ABSTRACT
Objectives
Recent biomedical research suggests that, in modern human populations, individuals may vary in their inherent tendency toward bone formation at skeletal and extra‐skeletal locations. However, the nature of this phenomenon is incompletely understood, and the extent to which it might apply to past populations is unclear. It is hypothesized that if there is inter‐individual variation in some overall tendency toward bone formation in skeletal and extra‐skeletal sites then there should be a positive relationship between ligamentous ossification and thickness of cortical bone. This work is a test of this hypothesis in an archaeological population.
Materials and Methods
The study material comprises adult skeletons (N = 137 individuals) of documented age at death from 18th to 19th century London. It examines the relationship between bone deposition in the anterior longitudinal ligament (ALL) in the thoracic spine and cortical index (CI) at the metacarpal measured by radiogrammetry.
Results
Controlling for the potential confounders age, sex, skeletal completeness, occupation (males) and parity (females), there was a positive association between ossification into the ALL and CI. This reflects lesser medullary cavity width in those showing ALL ossification.
Discussion
Ligamentous ossification in the axial skeleton and peripheral cortical bone status are linked, individuals with ALL ossification showing lesser resorption of cortical bone at the endosteal surface. This is consistent with the idea of inter‐individual variation in some general bone‐forming/bone‐losing tendency in this 200 year old study population, but there was no evidence of a link between ALL ossification and increased skeletal subperiosteal bone deposition. Am J Phys Anthropol 159:577–584, 2016. © 2015 The Authors American Journal of Physical Anthropology Published by Wiley Periodicals, Inc.
Keywords: DISH, osteoporosis, enthesophyte, anterior longitudinal ligament, cortical thickness
Almost 20 years ago, Juliet Rogers and co‐workers identified, in archaeological skeletons, a positive association between enthesophytes (bony spurring at ligamentous/tendinous insertions) and osteophytes (bony spurring at the margins of synovial joints). They suggested that this reflected inherent differences between individuals in a tendency toward bone formation at those anatomical sites. They termed individuals with a tendency toward these types of ossification as “bone‐formers.” Part of the association between enthesophytes and osteophytes noted by Rogers et al. (1997) was probably because both processes are age‐progressive and age could not be adequately controlled for in their undocumented archaeological material (Rogers et al., 2004; Felson and Neogi, 2004). Some (Kalichman et al., 2007; Hardcastle et al., 2014a, 2015) but not all (Gibson et al., 2012) studies on living populations have demonstrated an association between enthesophytes and osteophytes in the appendicular skeleton, which persists once the effects of age are controlled for. During the 1970s, it began to be noted (e.g., Foss and Byers, 1972) that there was an inverse relationship between osteoporosis (loss of bone mass with advancing age) and osteoarthritis (a proliferative arthropathy), and subsequent evidence has tended to support this view (Im and Kim, 2014; Hardcastle et al., 2014b, 2015). This may suggest that the bone‐former/bone‐loser continuum may have application not only for ossification at and around joints, but also in the skeleton more generally.
If individuals, and indeed populations, differ in their capacity to lose or form bone, then this has profound implications for osteoarchaeology. Estimation of age at death from skeletal remains plays a crucial role in palaeodemography, and also provides a framework for interpreting other skeletal data. Most age indicators in the adult involve the localized net deposition or resorption of bone at particular sites in the skeleton. Hence, the relationship between age indicators and age may potentially differ in individuals or populations lying at different points on a bone‐former/bone‐loser spectrum (Schmitt et al., 2007; Mays, 2015). The study of ancient disease and the study of past activity regimes are major foci of skeletal studies. Physiologically, the only way in which the skeleton can respond to disease or mechanical loads is via local or systemic alterations in the balance between bone formation and bone resorption. Therefore, an individual's location on a bone‐former/bone‐loser continuum will potentially influence the frequency and morphology of bony lesions that form in response to disease, and also the extent to which bone is added or removed in response to biomechanical stimuli. In this light, clarifying the validity of a bone‐loser/bone‐former continuum in archaeological populations is of prime importance. The extent to which findings in this regard from modern populations can be extrapolated to populations from the past is uncertain. There are great differences in lifestyle, including nutrition, drug‐treatment of disease, activity regimes and reproductive history which may potentially impact bone metabolism. Aside from the Rogers et al. (1997) study, cited above, there have been few been few empirical studies on archaeological skeletal populations, and the results of those that have been undertaken have been difficult to interpret. For example, some have focused on the relationship between osteoarthritis and osteoporosis (e.g., Burr et al., 1983; Brickley and Waldron, 1998; Weiss, 2013). They generally report a positive association, rather than the inverse relationship reported in modern biomedical studies but, as the original authors point out, the extent to which this reflects problems in adequately controlling for the effects of age and other potential confounders in undocumented archaeological populations is unclear.
Minor ossifications at ligamentous insertions are common in older individuals (Jurmain, 1999), but nowhere may they be more florid than in the anterior longitudinal ligament (ALL) in the spine. The ALL extends along the anterior surfaces of the vertebral bodies. It has several layers of closely interlaced fibers; the most superficial extend over four or five vertebrae, the second set between two or three vertebrae, whilst a deeper third set extend from one vertebra to the next. Progressive ossification into the ALL is frequent in middle aged and older people, particularly in the thoracic spine, in modern western populations (Resnick and Niwayama, 1976; Fornasier et al., 1983), and is also often seen in European archaeological skeletal series (Rogers, 2000). Bone is usually deposited initially at the enthesis of the ALL, which lies on anterior wall of the vertebral body. This ossification gradually extends so that the ossified ALL ankyloses neighbouring vertebrae (Resnick and Niwayama, 1976; Fornasier et al., 1983). This bone formation most often occurs in the thoracic segment, and when extensive is viewed as an identifier of diffuse idiopathic skeletal hyperostosis (DISH), a condition which appears to be an extreme expression of a bone‐forming tendency (Waldron, 2009: 73). Osteoporosis is characterized by progressive loss of bone mass from middle age onward, that is, it is a bone‐losing tendency. If the bone‐forming/bone‐losing continuum, which some have posited for living populations, has validity in the past, then we would hypothesize that a positive relationship between ossification into the ALL and measures of skeletal bone mass should be observable in ancient skeletal series, provided the effects of age and other potential confounders can be adequately controlled for. The current work investigates the relationship between ossification of the ALL and osteoporosis, as measured by cortical bone thickness, in a collection of 18th/19th century AD skeletal remains of documented age at death from London.
MATERIALS AND METHODS
The study material comprises human skeletal remains recovered from the crypt beneath Christ Church Spitalfields, London. They represent the interments of middle class Londoners who died in the 18th–19th century AD (Molleson and Cox, 1993). A subset of the burials have coffin plates giving name, age, and date of death; it is these burials that are the focus of the current work.
Ossification into the ALL was scored in the thoracic spine from macroscopic study of remains. Vertebral body osteophytes due to ossification of the ALL were distinguished from those due to other causes (e.g., intervertebral disc degeneration, sero‐negative spondyloarthropathies, etc.) using the following standard criteria described by Rogers et al. (1987): thoracic ALL ossifications produce flowing ossification on the anterolateral aspects of the vertebral body with a predilection for the right side; osteophytes are smooth and have a predominantly vertical orientation; they generally originate on the antero‐lateral vertebral body wall away from the disc margin; in the absence of co‐existent disease the vertebral facet joints and disc‐space are normal. The extent of ossification into the ALL considered sufficient to trigger a diagnosis of DISH is arbitrary (Resnick and Niwayama, 1988) and differs between investigators but, following Julkunen et al. (1971), ossification of the ALL sufficient to result in the formation of at least two complete bony bridges between vertebrae was recorded as DISH; less advanced ALL ossification, which had resulted in one or no complete bony bridges, was classified as subclinical DISH, representative of an earlier stage of the condition. The entry criterion for a skeleton to be included in the study were that at least three thoracic vertebral bodies were preserved.
Cortical bone thickness at the metacarpal has already been recorded for this collection (Mays, 2000, 2001), and it is these data that are used in the current study. Full details of methodology are given in the above publications, but in brief, an antero‐posterior radiograph was taken of one second metacarpal from each individual. Measurements of medullary width (M) and total width (T) were taken on the radiograph at the midshaft using callipers, following the methodology of Dequeker (1976). The cortical index (CI) was calculated as CI = 100× (T‐M)/T, and is a measure of cortical thickness standardized for bone width. Using this methodology about 3‐4% of sample variance likely reflects method error. This method was chosen as an indicator of osteoporosis for several reasons. Metacarpal cortical bone is often used to monitor osteoporosis in living subjects as it is a guide to bone mineral density at diverse skeletal sites, including the hip, spine and wrist (Adami et al., 1996; Dey et al., 2000; Boonen et al., 2005). Unlike bone mineral density measurements, provided that (as in the current work) bones suffering taphonomic damage are excluded, it is unaffected by post‐depositional alterations, and it enables study purely of cortical bone. In addition, measurements of T and M potentially enable focus specifically on the periosteal and endosteal surfaces.
Ossification into the ALL was not seen in young adults, so analysis was restricted to those aged 40+ years at death. 137 individuals (69 males, 68 females) were aged 40+years, met the entry criterion for scoring ALL ossification and had undamaged second metacarpals permitting cortical index to be measured. The date range of the burials is AD1729‐1852. Age at death ranges from 40 to 92 years. The age distribution is shown in Figure 1.
Figure 1.
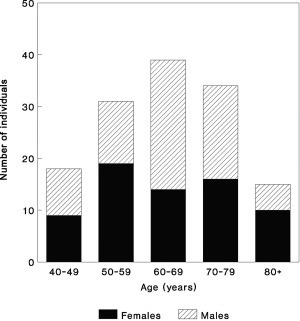
Age at death distribution of the study group.
Studies on living populations (Julkunen et al., 1971; Westerveld et al., 2008; Baraliakos et al., 2012) indicate that DISH is more frequent in males and, from middle age onward, is age progressive. These patterns have also been identified in some large archaeological skeletal collections (e.g., Waldron, 2007: 102). Therefore age and sex were controlled for in analysis. Biomedical studies have demonstrated a fairly consistent link between DISH and obesity (Mader et al., 2013), so this too will be a potential confounding variable. Various methods have been suggested for calculating body mass from skeletal parameters (Auerbach and Ruff, 2004), but when applied to archaeological material they may produce widely divergent results (Mays, 2007: 120‐121), which does not engender confidence in any of them. In addition, obesity may develop at any time in the life cycle and be a transient condition, so its effect (if any) on skeletal morphology may be rather variable. Save for a few individuals whose portraits survive, there is no direct evidence identifying obese individuals at Spitalfields. In the absence of reliable direct evidence for body mass, data on occupation are used as an indicator of exposure to some risk factors for obesity.
Information on the occupations of some of the Spitalfields men is available from documentary sources, specifically the London Trade Directories, the Christ Church Vestry Minute Books and the parochial registers (Molleson and Cox, 1993). The most frequent occupations were in the textile industry, particularly silk weaving. The occupations have been classified by Molleson and Cox (1993) as master weaver, journeyman weaver, miscellaneous non‐manual professions and manual trades. Master weavers would have served an apprenticeship but did little weaving thereafter. By contrast, journeyman weavers worked long hours at the loom, and this was demanding physical labour. To dichotomise workers into manual and non‐manual groups for the purposes of analysis, master weavers are grouped with the various other non‐manual professions, journeyman weavers with the manual trades. There is no simple correlation between occupation and wealth, but there was likely a tendency among those in professions classed as non‐manual (e.g., master weaver, lawyer, merchant, public servant) to be wealthier than those in manual trades (e.g., journeyman weaver, carpenter, bricklayer) (Molleson and Cox, 1993: 97‐101). Diets of the wealthy at that time were high in animal fat and protein (Molleson and Cox, 1993: 39). This coupled with more sedentary occupations, means that those in non‐manual professions were likely at increased risk of chronic obesity compared with those in manual occupations.
In females, calcium homeostasis is significantly altered during pregancy and lactation. Although consensus has yet to emerge, in well‐nourished populations, parity may have a long‐term protective effect on bone mass (Salari and Abdollahi, 2014). The potential impact of reproductive history on the balance between bone formation and resorption suggests that it could be a potential variable influencing ossification into the ALL in females. Cox (1989) reconstructed obstetric histories for some of the women buried at Spitalfields from documentary sources. Data on parity for female subjects used in the present study are taken from that work.
In palaeopathological study, skeletal completenesss is a potentially confounding variable, more complete burials being more likely to show skeletal lesions, other factors being equal. In this light, number of thoracic vertebral bodies available for observation was recorded for each skeleton.
The principal statistical approach to the data is logistic regression. This technique fits a regression surface to data for which the dependent variable is a dichotomy. It may be used to assess the effect of a set of independent variables, which may be categorical or numeric, on the state of a binary dependent variable. The logistic regression model may be written as:
| (1) |
where Ŷi is the probability that the ith case is in one of two categories, and u is given by
| (2) |
where A is a constant and Bn is the coefficient of the nth independent variable in the equation (Howell, 2002: 583‐593; Tabachnik and Fidell, 2013: 439‐509). In this study, the dependent variable (Y) is ossification status of the ALL, and the independent variables are metacarpal cortical index, sex, age, number of thoracic vertebral bodies available for study, parity (for females), and occupation (for males). In order to test our hypothesized relationship between thickness of cortical bone and ALL ossification, logistic regression is used to investigate whether metacarpal cortical index significantly affects the likelihood of observing ossification in the ALL, adjusting for the other independent variables. Several model‐building approaches are available for logistic regression. Direct logistic regression, in which all the independent variables are entered into the model simultaneously, involves no a priori assumptions concerning the relative importance of the independent variables in predicting the state of the binary dependent variable (Stoltzfus, 2011). It is, therefore, the principal approach used here.
RESULTS
The univariate statistics for the subgroups with and without ALL ossification are shown in Table 1. Examples of ALL ossification are illustrated in Figure 2. Fifty‐eight individuals showed ossification into the ALL. In 36 of these, no vertebrae were ankylosed; in 8 there was one complete bony bridge between neighbouring vertebrae, and 14 individuals showed two or more complete bony bridges and hence satisfied the criterion for DISH. The data split by age and sex are shown in Supporting Information Table 1. Of a total of 71 bony bridges observed, 10 are in the upper thoracic spine (C7/T1 – T3/4), 38 in the mid‐thoracic spine (T4/5 – T8/9) and 23 in the lower thoracic spine (T9/10 – T12/L1).
Table 1.
Univariate statisticsa
| N | Sex ratio | Age mean (SD) | CI Mean (SD) | N. thoracic vert Mean (SD) | |
|---|---|---|---|---|---|
| Individuals without ALL ossification | 79 | 30:49 | 61.6 (12.5) | 40.0 (8.5) | 11.0 (1.7) |
| Individuals with ALL ossification | 58 | 39:19 | 67.6 (11.2) | 42.2 (9.9) | 11.3 (1.2) |
Sex ratio, number of males: number of females; Age in years; CI, metacarpal cortical index; N. thoracic vert, number of thoracic vertebrae preserved.
Figure 2.
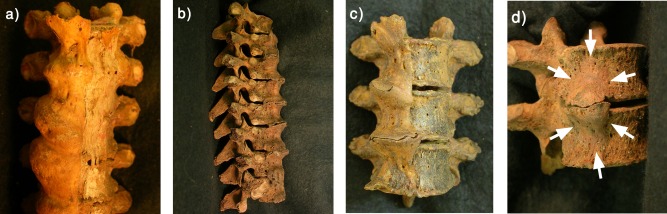
Ossification into the ALL in four of the Spitalfields skeletons. (a) Fourth through seventh thoracic vertebrae, Skel. 2829, anterior view, part of a sequence of nine thoracic vertebrae ankylosed by ossification into the ALL. Ossification is largely confined to the right side, and the unossified left side of the ALL (to the right in the photograph) partially survives as dessicated soft tissue. (b) Fifth through twelfth thoracic vertebrae, Skel. 2244, right side. Ossification into the ALL is extensive but nowhere are adjacent vertebrae ankylosed. (c). Seventh through ninth thoracic vertebrae, Skel. 2808, anterior view. Ossification into the ALL has ankylosed T7 and 8; the osteophytes on T8 and nine closely interdigitate. (d) Eighth and ninth thoracic vertebrae, Skel. 2643, right antero‐lateral view. Each bone bears a thick, smooth ossification that extends most of the height of the vertebral body. The osteophytes are not ankylosed.
In logistic regression, for the main analysis, ossification in the ALL was coded 1 = ossification observed, 0 = ossification not observed. For the independent variables, sex was coded 0 = female, 1 = male, and age, CI and number of thoracic vertebrae present were treated as continuous variables. The results are shown in Table 2. The Wald statistics indicate that for age, sex and CI, the coefficient in the logistic regression equation differs significantly from zero—that is, age, sex, and CI are each significantly associated with ossfication status of the ALL. For each independent variable, the odds ratio represents the change in odds of observing ALL ossification with a unit increase in that variable when the effects of the other independent variables are controlled for. The finding that for age, sex and CI, the odds ratios are greater than one means that ossification in the ALL is significantly associated with increasing age, male sex and greater CI. Since different algorithms for variable selection may potentially produce different models, the main logistic regression analysis was re‐run using another approach to model building, forward stepwise selection. The model produced was similar (Supporting Information Table 2), with sex, age, and CI being associated with ossification into the ALL, supporting the robustness of the results.
Table 2.
Logistic regression analysis of age, CI, sex and number of thoracic vertebrae preserved, with ALL ossification as the dependent variable (N = 137)a
| Variable | Coefficient (SE) | Wald | p | Odds ratio (95% conf. int.) |
|---|---|---|---|---|
| Age | 0.071 (0.020) | 12.89 | <0.001 | 1.073 (1.033–1.115) |
| CI | 0.065 (0.025) | 6.64 | 0.010 | 1.068 (1.016–1.122) |
| Sex | 1.249 (0.400) | 9.75 | 0.002 | 3.488 (1.592–7.638) |
| N. thoracic vert. | 0.198 (0.141) | 1.96 | 0.161 | 1.219 (0.924–1.608) |
| Constant | −10.428 (2.766) |
A number of parameters have been devised for measuring the strength of the relationship of the dependent with the independent variables in logistic regression, analogous to the coefficient of determination (R 2) in ordinary least squares models (Tabachnik and Fidell, 2013: 462–463). Although consensus has yet to emerge, it has been suggested that McFadden's ρ 2 may be the most consistently useful of these R 2 analogues (Menard, 2000). The McFadden's ρ 2 for the current logistic regression model is 0.16. Analogous to R 2 in multiple regression, McFadden's ρ 2 can take values from zero (indicating that independent variables play no part in predicting the state of the dependent variable) to one (the model predicts the dependent variable perfectly). In practice, values are generally lower than those of R 2 in good ordinary least squares regression models—values in the range 0.2–0.4 indicate an excellent fit (McFadden, 1978: 307). In the current case, the value is somewhat below this range, suggesting factors other than those included in the model are important contributors to variation in the dependent variable. To investigate the relative importance of age and CI in the model, the logistic regression was re‐run with age and CI transformed into z‐scores. The odds ratios of observing ossification in the ALL for a one standard deviation increment in age and CI were 2.38 and 1.82, respectively, suggesting that CI contributes less to the model than age. There was no relationship between number of thoracic vertebrae preserved for study and ossification in the ALL.
The main analysis (above) was re‐run with total width and medullary width of the metacarpal replacing CI. For total bone width, the Wald statistic was non‐significant, providing no evidence for a relationship with ossification in the ALL. For medullary width, the Wald statistic was 6.04, which is significant at p = 0.014; the coefficient was −0.768, indicating that greater medullary widths were associated with decline in odds of observing ossification in the ALL. In order to investigate whether CI was associated with the extent of ossification into the ALL, the logistic regression was run solely on those individuals showing ossification into the ALL (N = 58) with the dependent variable coded 0 = subclinical DISH, 1 = DISH. The Wald statistic for CI was non‐signficant (Wald = 1.11, p = 0.292), suggesting that, in the subgroup of individuals showing ALL ossification, CI does not significantly predict the extent of ossification.
Information on occupation was available for 47 of the males. In analysis, occupation was coded as 0 = non‐manual (N = 32), 1 = manual (N = 15). The results of the logistic regression with ALL ossification (coded 0 or 1, as in the main logistic regression analysis) versus age, CI, number of thoracic vertebrae and occupation as the independent variables are shown in Table 3. Data on parity was available for 47 females. It ranged from zero to 15 births, with a mean of 3.1. Logistic regression of ALL ossification versus age, CI, number of thoracic vertebrae and parity are shown in Table 4. The results indicate that although the associations between ossification into the ALL and age and CI persist when the sexes are analyzed separately, there is no evidence for association between ossification into the ALL and occupation in males or parity in females.
Table 3.
Logistic regression analysis of age, CI, occupation and number of thoracic vertebrae preserved, with ALL ossification as the dependent variable (N = 47 males)a
| Variable | Coefficient (SE) | Wald | p | Odds ratio (95% conf. int.) |
|---|---|---|---|---|
| Age | 0.083 (0.033) | 6.18 | 0.013 | 1.086 (1.018–1.159) |
| CI | 0.116 (0.052) | 5.02 | 0.025 | 1.124 (1.015–1.244) |
| Occupation | 0.485 (0.744) | 0.43 | 0.514 | 1.624 (0.378–6.976) |
| N. thoracic vert. | 0.313 (0.217) | 2.08 | 0.149 | 1.368 (0.894–2.094) |
| Constant | −13.946 (5.253) |
Abbreviations as Table 2.
Table 4.
Logistic regression analysis of age, CI, parity and number of thoracic vertebrae preserved, with ALL ossification as the dependent variable (N = 47 females)a
| Variable | Coefficient (SE) | Wald | p | Odds ratio (95% conf. int.) |
|---|---|---|---|---|
| Age | 0.110 (0.439) | 6.32 | 0.012 | 1.117 (1.025–1.217) |
| CI | 0.124 (0.050) | 6.19 | 0.013 | 1.132 (1.027–1.248) |
| Parity | −0.106 (0.135) | 0.62 | 0.432 | 0.899 (0.690–1.172) |
| N. thoracic vert. | 0.199 (0.295) | 0.46 | 0.500 | 1.220 (0.685–2.173) |
| Constant | −15.091 (5.904) |
Abbreviations as Table 2.
DISCUSSION
At Spitalfields, the prevalence of ossification into the ALL increases with age and is greater in males. These results echo those reported for DISH in living populations (Julkunen et al., 1971; Weinfeld et al., 1997; Kiss et al., 2002; Westerveld et al., 2008). Among those individuals who showed one or more complete bony bridges, the predilection for vertebral ankylosis the mid‐lower parts of the thoracic segment resembles the pattern seen today (Resnick and Niwayama, 1976; El Miedany et al., 2000). The prevalence of DISH in the current study group is 14.5% in males, 5.9% in females. There are some studies of living subjects where the criterion used for scoring DISH resembled that in the current work (ALL ossification sufficient to form at least two bony bridges) and where results are presented in a way to permit comparison. A study in Finland (Julkunen et al., 1971) found prevalences of 3.5% for males, 2.2% for females. That study was restricted to subjects aged over 40 yrs, but the sample had a younger age structure than the Spitalfields group, however even after controlling for this using the common odds ratio (ÔR) (Klaus, 2014: 303) the higher prevalence at Spitalfields persists (ÔR = 2.56). Another population‐based study, in Hungary, gave prevalence figures of 27.3 and 12.8% for males and females respectively (Kiss et al., 2002). A hospital‐based study in the Netherlands (Westerveld et al., 2008) produced similar results (26.6 and 17.2%). Both these latter confined themselves to subjects over 50 years, but the mean ages of their samples resemble that for the present study group. Although methodological differences preclude precise comparisons between skeletal and modern epidemiological studies, the prevalence of DISH at Spitalfields appears to fall within the range reported for modern European populations.
Controlling for the effects of sex and age, there was a relationship between ossification into the ALL and CI, greater CI being associated with a greater likelihood of observing ossification in the ALL. This supports the positive association between ALL ossification and CI hypothesized at the outset of the work. At Spitalfields, CI contributed less to the likelihood of observing ossification into the ALL than did age, and the analysis of goodness of fit of the logistic regression model indicates the potential importance of other, as yet unidentified variables in determining the likelihood of ossification into the ALL. In the subgroup showing ossification into the ALL, CI did not distinguish cases of DISH from subclinical DISH. Although the smaller sample size for this analysis may be in part responsible, it may indicate that greater CI was associated with the presence of a tendency to ossify the ALL, rather than the extent of that ossification.
Logistic regression allows confounders such as skeletal completeness to be included in the model, an important benefit in palaeopathology where skeletons studied normally vary in completeness. It was anticipated that completeness of the thoracic spine would be a potential confounding variable, the likelihood of observing ALL ossification being increased in more complete vertebral columns. This expectation was not met. This probably reflects the overall high level of completeness of the thoracic vertebral segment in the study group (Table 1).
Analysis of medullary cavity and total metacarpal width suggests that the association between CI and ossification into the ALL mainly or entirely reflects differences in medulary cavity width. Therefore, the association between CI and ALL ossification mainly or entirely reflects lesser resorption at the endosteal surface rather than increased periosteal apposition in those showing ALL ossification, but when in the life cycle this difference generally arose is unclear.
The current results support the suggestion that there is a link between bone formation in the ALL and retention of greater cortical bone thickness in older adults. This may be consistent with the notion that individuals lie along a continuum from bone‐formers (thicker cortical bone, ossification into the ALL) and bone‐losers who do not ossify their ALL and have thinner cortical bone due to greater endosteal resorption. However, for the current study group, it may be that the bone loser/bone former concept cannot be extended to embrace periarticular osteophyte formation. A previous study on the Spitalfields skeletons found that there was no association between ossification into the ALL and periarticular bone formation at the acetabular rim (Mays, 2012), that is, no evidence of a relationship between enthesophytes and osteophytes in the spine and hip respectively.
A few controlled studies have been done on living populations to investigate links between DISH (scored using of ossification into the ALL) and osteoporosis. The majority use bone mineral density (BMD), assessed using dual X‐ray absorptiometry or other methods, as a measure of osteoporotic bone loss. DiFranco et al. (2000) found higher BMD at the wrist in DISH cases, and Sahin et al. (2002), studying a female cohort, report greater BMD at the spine, hip and wrist in DISH patients. However Troillet et al. (1992) and Westerveld et al. (2009) report no evidence for greater BMD in DISH patients at the wrist and spine respectively. Eser et al. (2010) found no elevation of BMD in DISH cases at midshaft and distal epiphysial locations in the metacarpal, radius and tibia, although controls were not radiographed to confirm absence of DISH. Littlejohn et al. (1981) and Haara et al. (2007) studied metacarpal cortical bone and report greater cortical thickness in the metacarpal in DISH patients. Littlejohn et al.'s (1981) data suggested that the effect was due to lesser medulary width in DISH patients, but a difficulty with this study was that some of the controls had rheumatoid arthritis which may be associated with metacarpal osteopaenia (Bötcher and Pfeil, 2008). The current results are consistent with those of Littlejohn et al. (1981) and Haara et al. (2007) on modern subjects, and suggest that a positive relationship between ossification into the ALL and thickness of cortical bone is not restricted to modern populations but holds for a past population where aspects of lifestyle were rather different: for example, it is likely that, compared to living Western subjects, the Spitalfields population was less sedentary, disease load was higher, vitamin D status was poorer and, for women, parity was greater (Molleson and Cox, 1993).
Ossification into the ALL involves abnormal osteoblastic activity at affected sites in the ligament (El Miedany et al., 2000). Efforts have been made to identify the signalling pathways involved. Denko et al. (2002) reported elevated growth hormone and insulin‐like growth factor‐1 (IGF‐1), which stimulate osteoblastic proliferation, in DISH patients. DISH patients have also been reported as showing elevated platelet‐derived growth factor BB and transforming growth factor‐β1 (TGF‐β1), which influence osteoblastic differentiation in spinal ligament tissue (Kosaka et al., 2000). The Wnt/βcatenin pathway stimulates osteoblast differentiation and bone formation and is downregulated by a number of molecules including Dickkopf (Dkk) family members (Gosman, 2012). Serum Dkk‐1 levels were reported to be less in DISH patients (Senolt et al., 2012) The above signalling systems are also involved in osteoporosis (Corrado et al., 2013; Maruotti et al., 2013; Crane and Cao, 2014). This may suggest possible mechanisms underlying a relationship between skeletal bone mass and DISH, but further work is needed before firm inferences can be made.
CONCLUSIONS
In the 18th–19th century, Londoners represented by the Spitalfields skeletons, a positive association between metacarpal cortical thickness and ossification into the ALL was observed whilst controlling for the effects of age and sex and some other potential confounders. This supports the initial hypothesis that there should be a positive relationship between skeletal bone mass and tendency toward ligamentous ossification.
Bone comprises two major compartments within which modeling and remodeling occur, the periosteal and the endosteal envelope; the latter consists of three subdivisions, endocortical, intracortical, and trabecular. Each surface may have different age‐specific remodelling responses to hormonal, mechanical and other influences (Szulc and Seeman, 2009; Gosman et al., 2011). The relationship between ossification into the ALL and cortical thickness at Spitalfields appears bone compartment‐specific (at least as far as the metacarpal is concerned)—individuals with and without ALL ossification differ in the balance between bone formation and resorption at the endosteal rather than at the periosteal surface. Given that age‐related bone loss in osteoporosis occurs from the endosteal envelope (Parfitt, 2005), this may support the idea that individuals with a tendency toward ossification into the ALL show an overall lesser tendency toward osteoporotic bone loss, although the point in the life cycle at which differences in cortical thickness between those with and without ossification of ALL generally arose is unclear. An earlier study on the Spitalfields skeletons (Mays, 2012) failed to provide evidence for a relationship between ossification into the ALL and periarticular bone formation at the acetabulum. Bone formation at the acetabular rim may be subperiosteal (Corten et al., 2011), so this result is intelligible if, as suggested above, tendency toward ossification into the ALL is primarily associated with lesser resorption at the endosteal envelope rather than with increased deposition of bone subperiosteally.
The inference of an link between tendency toward ossification into the ALL and the balance between bone formation and resorption specifically at one or more surfaces in the endosteal envelope is one that requires further evaluation, but if it is generalizable it may have potential implications for the interpretation of age indicators in the adult skeleton that involve observations that relate to surfaces of the endosteal envelope. The so‐called “complex” ageing method of Acsádi and Nemeskéri (1970) involves study of trabecular bone involution in long‐bone metaphyses—that is, net resorption at the trabecular endosteal surface. Similarly, age markers such as pubic symphyseal and auricular surface morphology involve alterations in the balance between bone formation and resorption in subchondral trabecular bone and the subchondral bone plate (Mays, 2015). Histomorphometric ageing methods (Robling and Stout, 2008) evaluate indicators of bone remodelling in the intracortical component of the endosteal envelope. It might be hypothesized that age‐relationships for the above might differ in individuals with and without tendencies toward ossification into the ALL. Lastly, the observation that individuals with ossification into the ALL may show increased bone in cross‐section has implications for studies using cross‐sectional properties of long‐bones to attempt to reconstruct past activity regimes (Larsen, 2015: 214–255), especially those using methodology that includes measurements of endosteal as well as periosteal dimensions (Macintosh et al., 2013). In such studies, it may be prudent to include bone‐forming tendency, as indicated by ALL ossification status, as a potential confounding variable in analysis.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The author is grateful to the Natural History Museum, London, for access to the Spitalfields remains. Figure 2a–d appear courtesy of the Natural History Museum, London. Thanks are due to Robert Kruszynski for practical help in accessing the skeletal material. Louise Humphrey kindly supplied biographical details on the Spitalfields people. I am grateful to the journal editors and anonymous reviewers of this article for their pertinent comments.
LITERATURE CITED
- Acsádi G, Nemeskéri J. 1970. History of human lifespan and mortality. Budapest: Akademiai Kaido. [Google Scholar]
- Adami S, Zamberlan N, Gatti G, Zanfisi C, Braga V, Broginni M. 1996. Computed radiographic absorptiometry and morphometry in the assessment of post‐menopausal bone loss. Osteoporos Int 6:8–13. [DOI] [PubMed] [Google Scholar]
- Auerbach BM, Ruff CB. 2004. Human body mass estimation: a comparison of “morphometric” and “mechanical” methods. Am J Phys Anthropol 125:331–342. [DOI] [PubMed] [Google Scholar]
- Baraliakos X, Listing J, Buschmann J, von der Recke A, Braun J. 2012. A comparison of new bone formation in patients with ankylosing spondylitis and patients with diffuse idiopathic skeletal hyperostosis. A retrospective cohort study over six years. Arth Rheum 64:127–1133. [DOI] [PubMed] [Google Scholar]
- Boonen S, Nijs J, Borghs H, Peeters H, Vanderscheuren D, Luyten FP. 2005. Identifying post‐menopausal women with osteoporosis with calcaneal ultrasound, metacarpal digital radiogrammetry and phalangeal radiographic absorptiometry: a comparative study. Osteoporos Int 16:93–100. [DOI] [PubMed] [Google Scholar]
- Bötcher J, Pfeil A. 2008. Diagnosis of periarticular osteoporosis in rheumatoid arthritis using digital X‐ray radiogrammetry. Arth Res Ther 10:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley M, Waldron T. 1998. Relationship between bone density and osteoarthritis in a skeletal population from London. Bone 22:279–283. [DOI] [PubMed] [Google Scholar]
- Burr DR, Martin B, Schaffler MB, Jurmain RD, Harner EJ, Radin EL. 1983. Osteoarthrosis: sex‐specific relationship to osteoporosis. Am J Phys Anthropol 61:299–303. [DOI] [PubMed] [Google Scholar]
- Corrado A, Nene A, Macchiarola A, Gaudio A, Marucci A, Cantatore FP. 2013. RANKL/OPG ratio and DKK‐1 expression in primary osteoblastic cultures from osteoarthritic and osteoporotic subjects. J Rheumatol 40:684–694. [DOI] [PubMed] [Google Scholar]
- Corten K, Ganz R, Chosa E, Leunig M. 2011. Bone apposition of the acetabular rim in deep hips: a distinct finding of global pincer impingement. J Bone Jt Surg Am 93 (Suppl 2):10–16. [DOI] [PubMed] [Google Scholar]
- Cox MJ. 1989. An evaluation of the significance of “scars of parturition” in the Christ Church Spitalfields sample. PhD thesis, University College London.
- Crane JL, Cao X. 2014. Function of matrix IGF‐1 in coupling bone resorption and formation. J Mol Med 92:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denko CW, Boja B, Malemud CS. 2002. Growth hormone and insulin‐like growth factor‐I in symptomatic and asymptomatic patients with diffuse idiopathic skeletal hyperostosis (DISH). Front Biosci 7:a37–a43. [DOI] [PubMed] [Google Scholar]
- Dequeker J. 1976. Quantitative radiology: radiogrammetry of cortical bone. Br J Radiol 49:912–920. [DOI] [PubMed] [Google Scholar]
- Dey A, McClosky EV, Taune T, Cox R, Pande KC, Ashford RU, Forster M, de Takats D, Kanis JA. 2000. Metacarpal morphometry using a semi‐automated technique in the assessment of osteoporosis and vertebral fracture risk. Osteoporos Int 11:953–958. [DOI] [PubMed] [Google Scholar]
- El Miedany YM, Wassif G El Baddini M., 2000. Diffuse idiopathic skeletal hyperostosis (DISH): is it of vascular aetiology? Clin Expt Rheumatol 18:193‐200. [PubMed] [Google Scholar]
- Eser P, Bonel H, Seitz M, Villiger PM, Aeberli D. 2010. Patients with diffuse idiopathic skeletal hyperostosis do not have increased peripheral bone mineral density and geometry. Rheumatol 49:977–981. [DOI] [PubMed] [Google Scholar]
- Felson DT, Neogi T. 2004. Osteoarthritis: is it a disease of cartilage or bone? Arth Rheum 50:341–344. [DOI] [PubMed] [Google Scholar]
- Fornasier VL, Littlejohn G, Urowitz MB, Keystone EC, Smythe HA. 1983. Spinal entheseal new bone formation: the early changes of spinal diffuse idiopathic skeletal hyperostosis. J Rheumatol 10:939–947. [PubMed] [Google Scholar]
- Foss MV, Byers PD. 1972. Bone density, osteoarthrosis of the hip, and fracture of the upper end of the femur. Ann Rheum Dis 31:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranco M, Mauceri MT, Sili‐Scavalli A, Iagnocco A, Ciocci A. 2000. Study of peripheral bone mineral density in patients with diffuse idiopathic skeletal hyperostosis. Clin Rheumatol 19:188–192. [DOI] [PubMed] [Google Scholar]
- Gibson N, Guermazi A, Clancy M, Niu J, Grayson P, Aliabadi P, Roemer F, Felson DT. 2012. Relation of hand enthesophytes and knee enthesopathy: is osteoarthritis related to a systemic enthesopathy? J Rheumatol 39:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosman JH. 2012. The molecular biological approach to palaeopathology In: Grauer A, editor. A companion to palaeopathology. Chichester: Wiley‐Blackwell; p 76–96. [Google Scholar]
- Gosman JH, Stout SD, Larsen CS. 2011. Skeletal biology over the life span: a view from the surfaces. Ybk Phys Anthopol 54:86–98. [DOI] [PubMed] [Google Scholar]
- Haara MM, Arokoski JPA, Kröger H, Kärkkäinen A, Manninnen P, Knekt P, Heliövaara M. 2007. Relative bone mineral density measured by metacarpal index (MCI) and chronic spinal syndromes: a epidemiological study. Scand J Rheumatol 36:466–469. [DOI] [PubMed] [Google Scholar]
- Hardcastle SA, Dieppe P, Gregson CL, Arden NK, Spector TD, Hart DJ, Edwards MH, Dennison EM, Cooper C, Sayers A, Williams M, Davey Smith G, Tobias JH. 2015. Individuals with high bone mass have an increased prevalence of radiographic knee osteoarthritis. Bone 71:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle SA, Dieppe P, Gregson CL, Arden NK, Spector TD, Hart DJ, Edwards MH, Dennison EM, Cooper C, Williams M, Davey Smith G, Tobias JH. 2014a. Osteophytes, enthesophytes, and high bone mass. A bone forming triad with potential relevance in osteoarthritis. Arth Rheumatol 66:2429–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle SA, Dieppe P, Gregson CL, Hunter D, Thomas GER, Arden NK, Spector TD, Hart DJ, Laugharne MJ, Clague GA, Edwards MH, Dennison EM, Cooper C, Williams M, Davey Smith G, Tobias JH. 2014b. Prevalence of radiographic hip osteoarthritis is increased in high bone mass. Osteoarth Cartil 22:1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DC. 2002. Statistical methods for psychology, 5th edn Pacific Grove: Duxbury. [Google Scholar]
- Im G‐I, Kim M‐K. 2014. The relationship between osteoarthritis and osteoporosis. J Bone Miner Metab 32:101–109. [DOI] [PubMed] [Google Scholar]
- Julkunen H, Heionen OP, Pyorala K. 1971. Hyperostosis of the spine in an adult population. Ann Rheum Dis 30:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurmain R. 1999. Stories From the Skeleton. Behavioral Reconstruction in Human Osteology. London: Gordon and Breach. [Google Scholar]
- Kalichman L, Malkin I, Kobyliansky E. 2007. Hand bone midshaft enthesophytes: the influence of age, sex and heritability. Osteoarth Cartilage 15:1113–1119. [DOI] [PubMed] [Google Scholar]
- Kiss C, O'Neil TW, Mituszova M, Szilágyi M, Donath J, Poór G. 2002. Prevalence of diffuse idiopathic skeletal hyperostosis in Budapest, Hungary. Rheumatol 41:1335–1336. [DOI] [PubMed] [Google Scholar]
- Klaus HD. 2014. Frontiers in the bioarchaeology of stress and disease: cross‐disciplinary perspectives from pathophysiology, human biology, and epidemiology. Am J Phys Anthropol 155:294–308. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Imakiire A, Mizuno F, Yamamoto K. 2000. Activation of nuclear factor κB at the onset of ossification of the spinal ligaments. J Orthop Sci 5:572–578. [DOI] [PubMed] [Google Scholar]
- Larsen CS. 2015. Bioarchaeology. Interpreting behavior from the human skeleton, 2nd ed. Cambridge: Cambridge University Press. [Google Scholar]
- Littlejohn GO, Urowitz MB, Smythe HA, Keystone EC. 1981. Radiographic features of the hand in diffuse idiopathic skeletal hyperostosis (DISH). Radiol 140:623–629. [DOI] [PubMed] [Google Scholar]
- Macintosh AA, Davies TG, Ryan TM, Shaw CN, Stock JT. 2013. Periosteal versus true cross‐sectional geometry: a comparison among humeral, femoral, and tibial diaphyses. Am J Phys Anthropol 150:442–452. [DOI] [PubMed] [Google Scholar]
- Mader R, Verlan J‐J, Buskila D. 2013. Diffuse idiopathic skeletal hyperostosis: clinical features and pathogenic mechanisms. Nat Rev Rheumatol 9:741–750. [DOI] [PubMed] [Google Scholar]
- Maruotti N, Corrado A, Neve A, Canatore FP. 2013. Systemic effects of Wnt signalling. J Cell Physiol 228:1428–1432. [DOI] [PubMed] [Google Scholar]
- Mays S. 2000. Age‐dependent cortical bone loss in women from 18th and early 19th century London. Am J Phys Anthropol 112:349–361. [DOI] [PubMed] [Google Scholar]
- Mays S. 2001. Effects of age and occupation on cortical bone in a group of 18th–19th century British men. Am J Phys Anthropol 116:34–44. [DOI] [PubMed] [Google Scholar]
- Mays S. 2007. The human remains In: Mays S, Harding C, Heighway C. Wharram. A study of settlement on the Yorkshire Wolds XI: The Churchyard. York University Archaeological Publications 13. York: York University; p 77‐192, 337–397. [Google Scholar]
- Mays S. 2012. An investigation of age‐related changes at the acetabulum in 18th–19th century AD adult skeletons from Christ Church Spitalfields, London. Am J Phys Anthropol 149:485–492. [DOI] [PubMed] [Google Scholar]
- Mays S. 2015. The effect of factors other than age upon skeletal age indicators in the adult. Ann Hum Biol 42:330–339. [DOI] [PubMed] [Google Scholar]
- McFadden D. 1978. Quantitative methods for analysing travel behaviour of individuals: some recent developments In: Hensher DA, Stopher PR, editors. Behavioural travel modelling. London: Croom‐Helm; p 279–318. [Google Scholar]
- Menard S. 2000. Coefficients of determination for multiple logistic regression analysis. Am Stat 54:17–24. [Google Scholar]
- Molleson T, Cox M. 1993. The Spitalfields project, Volume 2: The anthropology. CBA Research Report 86. York: Council for British Archaeology. [Google Scholar]
- Parfitt AM. 2005. New concepts of bone remodelling: a unified spatial and temporal model with physiologic and pathophysiologic implications In: Agarwal SC, Stout SD, editors. Bone loss and osteoporosis. An anthropological perspective. New York: Kluwer Academic/Plenum; p 3–17. [Google Scholar]
- Resnick D, Niwayama G. 1976. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiol 119:559–568. [DOI] [PubMed] [Google Scholar]
- Resnick D, Niwayama G. 1988. Diffuse idiopathic skeletal hyperostosis (DISH): ankylosing hyperostosis of Forestier and Rotes‐Querol In: Resnick D, Niwayama G, editors. Diagnosis of Bone and Joint Disorders, 2nd ed. London: WB Saunders; p 1563–1602. [Google Scholar]
- Robling AG, Stout SG. 2008. Histomorphometry of human cortical bone: applications to age estimation In: Katzenberg MA, Saunders SR, editors. Biological anthropology of the human skeleton, 2nd ed. Chichester: Wiley; p 149–182. [Google Scholar]
- Rogers J. 2000. The palaeopathology of joint disease In: Cox M, Mays S, editors. Human osteology in archaeology and forensic science. London: Greenwich Medical Media; p 163–182. [Google Scholar]
- Rogers J, Shepstone L, Dieppe P. 1997. Bone formers: osteophyte and enthesophyte formation are positively associated. Ann Rheum Dis 56:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Shepstone L, Dieppe P. 2004. Is osteoarthritis a systemic disorder of bone? Arth Rheum 50:452–457. [DOI] [PubMed] [Google Scholar]
- Rogers J, Waldron T, Dieppe P, Watt I. 1987. Arthropathies in palaeopathology: the basis of classification according to most probable cause. J Archaeol Sci 14:179–193. [Google Scholar]
- Sahin G, Polat G, Bagis S, Milcan A, Erdogan C. 2002. Study of axial bone mineral density in postmenopausal women with diffuse idiopathic skeletal hyperostosis related to type 2 diabetes mellitus. J Women's Health 11:801–804. [DOI] [PubMed] [Google Scholar]
- Salari P, Abdollahi M. 2014. The influence of pregnancy and lactation on maternal health: a systematic review. J Family Reproduct Health 8:135–148. [PMC free article] [PubMed] [Google Scholar]
- Senolt L, Julejova H, Krystufkova O, Forejtova S, Andres Cerezo L, Gatterova J, Pavelka K, Vencovsky J. 2012. Low circulating Dickkopf‐1 and its link with severity of spinal involvement in diffuse idiopathic skeletal hyperostosis. Ann Rheum Dis 71:71–74. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Wapler U, Couallier V, Cunha E. 2007. Are bone losers distinguishable from bone formers in a skeletal series? Implications for adult age at death assessment methods. Homo‐J Comp Hum Biol 58:53–66. [DOI] [PubMed] [Google Scholar]
- Stoltzfus JC. 2011. Logistic regression: a brief primer. Acad Emerg Med 18:1099–1104. [DOI] [PubMed] [Google Scholar]
- Szulc P, Seeman E. 2009. Thinking inside and outside the envelopes of bone. Osteoporos Int 20:1281–1288. [DOI] [PubMed] [Google Scholar]
- Tabachnik BG, Fidell LS. 2013. Using multivariate statistics, 6th edn London: Pearson. [Google Scholar]
- Troillet N, Burckhardt P, Gerster JC. 1992. Etude controlee du metabolisme phosphocalcique et de la densitometrie osseuse de l'avant bras au cours de la maladie de Forestier. Rhumatol 44:150–152. [Google Scholar]
- Waldron T. 2007. St Peter's Barton‐upon‐Humber, Lincolnshire. A Parish Church and its Community. Volume 2 The human remains. Oxford: Oxbow. [Google Scholar]
- Waldron T. 2009. Palaeopathology. Cambridge: Cambridge University Press. [Google Scholar]
- Weinfeld RM, Olson PN, Maki DD, Griffiths HJ. 1997. The prevalence of diffuse idiopathic skeletal hyperostosis in two large American Midwest metropolitan hospital populations. Skel Radiol 26:222–225. [DOI] [PubMed] [Google Scholar]
- Weiss E. 2013. Hand osteoarthritis and bone loss: is there an inverse relationship? Homo J Comp Hum Biol 64:357–365. [DOI] [PubMed] [Google Scholar]
- Westerveld LA, Quarles van Ufford HME, Verlaan J‐J, Oner FC. 2008. The prevalence of diffuse idiopathic skeletal hyperostosis in an outpatient population in the Netherlands. J Rheumatol 35:1635–1638. [PubMed] [Google Scholar]
- Westerveld LA, Verlaan J‐J, Lam MG, Scholten WP, Bleys RL, Dhert WJ, Oner FC. 2009. The influence of diffuse idiopathic skeletal hyperostosis on bone mineral density measurements of the spine. Rheumatol 48:1133–1136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


