 24. Gutzwiller FS, Schwenkglenks M, Blank PR, Braunhofer PG, Mori C, Szucs TD, Ponikowski P, Anker SD. Health economic assessment of ferric carboxymaltose in patients with iron deficiency and chronic heart failure based on the FAIR‐HF trial: an analysis for the UK. Eur J Heart Fail 2012; 14: 782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD. Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GD, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012; 14: 803–869. [DOI] [PubMed] [Google Scholar]
- 27. Thankachan P, Walczyk T, Muthayya S, Kurpad AV, Hurrell RF. Iron absorption in young Indian women: the interaction of iron status with the influence of tea and ascorbic acid. Am J Clin Nutr 2008; 87: 881–886. [DOI] [PubMed] [Google Scholar]
- 28. Benyamin B, Ferreira MAR, Willemsen G, Gordon S, Middelberg RPS, McEvoy BP, Hottenga JJ, Henders AK, Campbell MJ, Wallace L, Frazer IH, Heath AC, de Geus EJC, Nyholt DR, Visscher PM, Penninx BW, Boomsma DI, Martin NG, Montgomery GW, Whitfield JB. Common variants in TMPRSS6 are associated with iron status and erythrocyte volume. Nat Genet 2009; 41: 1173–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chambers JC, Zhang W, Li Y, Sehmi J, Wass MN, Zabaneh D, Hoggart C, Bayele H, McCarthy MI, Peltonen L, Freimer NB, Srai SK, Maxwell PH, Sternberg MJE, Ruokonen A, Abecasis G, Jarvelin MR, Scott J, Elliott P, Kooner JS. Genome‐wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nat Genet 2009; 41: 1170–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kamatani Y, Matsuda K, Okada Y et al Genome‐wide association study of hematological and biochemical traits in a Japanese population. Nat Genet 2010; 42: 210–215. [DOI] [PubMed] [Google Scholar]


