Abstract
Aims
It has been shown that electrical stimulation can improve tissue repair in patients. Imbalances in the extracellular matrix composition induce manifestation of heart failure. Here we investigated the application of microcurrent (MC) to modulate the expression of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) in cardiomyocytes in vitro and in vivo to reverse remodelling in the heart in spontaneous hypertensive rats (SHR).
Methods
Cardiomyocytes from young SHR (7 months) and old SHR (14 months) were stimulated in vitro and in vivo with MC. MMP and TIMP expression were analysed by qPCR and immunofluorescence to evaluate the modulation of MC treatment.
Results
Modulation of cardiomyocytes with MC enhances proliferation with no morphological changes in vitro. By electrical stimulation dual effects, increase and decrease, on MMP‐2, MMP‐9, TIMP‐3, and TIMP‐4 mRNA as well as protein expression were observed, depending on the age of the cardiomyocytes. In our in vivo study, MC down‐regulated MMP‐2, MMP‐9, and TIMP‐4 and increased TIMP‐3 in young SHR. In old SHR MMP‐2, MMP‐9, and TIMP‐4 were up‐regulated, whereas TIMP‐3 was unaffected.
Conclusions
Our data indicate that treatment of MC can modulate the expression of MMPs and TIMPs in vitro and in vivo in SHR. Based on these results new treatments for heart failure could be developed.
Keywords: Cardiomyocytes, Microcurrent, Extracellular matrix, MMP/TIMP
Introduction
Heart failure (HF) is manifested by defects in cardiac pump function (ejection, filling, or a combination of both), which in turn will cause clinical signs and symptoms that are often progressive and result in emergent presentation and hospitalization.1
The structural and functional manifestation of HF has been associated with structural changes in the extracellular matrix (ECM) composition.2 The cardiac ECM assigns the cellular environment and serves as reservoir for signalling molecules such as growth factors, hormones, and cytokines. Modulation of these components provides correct mechanical, chemical, and electrical signalling between cells.3, 4 An imbalance of the ECM composition and organization results in cardiac dysfunction, remodelling, and progression of HF.
Organization of the cardiac ECM is a sensitive balance between matrix synthesis and degradation, which requires matrix metalloproteinases (MMPs) as well as tissue inhibitor of MMPs (TIMPs), which are produced and released by a number of myocardial cell types, including myocytes. 5 The gelatinases, MMP‐2 and MMP‐9, are responsible for collagen turnover, the major part of the cardiac ECM. Increased MMP‐2 expression is associated with cardiac dysfunction under pathophysiologic conditions.6 MMP‐9, which is also expressed in cardiomyocytes is up‐regulated in HF.7, 8 TIMP‐3 is the TIMP that is ECM bound and could thereby exert tissue‐specific effects.9 Comparative studies in vitro have identified that TIMP4 affects transdifferentiation independent of MMP inhibitory effect.10 Vanhoutte and Heymans found that TIMP‐3 expression is reduced and TIMP‐4 is highly expressed in failing hearts.11 Mujumdar and Tyagi demonstrated that TIMP‐4 levels increased with hypertrophy and decreased with the onset of HF in experimental animals.12
In vivo and in vitro studies in other organs have shown that electric stimulation can modulate a number of factors relevant for tissue remodelling.13, 14, 15 Electric stimulation has proven clinical improvement in the treatments of bone fracture, wound healing, and spinal cord injury.16 Electrically stimulating cartilage explanted from patient with osteoarthritis resulted in increased collagen deposition and reduced mRNA expression of certain MMPs.17 In fibroblast culture, cellular viability, migration, and rate of protein synthesis, including matrix proteins, have been shown to be increased with electrical stimulation.18
Therefore we were wondering whether microcurrent (MC) application would influence the ECM in hypertrophic hearts.
It is the goal of the present study to investigate whether MC can change the expression of MMPs and TIMPs in cardiomyocytes to reverse remodelling in the heart.
MC was applied (a) in vitro by using cardiomyocytes, isolated from young and old spontaneously hypertensive rats (SHR) and (b) in vivo using young and old SHR rats.
Methods
Ethics statement
All experiments were approved by the local Institutional Animal Care and Use Committee (IACUC) of the Medical University of Vienna. Housing, handling, and the experimental procedures were accredited by Austrian authorities according to the Austrian Law of Animal experiments and the DIRECTIVE 2010/63/EU on the protection of animals used for scientific purposes.
Animal groups
Sixteen male spontaneous hypertensive rats (SHR) were divided into four groups: SHR young with MC (SHR MC; n = 5; 8.8 ± 2.7 months), SHR young without MC (SHR w/o MC; n = 3; 7.0 ± 0.0 months), SHR old with MC (SHR MC; n = 5; 14.2 ± 1.3 months), and SHR old without MC (SHR w/o MC; n = 3; 14.3 ± 2.0 months). As healthy controls male Wistar Kyoto (WKY) were used in the in vivo experiments (young WKY, n = 3; 6.6 ± 0.4 months and old WKY; n = 3; 14.0 ± 0.0 months). All rats were obtained from Harlan‐Winkelmann (Germany).
In vivo microcurrent setting
Rats were anaesthetized intraperitoneally with ketamine (100 mg/kg) and xylazine (5 mg/kg). After endotracheal intubation and controlled ventilation with 40% O2 and 1% isoflurane electrodes were implanted. Antibiotic therapy was given with enrofloxacine (10 mg/kg, Baytril®, Bayer, Vienna, Austria). Piritramide (4.5 mg/kg, Dipidolor®, Janssen‐Cilag Pharma, Vienna, Austria) was used for analgesia.
In the five young and old SHR rats a platinum patch electrode was fixed on the left ventricular epicardium of the heart with 7.0 monophilic single stitch technique via a left side thoracotomy. The counter electrode was placed subcutaneous on the contra lateral side of the chest. Direct MC (~1 μA) was applied to the epicardium via the implant patch over a period of 7.7 ± 0.9 h per day. In average MC was applied for 24.3 ± 6.1 days. The two groups without MC were not exposed to MC. The animals were not anaesthetized during the treatment. Each animal was placed during the treatment in a separate cage with a grid‐lid where the electric cable could be conducted to the power supply.
At the end of the study all rats were sacrificed under anaesthetic by 100 mg/kg ketamine and 10 mg xylazine i.p. Rat hearts were removed under sterile conditions and placed in cold Ringer Solution (Mayrhofer Pharmazeutika, Austria). Myocardial samples for histology were stored in 7.5% formalin; heart tissue for molecular analysis was frozen in liquid nitrogen and stored at −80°C immediately after excision.
Primary culture of cardiomyocytes
Cardiac myocytes were isolated from hearts of 7 ‐month ‐old (SHR young) and 14 ‐month ‐old male SHR (old). Briefly, rats were sacrificed under anaesthetic by ketamine i.p., rat hearts were rinsed with Ringer Solution, and the left ventricle was digested mechanically. After a preplating procedure for 60 min to eliminate fibroblasts, the supernatant was transferred to a six‐well culture plate (Corning, USA), and cells were cultivated in Dulbecco's modified Eagle's medium (DMEM, Invitrogen™, UK) with 10% fetal calf serum (FCS, PAA, Austria), ascorbic acid (5 mg/mL, Sigma, USA), transferrin (10 µg/mL, Sigma), NEAA (Non‐Essential Amino Acids, 10 mM, Sigma), sodium‐selenite (20 µg/mL, Sigma, USA), insulin (10 µg/mL, Sigma), Endothelial Cell Growth Supplement (50 µg/mL, Becton Dickinson, Austria), and penicillin/streptomycin (100 U/mL‐100 µg/mL, Gibco, UK). Medium was replaced once a week.
As control H9c2 cells (CRL‐1446, ATCC, USA) were cultivated in DMEM supplemented with 10% FCS, and penicillin/streptomycin (100 U/mL–100 µg/mL). Cell cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Characterization of cardiomyocytes
Morphological adaptations of rat cardiomyocytes in vitro were evaluated by immunofluorescence staining. Cells were grown on slides, washed with PBS, and fixed in cooled acetone. Cells were permeabilized with 1% H2O2, blocked in blocking solution [2% goat serum or 2% horse serum, 1% bovine serum albumin, 0.1% gelatine, 0.1% Triton X‐100, 0.05% Tween 20, and 1× Tris Buffered Saline (TBS), all reagents from Sigma] and incubated with the primary antibody at 4°C overnight (Supporting Information, Table S1 ). The following day, cells were washed three times in TBS and incubated with the secondary antibody for 1 h at room temperature. Cells were counterstained with DAPI (4′,6‐diamidino‐2‐phenylindole dihydrochloride, 1 µg/mL, Sigma), mounted in fluorescence mounting medium (Dako, Austria), and visualized by fluorescence microscopy. Only cultured cells which were more than 95% positive for cardiac markers were used in the experiments.
In vitro microcurrent setting
Cells from SHR young and SHR old were stimulated using a direct MC power generator with two electrodes which were integrated into the top cover of the culture plate. The cells were treated without MC (w/o MC) and with ~1 μA (MC). For proliferation experiments 1 × 10E4 cells were seeded in 24‐well plates in triplicates and counted daily. For immunofluorescence staining 1.5 × 10E4 cells were seeded on cover slips. After MC treatment cells were washed with 1× Phosphate Buffered Saline (PBS), fixed in acetone for 10 min at 4°C and air dried. For qPCR 1 × 105 cells were seeded in 24‐well plates and harvested with a rubber policeman for RNA isolation.
Histology and immunohistochemistry of tissue
Sections (5 µm thick) were stained with haematoxylin/eosin and Goldner's Masson trichrome stain to determine the percentage of collagen. For immunohistochemistry tissue sections were stained using an avidin–biotin–immunoperoxidase method according to the manufacturer's protocol (Vectastain Elite ABC Kit, Vector Laboratories, USA). Briefly, sections were incubated with diaminobenzidine (DAB substrate Kit, Vector Laboratories) to visualize positive immunoreactions, counterstained with haematoxylin covered in mounting medium (Dako). Tissue staining was analysed by using Image J software 1.49n (http://rsbweb.nih.gov/ij/) and proteins were calculated in per cent of pixel relative to age matched control.
Double staining of cells
Double staining for MMPs and TIMPs was carried out by incubation of both primary antibodies overnight (Table S1). After washing the cells with 1× TBS corresponding secondary antibodies were incubated for 1 h at room temperature. Cells were counterstained with DAPI and mounted with fluorescence mounting medium (Dako). The cells were analysed by using Nuance™ FX multispectral imaging system 2.8.0 (PerkinElmer, USA).
RNA isolation and qPCR
Total RNA from cells was isolated using a Gene Elute Mammalian Total RNA Isolation Kit (Sigma). Eight randomly picked RNA samples from cells were pooled, and 1 µg was used for cDNA‐synthesis using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Austria).
RNA from snap frozen tissue was extracted using Trizol (Invitrogen, Austria) according to the manufacturer's protocol.
QPCR was performed using Power SYBR Green PCR Master Mix (Applied Biosystems) and 7500 Real Time PCR System, according to the manufacturer procedure using gene specific primer listed in Table S2. Relative signal quantifications were carried out by signal normalization of different genes with the glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) signal used as endogenous control by 2(−ΔCT) method. Measurements were performed in triplicates.
Statistical analysis
All results are expressed as means ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism software (Prism5; GraphPad Software, San Diego, USA). Different groups were compared by using paired t‐test or one‐way ANOVA followed by Bonferroni's post hoc test. Statistical significance was recognized at P < 0.05.
Results
Microcurrent induces proliferation in cardiomyocytes in vitro
Rat cardiomyocytes (young SHR and old SHR) as well as control cells were grown without and with MC for up to 96 h. All cells were adherent within 24 h after seeding and showed no morphological changes during the indicated time period (Figure S1).
A significant increase in the cell number of cardiomyocytes of young SHR treated with MC at the time points 24 h, 48 h, and 72 h compared with no treatment was observed (*P < 0.05;***P < 0.001; Figure 1 A). In H9c2 similar cell amplification was detected during MC treatment (data not shown). In SHR old cardiomyocytes a slight increase in the cell number but no statistical difference was observed (Figure 1 B).
Figure 1.
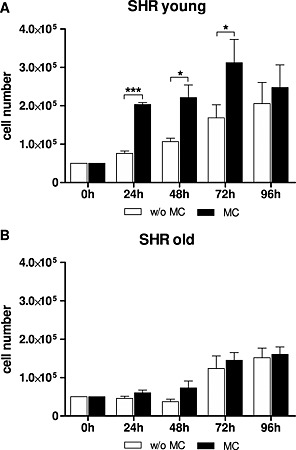
Cell number of cardiomyocytes from (A) young spontaneous hypertensive rats and (B) old spontaneous hypertensive rats treated without and with MC determined at the indicated time points. Mean ± standard deviation in triplicates,*P < 0.05; ***P < 0.001. MC, microcurrent.
Analysis of matrix metalloproteinase‐2 expression in vitro and in vivo after microcurrent stimulation
In vitro, treatment with MC decreased MMP‐2 mRNA expression in young SHR cardiomyocytes 0.81‐fold, whereas in control cardiomyocytes and old SHR cardiomyocytes MMP‐2 mRNA levels increased (2.99‐fold and 1.30‐fold, respectively; Table S3). Immunofluorescence staining revealed that MMP‐2 expression was slightly decreased upon MC treatment in old SHR cardiomyocytes (97.6 ± 10.3%), whereas in control and young SHR cardiomyocytes an increase compared with no treatment was observed (113.0 ± 8.0% and 142.0 ± 11.8%, respectively; Figure 2 A).
Figure 2.
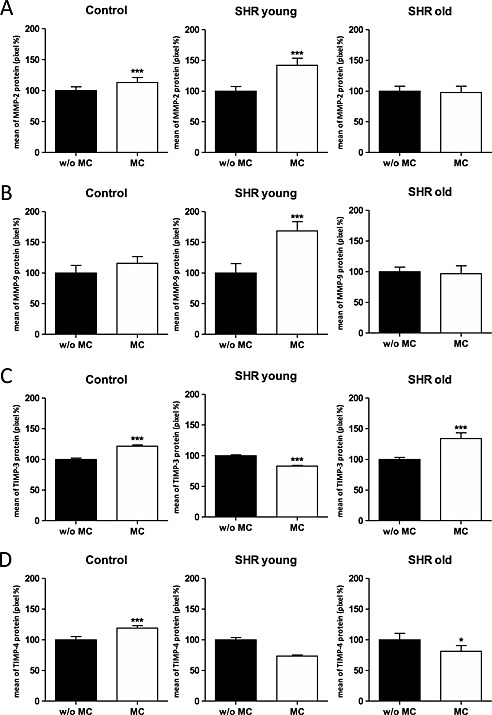
In vitro immunofluorescence staining of control, spontaneous hypertensive rats young, and spontaneous hypertensive rats old cells without and with MC stimulation. Bar charts are showing the quantification of positive stained cells. (A) MMP‐2; (B) MMP‐9; (C) TIMP‐3 and (D) TIMP‐4. In all cases, data shown are the mean ± standard deviation of three different experiments. *P < 0.05; ***P < 0.001; MC, microcurrent; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase.
In vivo, we observed that in heart tissue of non‐treated young SHR and old SHR MMP‐2 mRNA levels (0.58‐fold and 0.45‐fold, respectively) as well as protein levels (98.8 ± 3.7% and 92.6 ± 3.5%, respectively) were lower compared with non‐treated age‐matched WKY rats (Table S4).
Treatment with MC decreased MMP‐2 mRNA expression in young SHR (0.78‐fold) as well as in old SHR (0.24‐fold) compared with the non‐treated SHR (Figure 3 A). Also the MMP‐2 protein level decreased in young SHR (97.1 ± 5.2%), and increased in old SHR (99.4 ± 2.5%, P < 0.001) upon MC treatment (Figure 4 A).
Figure 3.
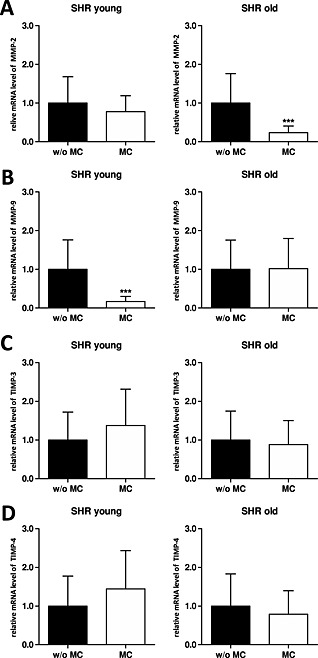
In vivo mRNA expression data from young and old spontaneous hypertensive rats without and with microcurrent treatment. Graphs showing transcript changes using qPCR analysis as fold change relative to glyceraldehyde‐3‐phosphate dehydrogenase. (A) Matrix metalloproteinase (MMP)‐2; (B) MMP‐9; (C) tissue inhibitor of metalloproteinase (TIMP)‐3 and (D) TIMP‐4. In all cases, data shown are the mean ± standard deviation of five animals per group, *P < 0.05; ***P < 0.001.
Figure 4.
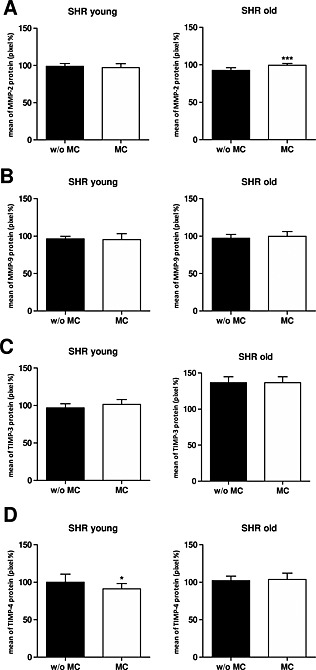
In vivo protein expression data from young and old spontaneous hypertensive rats without and with microcurrent treatment. Bar charts are showing the quantification of positive stained cells. (A) Matrix metalloproteinase (MMP)‐2; (B) MMP‐9; (C) tissue inhibitor of metalloproteinase (TIMP)‐3 and (D) TIMP‐4. In all cases, data shown are the mean ± standard deviation of five animals per group, *P < 0.05; ***P < 0.001.
Effect of microcurrent on matrix metalloproteinase‐9 expression in vitro and in vivo
In vitro, treatment with MC slightly increased MMP‐9 mRNA expression in control as well as young SHR cardiomyocytes (1.05‐fold and 1.16‐fold, respectively) compared with the non‐treated cardiomyocytes (Table S3). Similar results were obtained at the protein level (115.8 ± 10.8% and 168.4 ± 15.3%, respectively; Figure 3 B). In old SHR cardiomyocytes we observed a slight increase of MMP‐9 mRNA expression (1.06‐fold) upon MC treatment, but a slight decrease on protein level (96.7 ± 12.9%, Figure 2 B).
In vivo, we observed that in heart tissue of non‐treated young SHR MMP‐9 mRNA levels were increased (16.50‐fold) whereas non‐treated old SHR showed decreased mRNA expression (0.68‐fold) compared with the appropriate non‐treated age‐matched WKY rats (Table S4). Protein levels were slightly lower in young and old SHR (96.4 ± 3.4% and 97.3 ± 4.7%, respectively) when compared with non‐treated age‐matched WKY rats (data not shown).
Treatment with MC decreased MMP‐9 mRNA expression in young SHR (0.17‐fold) but not in old SHR (1.02‐fold) compared with the non‐treated SHR (Figure 3 B). Changes on protein level were marginal in young SHR (95.3 ± 7.7%) as well as in old SHR (99.8 ± 6.1%, Figure 4 B).
Analysis of tissue inhibitor of metalloproteinase‐3 expression in vitro and in vivo after microcurrent stimulation
In vitro, treatment with MC decreased TIMP‐3 mRNA expression in the different cardiomyocytes (control, young SHR, and old SHR; 0.93‐fold, 0.98‐fold, and 0.57‐fold, respectively; Table S3). Immunofluorescence staining revealed that TIMP‐3 expression was decreased upon MC treatment in young SHR cardiomyocytes (83.1 ± 1.1%), whereas in control and old SHR cardiomyocytes an increase compared with no treatment was observed (121.6 ± 2.2% and 133.9 ± 9.5%, respectively; Figure 2 C).
In vivo, we observed that in heart tissue of non‐treated young SHR TIMP‐3 mRNA levels were slightly increased (1.11‐fold) whereas non‐treated old SHR showed decreased mRNA expression (0.55‐fold) compared with the appropriate non‐treated age‐matched WKY rats (Table S4). In comparison to age‐matched WKY rats, the protein levels of TIMP‐3 were lower in young SHR (97.0 ± 5.3%), but higher in old SHR (136.5 ± 8.3%, data not shown). In young SHR the treatment with MC lead to an increase of TIMP‐3 mRNA as well as TIMP‐3 protein expression (1.37‐fold and 101.5 ± 6.6%, respectively; Figures 3 C and 4 C). In contrast, in old SHR we could observe a decrease in TIMP‐3 mRNA expression (0.73‐fold), but interestingly no change on protein level (136.5 ± 8.3%) upon MC treatment (Figures 3 C and 4 C).
Effect of microcurrent on tissue inhibitor of metalloproteinase‐4 expression in vitro and in vivo
In vitro, treatment with MC increased TIMP‐4 mRNA expression in the different cardiomyocytes (control, young SHR, and old SHR; 5.10‐fold, 1.13‐fold, and 2.05‐fold, respectively; Table S3). Immunofluorescence staining revealed that TIMP‐4 expression was decreased upon MC treatment in young and old SHR cardiomyocytes (73.5 ± 1.8% and 81.3 ± 9.4%, respectively), whereas in control cardiomyocytes an increase compared with no treatment was observed (119.0 ± 3.9%, Figure 2D).
In vivo, we observed that in heart tissue of non‐treated young SHR and old SHR TIMP‐4 mRNA levels were lower (0.63‐fold and 0.31‐fold, respectively) whereas the protein levels (100.0 ± 10.9% and 102.0 ± 6.2%, respectively) were similar compared with non‐treated age‐matched WKY rats (Table S4). When we treated with MC, we observed increased TIMP‐4 mRNA levels in young SHR (1.44‐fold), but decreased mRNA levels in old SHR (0.69‐fold; Figure 3 D). At the protein level we saw an opposite effect: TIMP‐4 protein levels were decreased in young SHR (91.3 ± 7.0%, P < 0.05) and slightly increased in old SHR (103.7 ± 8.5%) upon treatment with MC (Figure 4 D).
Discussion
Cardiac remodelling is a major determinant in the clinical progress of HF. New approaches to promote reverse remodelling are being evaluated. Although several studies have described the effect of electrical application on tissue repair,19, 20 no data are available so far on the effects of MC on cardiomyocytes or remodelling of the heart tissue. ECM remodelling in the heart implies changes in the rearrangement of normal existing structures.21 Organization of the cardiac ECM is a sensitive balance between matrix synthesis and degradation, which requires MMPs as well as tissue inhibitor of MMPs (TIMPs), which are produced and released by a number of myocardial cell types, including myocytes.
In the present study, we investigated the influence of MC on the modulation of MMPs and TIMPs in cardiomyocytes in vitro and in heart tissue of young and old SHR in vivo. In vitro, on the one hand, modulation with MC did not change the morphology. On the other hand, enhanced proliferation in young and old SHR cardiomyocytes was observed, but only in young SHR with a statistical significance. Mechanical instead of enzymatic cell isolation increases the efficiency to obtain old primary cells in culture that would further proliferate. Furthermore, our in vitro results demonstrate that stimulation with MC had different effects on MMP‐2, MMP‐9, and TIMP‐3 mRNA as well as protein expression in cardiomyocytes dependent on the age of the SHR they were derived from. Decreased TIMP‐4 protein levels were observed upon MC treatment in cardiomyocytes from young as well as old SHR. Using different culture media (e.g. with high or low glucose) the expression of MMP2/MMP9 can be modified.22 Our data show that MC can modulate expression of MMPs and TIMPs on mRNA and protein level in cardiomyocytes in vitro. All these in vitro data point into the direction that MC leads to a statistically significant enhanced proliferation in young and to a lesser extent in old cardiomyocytes and an antifibrotic stimulation in young and old SHR cells. Our results conform data published by Brighton et al. reporting the beneficial effect of electric stimuli on mRNA and protein synthesis of matrix proteins in osteoarthritic implants.13
Numerous studies have demonstrated increased levels of MMPs, especially MMP‐2 and MMP‐9, at sites of cardiovascular diseases, including hypertension.23, 24, 25 Also it has been commonly observed that in ageing, fibrosis leads to increased passive stiffness in the myocardium and impaired diastolic dysfunction. Therefore, changes in the MMP and TIMP profile and the consequent effects on myocardial ECM are found in ageing.26 MMP‐2 is also known to induce MMP‐9 and in consequence contributing to tissue gelatinase activities, which were associated with cardiac disease.27 Li et al. showed increased MMP‐9 activity in compensatory hypertrophy in SHR rats.28
In our in vivo study, we observed that MC treatment decreased MMP‐2 protein expression in young SHR but increased MMP‐2 protein expression in old SHR. In contrast, we found that MMP‐9 expression was decreased in young SHR but increased in old SHR after MC treatment. Our results conform data published by Spinale et al. reporting that different MMP‐2 levels are existing in dilated cardiomyopathy, depending on the stimulation of different intracellular signalling pathways and selective activation of MMPs29. Because MMP‐9 acts downstream of MMP‐2 and old SHR act as a model for hypertension in humans, MC treatment may have a positive effect on reverse remodelling. Local proteolytic activity in cardiac ECM is important for decreasing collagen accumulation and prevents fibrosis.
TIMP‐3 has been identified as a key factor in the regulation of remodelling and is associated with HF.30 A reduction of TIMP‐3 is accompanied by MMP activity, matrix turn over, and significant changes in the ECM.31 TIMP‐4 is predominantly expressed in the cardiovascular system and may have relevance to the myocardial remodelling process.32
In our study, we observed that MC treatment increased TIMP‐3 protein expression in young SHR whereas no change was observed in old SHR. TIMP‐4 expression was decreased in young SHR but increased in old SHR upon MC treatment. Because TIMP‐4 serves as a regulator of MMP‐2 activity, MC treatment might contribute to ECM regeneration and reverse remodelling in old SHR through up‐regulation of TIMP‐4.
All in all, our in vivo data point into the direction that the effect of MC is more prominent in young SHR. In old SHR, the group of our interest, a modulation by treatment of MC to prevent remodelling is also observed, but less prominently.
In summary, we could show that MC stimulation can modulate the expression of MMPs and TIMPs in cardiomyocytes in vitro and in vivo and might be a potential therapeutic tool to induce reverse remodelling processes for heart regeneration.
Limitations
The following limitation has to be indicated:
In the in vitro experiments other physiological conditions persist than in the in vivo situation. There are no other cell types in connection to each other and therefore protein turnover is different.
Changes on mRNA levels were more prominent compared with the changes in protein levels in vitro as well in vivo. It might be possible to enhance the modulation of protein levels by applying a more extended MC treatment.
Conclusions
In this study, using a novel methodology, we show that application of MC can modulate the expression of MMPs and TIMPs in cardiomyocytes in vitro and in heart tissue in vivo.
Although further studies are needed, e.g. extended MC treatment in vivo, our findings support the notion that this technology could have clinical implication for patients with HF.
Conflict of Interest
None declared.
Supporting information
Supporting info item
Supporting info item
Kapeller, B. , Mueller, J. , Losert, U. , Podesser, B. K. , and Macfelda, K. (2016) Microcurrent stimulation promotes reverse remodelling in cardiomyocytes. ESC Heart Failure, 3: 122–130. doi: 10.1002/ehf2.12080.
References
- 1. Ponikowksi P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Siswanto BB, Sliwa K, Filipaos G. Heart failure: preventing disease and death worldwide. ESC Heart Failure 2014; 1: 4–25. [DOI] [PubMed] [Google Scholar]
- 2. Spinale FG, Zile MR. Integrating the myocardial matrix into heart failure recognition and management. Circ Res 2013; 113: 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bowers SL, Banerjee I, Baudino TA. The extracellular matrix: at the center of it all. J Mol Cell Cardiol 2010; 48: 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol 2011; 12: 308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bax NA, van Marion MH, Shah B, Goumans MJ, Bouten CV, van der Schaft DW. Matrix production and remodeling capacity of cardiomyocyte progenitor cells during in vitro differentiation. J Mol Cell Cardiol 2012; 53: 497–508. [DOI] [PubMed] [Google Scholar]
- 6. Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 2007; 87: 1285–1342. [DOI] [PubMed] [Google Scholar]
- 7. He BJ, Joiner MA, Singh MV, Luczak ED, Swaminathan PD, Koval OM, Kutschke W, Allamargot C, Yang J, Guan X, Zimmerman K, Grumbach IM, Weiss RM, Spitz DR, Sigmund CD, Blankesteijn WM, Heymans S, Mohler PJ, Anderson ME. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nature Med 2011; 17: 1610–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase‐9: many shades of function in cardiovascular disease. Physiology (Bethesda) 2013; 28: 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Troeberg L, Lazenbatt C, Anower‐E‐Khuda MF, Freeman C, Federov O, Habuchi H, Habuchi O, Kimata K, Nagase H. Sulfated glycosaminoglycans control the extracellular trafficking and the activity of the metalloprotease inhibitor TIMP‐3. Chem Biol 2014; 21: 1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lovelock JD, Baker AH, Gao F, Dong JF, Bergeron AL, McPheat W, Sivasubramanian N, Mann DL. Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. Am J Physiol Heart Circ Physiol 2005; 288: H461–H468. [DOI] [PubMed] [Google Scholar]
- 11. Vanhoutte D, Heymans S. TIMPs and cardiac remodeling: ‘Embracing the MMP‐independent‐side of the family’. J Mol Cell Cardiol 2010; 48: 445–453. [DOI] [PubMed] [Google Scholar]
- 12. Mujumdar VS, Tyagi SC. Temporal regulation of extracellular matrix components in transition from compensatory hypertrophy to decompensatory heart failure. J Hypertens 1999; 17: 261–270. [DOI] [PubMed] [Google Scholar]
- 13. Brighton CT, Wang W, Clark CC. The effect of electrical fields on gene and protein expression in human osteoarthritic cartilage explants. J Bone Joint Surg Am 2008; 90: 833–848. [DOI] [PubMed] [Google Scholar]
- 14. Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol‐3‐OH kinase‐gamma and PTEN. Nature 2006; 442: 457–460. [DOI] [PubMed] [Google Scholar]
- 15. Houghton PE. Clinical trials involving biphasic pulsed current, microcurrent, and/or low‐intensity direct current. Adv Wound Care (New Rochelle) 2014; 3: 166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang ET, Zhao M. Regulation of tissue repair and regeneration by electric fields. Chin J Traumatol 2010; 13: 55–61. [PubMed] [Google Scholar]
- 17. Haddad JB, Obolensky AG, Shinnick P. The biologic effects and the therapeutic mechanism of action of electric and electromagnetic field stimulation on bone and cartilage: new findings and a review of earlier work. J Altern Complement Med 2007; 13: 485–490. [DOI] [PubMed] [Google Scholar]
- 18. Kloth LC. Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Int Low Extrem Wounds 2005; 4: 23–44. [DOI] [PubMed] [Google Scholar]
- 19. Aaron RK, Boyan BD, Ciombor DM, Schwartz Z, Simon BJ. Stimulation of growth factor synthesis by electromagnetic fields. Clin Orthop 2004; 419: 30–37. [DOI] [PubMed] [Google Scholar]
- 20. Ahadian S, Ostrovidov S, Hosseini V, Kaji H, Ramalingam M, Bae H, Khademhosseini A. Electrical stimulation as a biomimicry tool for regulating muscle cell behavior. Organogenesis 2013; 9: 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fedak PW, Verma S, Weisel RD, Li RK. Cardiac remodeling and failure: from molecules to man (Part I). Cardiovasc Pathol 2005; 14: 1–11. [DOI] [PubMed] [Google Scholar]
- 22. Ho FM, Liu SH, Lin WW, Liau CS. Opposite effect of high glucose on MMP‐2 and MMP‐9 in human endothelial cells. J Cell Biochem 2007; 101: 442–450. [DOI] [PubMed] [Google Scholar]
- 23. Lovett DH, Chu C, Wang G, Ratcliffe MB, Baker AJ. A N‐terminal truncated intracellular isoform of matrix metalloproteinase‐2 impairs contractility of mouse myocardium. Front Physiol 2014; 5: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roldán V, Marín F, Gimeno JR, Ruiz‐Espejo F, González J, Feliu E, García‐Honrubia A, Saura D, de la Morena G, Valdés M, Vicente V. Matrix metalloproteinases and tissue remodeling in hypertrophic cardiomyopathy. Am Heart J 2008; 156: 85–91. [DOI] [PubMed] [Google Scholar]
- 25. Bergman MR, Teerlink JR, Mahimkar R, Li L, Zhu BQ, Nguyen A, Dahi S, Karliner JS, Lovett DH. Cardiac matrix metalloproteinase‐2 expression independently induces marked ventricular remodeling and systolic dysfunction. Am J Physiol Heart Circ Physiol 2007; 292: H1847–1860. [DOI] [PubMed] [Google Scholar]
- 26. Horn MA, Graham HK, Richards MA, Clarke JD, Greensmith DJ, Briston SJ, Hall MC, Dibb KM, Trafford AW. Age‐related divergent remodeling of the cardiac extracellular matrix in heart failure: collagen accumulation in the young and loss in the aged. J Mol Cell Cardiol 2012; 53: 82–90. [DOI] [PubMed] [Google Scholar]
- 27. DeCoux A, Lindsey ML, Villarreal F, Garcia RA, Schulz R. Myocardial matrix metalloproteinase‐2: inside out and upside down. J Mol Cell Cardiology 2014; 77: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li H, Simon H, Bocan TM, Peterson JT. MMP/TIMP expression in spontaneously hypertensive heart failure rats: the effect of ACE‐ and MMP‐inhibition. Cardiovasc Res 2000; 46: 298–306. [DOI] [PubMed] [Google Scholar]
- 29. Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation 2000; 102: 1944–1949. [DOI] [PubMed] [Google Scholar]
- 30. Fedak PW, Altamentova SM, Weisel RD, Nili N, Ohno N, Verma S, Lee TY, Kiani C, Mickle DA, Strauss BH, Li RK. Matrix remodeling in experimental and human heart failure: a possible regulatory role for TIMP‐3. Am J Physiol Heart Circ Physiol 2003; 284: H626–34. [DOI] [PubMed] [Google Scholar]
- 31. Kassiri Z, Defamie V, Hariri M, Oudit GY, Anthwal S, Dawood F, Liu P, Khokha R. Simultaneous transforming growth factor‐tumor necrosis factor activation and cross‐talk cause aberrant remodeling response and myocardial fibrosis in Timp3‐deficient heart. J Biol Chem 2009; 284: 29893–29904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, Lowry AS, Escobar GB, Lucas DG, Yarbrough WM, Zile MR, Spinale FG. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation 2003; 107: 618–625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item
Supporting info item


