Summary
Background
Helicobacter pylori is one of the most prevalent global pathogens and can lead to gastrointestinal disease including peptic ulcers, gastric marginal zone lymphoma and gastric carcinoma.
Aim
To review recent trends in H. pylori antibiotic resistance rates, and to discuss diagnostics and treatment paradigms.
Methods
A PubMed literature search using the following keywords: Helicobacter pylori, antibiotic resistance, clarithromycin, levofloxacin, metronidazole, prevalence, susceptibility testing.
Results
The prevalence of bacterial antibiotic resistance is regionally variable and appears to be markedly increasing with time in many countries. Concordantly, the antimicrobial eradication rate of H. pylori has been declining globally. In particular, clarithromycin resistance has been rapidly increasing in many countries over the past decade, with rates as high as approximately 30% in Japan and Italy, 50% in China and 40% in Turkey; whereas resistance rates are much lower in Sweden and Taiwan, at approximately 15%; there are limited data in the USA. Other antibiotics show similar trends, although less pronounced.
Conclusions
Since the choice of empiric therapies should be predicated on accurate information regarding antibiotic resistance rates, there is a critical need for determination of current rates at a local scale, and perhaps in individual patients. Such information would not only guide selection of appropriate empiric antibiotic therapy but also inform the development of better methods to identify H. pylori antibiotic resistance at diagnosis. Patient‐specific tailoring of effective antibiotic treatment strategies may lead to reduced treatment failures and less antibiotic resistance.
Introduction
Helicobacter pylori is one of the most prevalent global pathogens and colonises an estimated 50% of the world's population.1, 2 It was first described in gastric biopsies by Warren and Marshall in Australia in 1983.3, 4, 5 H. pylori is a Gram‐negative bacillus that infects the human stomach mucosa and produces diseases of the upper gastrointestinal tract such as chronic gastritis, peptic ulcer disease, gastric marginal zone/mucosa‐associated lymphoid tissue (MALT) lymphoma and gastric carcinoma.3, 4, 6, 7 More recently, it has been suggested that H. pylori may be associated with extraintestinal diseases, including immune thrombocytopenic purpura, refractory iron deficiency anaemia and vitamin B12 deficiency.1, 8, 9
The precise epidemiology of H. pylori infection still remains unclear; however, studies have shown that ingestion of contaminated food may increase the risk of H. pylori infection. Person‐to‐person transmission by oral‐oral, faecal‐oral or gastro‐oral exposure is suggested to be the most likely route of transmission.10 Accordingly, improvements in hygiene and living conditions are important factors in decreasing the prevalence of infection.11 In addition, mammal and insect reservoirs have been suggested such as pigtailed monkeys, rhesus monkeys, cats, sheep and cockroaches.12 There have also been association studies with maternal infection and socioeconomic status being an important risk factor for paediatric infection.13, 14, 15, 16, 17
Current treatment of H. pylori in the United States is empiric despite relatively high failure rates in more than 20% of cases.18 In the early 1990s the eradication rate of H. pylori was greater than 80%.19, 20, 21 However, the antimicrobial eradication rates are decreasing – as low as 60% in some countries – and are inversely correlated with antibiotic resistance rates reported worldwide.19, 20 Eradication failures are concerning at the present time, but they are likely to be more critical in the future, given that global rates of antibiotic resistance are increasing and therapy for H. pylori infection is increasingly prescribed.21, 22, 23, 24 This is reflected in the most recent Maastricht recommendations which state that susceptibility testing should be performed prior to therapy in regions with high clarithromycin resistance rates.1 According to the recent Kyoto Global Consensus Meeting, only regimens shown to result in at least a 90% eradication rate in a particular region should be used as empiric therapy.25 Therefore, the goal in designing a treatment regimen should focus on a strategy which results in a cure rate approaching 100%.21, 26, 27, 28
Prevalence of H. pylori infection
Infection with H. pylori occurs worldwide, but there are substantial geographic differences in the prevalence of infection both within and between countries.29 Multiple studies have demonstrated that low socioeconomic status is associated with increased risk of H. pylori infection.30, 31 Additionally, an age‐related cohort effect has been observed with prevalence of infection increasing with age.31, 32, 33 Within Europe, H. pylori prevalence rates range from 11% in Sweden to 60.3% in Spain.29, 34 In China, H. pylori prevalence has been reported as high as 83.4%.35 Additionally, many countries such as China, Japan and Bulgaria have experienced an overall increase in the prevalence of H. pylori infection over the last 20 years (Figure 1).29, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 In Canada, the prevalence of H. pylori is approximately 30%; however, within the Aboriginal populations living in Canada, the prevalence of H. pylori has been reported as high as 95%.54, 55 In the USA, cross‐sectional studies of the participants in the National Health and Nutrition Examination Survey (NHANES) III and NHANES 1999–2000 demonstrate an overall seropositivity rate of approximately 30%.29, 56 In populations with high infection rates, it is likely that patients are infected with more than one strain of H. pylori.
Figure 1.
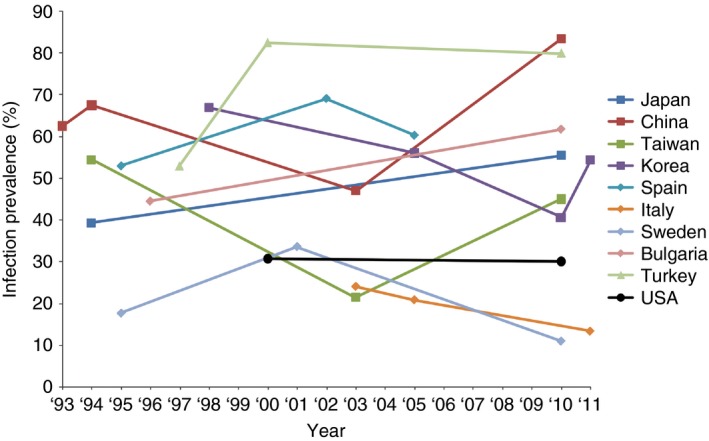
Global prevalence of H. pylori infection by country and year.29, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53
Prevalence of antibiotic resistance
The prevalence of bacterial resistance varies in different geographic areas and appears to be increasing with time in many countries.57, 58 While the overall prevalence of H. pylori in the USA has been similar in studies from both 2000 and 2010, the antimicrobial eradication rates for H. pylori have been decreasing over that interval for several reasons; the most likely primary reasons for treatment failure were found to be H. pylori resistance to one or more of the antibiotics and patient compliance.1, 23, 29, 53, 59, 60, 61
The European Multicentre Study Group included 2204 patients from 2008 to 2009, spanning 18 European countries and demonstrated H. pylori resistance rates to clarithromycin, metronidazole and levofloxacin at 17.5%, 34.9% and 14.1% respectively.22 The Japanese National Surveillance Study looked at 3707 H. pylori isolates from 2002 to 2005. Kobayashi et al. found that clarithromycin resistance rates increased from 18.9% to 27.7% between this 3‐year interval. Metronidazole resistance remained fairly consistent, ranging from 3.3% to 5.3%. Amoxicillin resistance rates were negligible.62 The Surveillance of H. pylori Antimicrobial Resistance Partnership (SHARP) program meta‐analysis conducted between 1993 and 1999 demonstrated that the resistance rates to clarithromycin, metronidazole and amoxicillin are 10.1%, 36.9% and 1.4% respectively.57 The prospective multicenter Helicobacter pylori Antimicrobial Resistance Monitoring Program (HARP) study examined the national incidence rates of H. pylori antimicrobial resistance between 1998 and 2002; this study demonstrated the resistance to clarithromycin, metronidazole and amoxicillin as 12.9%, 25.1% and 0.9% respectively.63
There is evidence that bacterial resistance is correlated with the general consumption of antibiotics in the general population.22, 64 In a study focused on the Alaskan Native population, prior use of a macrolide was associated with increased H. pylori clarithromycin resistance. Overall, 37% of this Alaskan Native population was infected with clarithromycin‐resistant strains of H. pylori and, of these patients, 92% had previously been treated with a macrolide. Additionally, McMahon et al. found a dose–response relationship between clarithromycin resistance and the number of prior macrolide courses. Within this population, a similar association was found with infection by metronidazole‐resistant H. pylori strains and prior metronidazole use.65, 66, 67 Evaluating and understanding this relationship between previous antimicrobial use and H. pylori antimicrobial resistance would help to increase cure rates.68, 69
Clarithromycin
Numerous studies have been performed to determine the prevalence of H. pylori resistance to particular antibiotics. In particular, clarithromycin‐resistant H. pylori has been extensively studied. The prevalence has been increasing in many countries (Figure 2a).22, 48, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85 Studies have shown that in countries with low rates of H. pylori seropositivity, the prevalence of antibiotic resistance does not appear to change considerably over time. For instance, H. pylori seropositivity rates in Sweden have remained approximately 20% since 1995.29, 48, 49 The prevalence of clarithromycin‐resistant strains of H. pylori has also remained low (below 5%) over this period of time.48, 74, 79 In contrast, countries with higher rates of H. pylori seropositivity are associated with dramatically increasing rates of clarithromycin resistance. For example, Horiki et al. demonstrated that the prevalence of clarithromycin resistance has increased considerably from 1.8% in 1996 to 27.1% in 2008 in the Japanese population.70 Okamura et al. described an overall resistance rate of 31.1% in patients studied between 2000 and 2013.71 The prevalence of H. pylori seropositivity over this time period has increased from approximately 40% to 55%.29, 36, 70 Similarly, China has experienced an increase in clarithromycin resistance from 14.8% in 2000 to 52.6% in 2014 with an increase in seropositivity rates from approximately 65% to 83%.35, 37, 38, 72, 73, 74 In addition, a marked increase in prevalence of clarithromycin resistance was seen in Korea from 11% in 2005 to 60% in 2009.76, 77, 78 The variability of clarithromycin resistance seen in different regions emphasises the need to examine resistance rates in each geographic area to better guide treatment regimens. Within the USA, the prevalence of clarithromycin resistance has increased from 6.1% in 1993 to 12.9% in 2002.58, 61, 84 A recent report studying a population of military veterans from the USA between 2009 and 2013 demonstrated an overall 17.8% clarithromycin resistance rate.85 Within the paediatric population, the rate of clarithromycin resistance has been reported to be as high as 50%.86 The dramatic increase in clarithromycin resistance observed in recent reports from the USA suggests the need for an updated national survey.
Figure 2.
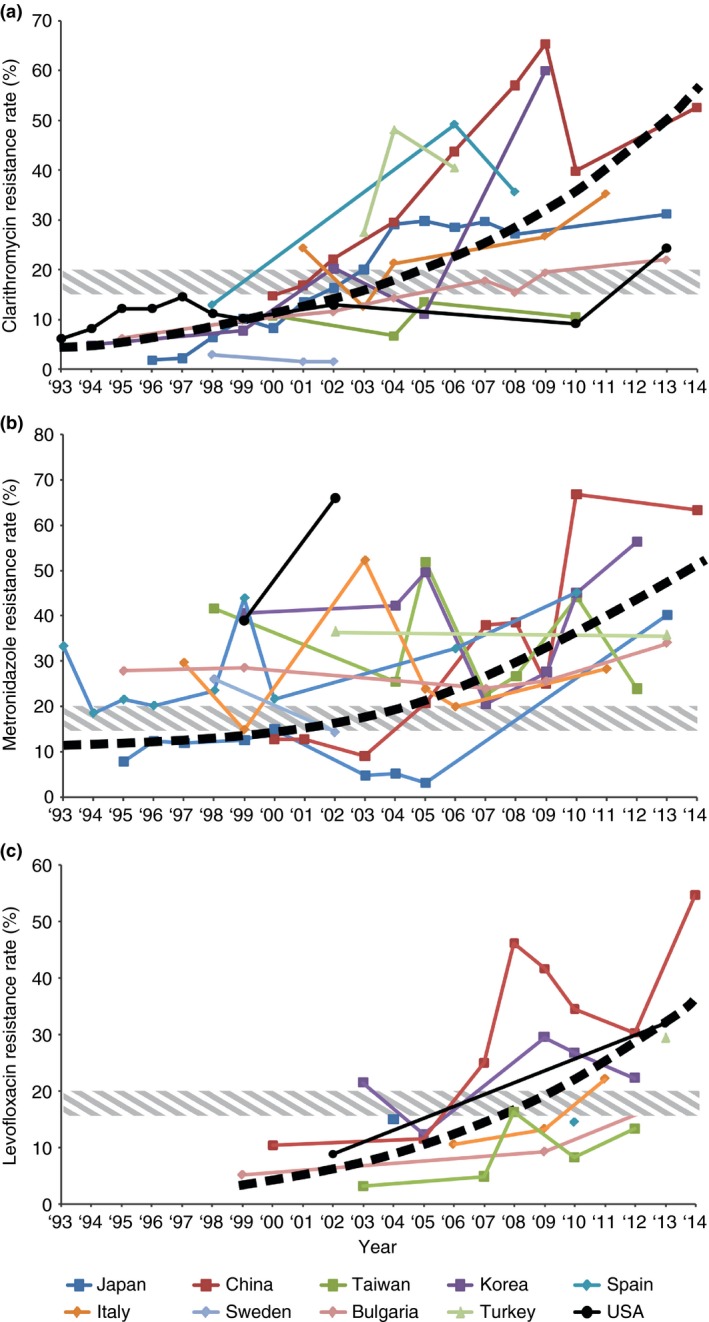
(a) Global prevalence of clarithromycin antibiotic resistance by country and year.22, 48, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85 (b) Global prevalence of metronidazole antibiotic resistance by country and year.62, 65, 71, 72, 73, 74, 75, 76, 77, 79, 81, 82, 83, 84, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106 (c) Global prevalence of levofloxacin antibiotic resistance by country and year.22, 67, 72, 73, 74, 77, 81, 82, 83, 85, 87, 88, 90, 95, 96, 106, 117, 118, 119, 120, 121 Hashed band indicates threshold for altering therapeutic intervention.
Metronidazole
Overall, metronidazole resistance rates have been increasing in many countries (Figure 2b).62, 65, 71, 72, 73, 74, 75, 76, 77, 79, 81, 82, 83, 84, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106 The prevalence of H. pylori resistance to metronidazole ranges from 20% to 40% in Europe and the USA, with one exception in Northern Italy (14.9%).100 The overall resistance rate to metronidazole was 33.1% in Europe with no substantial difference between Northern and Southern Europe; however, a noticeably lower prevalence was found in Central and Eastern Europe.100, 107, 108, 109, 110, 111 A higher prevalence has been reported in developing countries (50%–80%), for example Mexico (76.3%).110 The prevalence of metronidazole resistance tends to be lower in Japan (9–12%).94 The prevalence in Canada was reported from 18% to 22%.112 Metronidazole resistance rates in a US population was recently reported as 21.5%.85 One possible explanation includes different rates of prior metronidazole use in various countries. As seen in Alaskan Native populations, women have higher rates of metronidazole resistance than men, and prior use of metronidazole was associated with increased metronidazole resistance.65 The finding of higher rates in Alaskan Native women may be related to antibiotic treatment of gynecological infections, although this was not specifically studied.65, 66, 67
Other antibiotics
It has been reported that resistance to tetracycline is as low as 0.7% in Spain, 0.5% in the UK and 0.5% in the Hong Kong, or even absent in most countries.22, 113, 114, 115
The prevalence of resistance to fluoroquinolones has been determined in only a limited number of studies.1 In China and Italy, levofloxacin resistance rates have been reported as 34.5% and 22.1% respectively.73, 81 Surprisingly, a recent study from the USA demonstrated a levofloxacin resistance rate of 31.9%; this resistance rate needs to be confirmed with additional studies.85 Portugal, has reported a high resistance rate of 20.9% in strains isolated from 110 adult patients.116 Although this resistance to this antibiotic has not been studied as extensively as other antimicrobials, there is a trend of increasing levofloxacin‐resistant H. pylori (Figure 2c).22, 67, 72, 73, 74, 77, 81, 82, 83, 85, 87, 88, 90, 95, 96, 106, 117, 118, 119, 120, 121 In the Netherlands, a rate of 4.7% resistance was reported with trovafloxacin, a drug not yet introduced to the Dutch market; this finding suggests cross resistance between the different molecules of this antibiotic group.122 In France, a rate of 3.3% was reported; in five Eastern European countries, the rate was similar (3.9%).123, 124 Sitafloxacin appears not to be affected by prior fluoroquinolones use and has also been shown to achieve high H. pylori eradication rates.125, 126
Resistance to amoxicillin has been shown to be negligible (0 to <2%) in European countries, such as Germany and the Netherlands.122, 127 In Alaskan Native populations, 6% of patients were infected with amoxicillin‐resistant strains of H. pylori.65 In Asia and South America, amoxicillin resistance rates have been reported to be up to 38%.128, 129 However, it has been suggested that H. pylori resistance to amoxicillin does not reduce treatment efficacy.95
Diagnosis of H. pylori infection
H. pylori testing has become more common over the last decade in patients with epigastric pain and dyspepsia.130 H. pylori is considered a group I carcinogen by the International Agency for Research on Cancer, eradication of which should reduce the incidence of gastric carcinoma and MALT/marginal zone lymphoma.4, 6, 131, 132 Investigations to assess for H. pylori infection are broadly divided into non‐invasive and invasive methods.133, 134, 135
Non‐invasive methods for H. pylori detection with subsequent treatment is recommended for patients younger than 55 years of age presenting with new‐onset dyspepsia without alarm symptoms.134, 135 These tests include peripheral blood serology, urea breath test and stool antigen test. Serological testing detects immunoglobulin G antibodies to H. pylori infection; however, seroconversion for H. pylori is rare and serologic tests are not recommended by current guidelines.1, 136, 137 This test is not suitable for monitoring post‐eradication because successful treatment does not alter IgG levels immediately.138 Additionally, serologic tests may perform differently in different ethnic groups. Serological testing for immunoglobulin M antibodies directed against H. pylori can detect active infection, however, IgM levels are only elevated shortly after infection.139 Urea breath testing is currently the gold standard for determining H. pylori status. This test uses either nonradioactive 13C or radioactive 14C to detect urease activity produced by H. pylori.140 One of the advantages of this test is its ability to monitor eradication after treatment. Stool antigen testing detects H. pylori antigen in the stool via monoclonal and polyclonal anti‐H. pylori antibodies.141 Like the urea breath test, stool antigen testing is capable of monitoring patients in the post‐treatment period.142 This test is a suitable substitute detection modality in areas where the urea breath test is not available.133, 143
Upper endoscopy (an invasive procedure) is recommended in the evaluation of all patients presenting with new‐onset dyspepsia with alarm symptoms, including unintended weight loss, gastrointestinal bleeding, unexplained iron‐deficiency anaemia and progressive dysphagia.133, 134 Patients with new‐onset dyspepsia in the absence of alarm symptoms are recommended to undergo upper endoscopy if they are 55 years of age or greater.133, 134 However, this age criterion is controversial and may vary by population.135 Determination of an appropriate age cut‐off should take into consideration the presence of persistent symptoms and the local prevalence of gastric cancer.1, 134 If biopsy samples can be easily obtained, the rapid urease test is an option. In this test, two biopsy specimens from the gastric antrum and body are tested to detect the presence of urease from a H. pylori infection.144 Although this test is cheaper than histology, the practice of utilising this test without a specimen also being sent for histopathologic evaluation is not recommended due to the diagnostic yield of histopathology in detecting severity of inflammation and related pathology, including lymphoma or carcinoma.25 Several ancillary staining methods can be used in conjunction with routine histological evaluation by hematoxylin and eosin stained tissue sections.145 These include special stains (e.g. modified Giemsa or Warthin–Starry) and H. pylori immunohistochemical stains.
Antibiotic therapeutic regimens
Multiple therapeutic regimens have been developed in order to treat H. pylori infection (Figure 3).133 If eradication is not achieved, salvage treatment regimens have been proposed.
Figure 3.
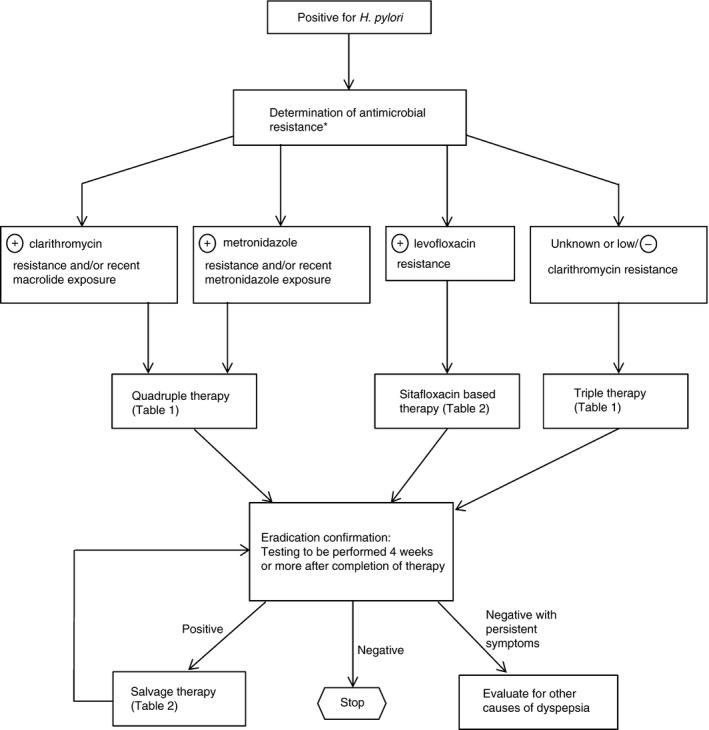
Recommended algorithm for the management of H. pylori.134 *In patients who have failed 2 or more courses of therapy, culture and sensitivity testing may be considered.
First line therapy (Table 1)
Table 1.
Current initial therapy regimens (Disclaimer: Local resistance rates can markedly impact the choice of therapy)
| Recommendations & comments | Regimen | Duration of therapy | |
|---|---|---|---|
| Triple therapy133 | Consider in areas of low clarithromycin resistance or patients with no prior recent macrolide exposure |
|
10–14 days |
| Quadruple therapy133 | Consider in areas of high clarithromycin resistance or patients with recent or repeated macrolide exposure |
|
10–14 days |
| Alternative regimens: | |||
| Sequential therapy133 | Needs validation in USA |
|
5 days 5 days |
| Concomitant therapy163 | Needs validation in USA |
|
10 days |
| Hybrid therapy167, 168, 169 | Needs validation in USA |
|
7 days 7 days |
PPI, proton pump inhibitor; b.d., twice daily; q.d., daily; q.d.s., four times daily.
Metronidazole 500 mg b.d. can be substituted for patients with a penicillin allergy.
Triple therapy
The effectiveness of antibiotics is related to many biologic and non‐biologic factors including antimicrobial strength, cost, side effects, duration, tolerability of drugs, local antibiotic use, and bacterial resistance.146, 147 The current standard regimen for H. pylori treatment in areas where clarithromycin resistance is below 15% is PPI‐based triple therapy which involves the use of a PPI, clarithromycin and amoxicillin.133, 146, 147 This regimen has been used widely for more than a decade, however, its efficacy has decreased globally.12 In cases of penicillin allergy, metronidazole can substitute for amoxicillin with equivalent effectiveness to amoxicillin.146 Increased antibiotic resistance, especially to clarithromycin, is thought to be the main cause of eradication failure for standard PPI‐containing triple therapy. Additional factors which may impact cure rates include, but are not limited to, patient compliance, body weight, type of H. pylori strains, high bacterial load, gastric acidity and atrophic gastritis.12, 148 As a result, some authors have suggested avoiding clarithromycin in first‐line empiric therapies for areas with high rates of resistance.59, 149 Per the Maastricht guidelines, sensitivity testing is recommended prior to treatment when H. pylori antibiotic resistance rates exceed 20% in the population of interest.1 In addition, a review of patients' prior antibiotic use is important to increase cure rates as prior exposure to macrolides increase H. pylori resistance rates to clarithromycin65, 66, 67
Nevertheless, 7 days of triple therapy is recommended in areas where the rate of clarithromycin resistance is less than 15% based on European guidelines; 14 days of triple therapy is suggested for areas with clarithromycin resistance >20%.1, 150 A randomised trial conducted by Paoluzi et al. compared H. pylori eradication rate between patients treated with 7 days vs. 14 days of standard therapy containing amoxicillin, clarithromycin and omeprazole.151 Although antibiotic resistance rates were not determined in this study population, the overall results suggested that 14 days of standard therapy resulted in increased eradication (77% vs. 66%).151
Bismuth‐containing quadruple therapy
Bismuth‐containing quadruple therapy consists of a PPI, bismuth and two antibiotics, such as tetracycline, clarithromycin and metronidazole.1, 133, 152 The regimen has been proven effective as the first line of treatment in areas of high clarithromycin or metronidazole resistance and for patients with recent or repeated exposure to these antibiotics.1 The main advantage of this regimen is that it can overcome clarithromycin or metronidazole resistance, especially when therapy contains both metronidazole and clarithromycin, concomitantly.152 A meta‐analysis performed by Fisch and Evans has shown better outcomes for bismuth‐containing quadruple therapy as compared to the standard triple therapy in areas with increased clarithromycin or metronidazole resistance.153
Doxycycline or amoxicillin could be substitutes in countries where tetracycline and bismuth salts are not available, although the efficacy data are conflicting.153, 154, 155, 156, 157, 158 Limited compliance has been reported due to the increased number of drugs and frequent dosing.159 Quadruple therapy using combination capsules has shown promising eradication rates.159
Sequential therapy
Sequential therapy was first introduced by Zullo et al. and consists of a 10‐day therapy comprising 5 days of PPI plus amoxicillin followed by 5 days of triple treatment of a PPI, clarithromycin and metronidazole.1, 26, 133 Levofloxacin can be used in patients with penicillin allergy or in areas of high clarithromycin resistance.160 Although this regimen includes clarithromycin, it is considered an alternative to standard triple therapy in high clarithromycin‐resistant areas.1, 152 This therapeutic regimen employs the use of amoxicillin prior to clarithromycin in order to overcome clarithromycin resistance. Amoxicillin disrupts H. pylori cell walls and prevents activation of efflux channels, one of the mechanisms of clarithromycin resistance.160, 161 The drawback of this therapy is its complex regimen which results in decreased patient compliance.59, 162 If the patient fails treatment, there is potential to develop multidrug resistance. Additionally, sequential therapy utilises the main antibiotics effective against H. pylori, limiting salvage therapy options.
Concomitant therapy
Concomitant therapy consists of a PPI, clarithromycin, amoxicillin and metronidazole for at least 10 days. This regimen has shown superiority over standard triple therapy, especially in cases of clarithromycin resistance. A better result with concomitant treatment was observed vs. standard triple therapy in recent randomised clinical trials.163, 164 A meta‐analysis of nine studies (conducted in Japan, UK, Germany, Spain and Italy) showed >90% per protocol analysis (PP) and >80% intention‐to‐treat (ITT) eradication rates for concomitant therapy, which was an improvement over the standard triple therapy.162
Additionally, a recent study conducted in Taiwan evaluated concomitant and sequential therapy regimens using a PPI, levofloxacin, amoxicillin and metronidazole. The population studied was found to have the following resistance rates: levofloxacin – 10.2%, amoxicillin – 0.6%, clarithromycin – 6.6% and metronidazole – 33.5%. ITT analysis showed comparable eradication rates for levofloxacin‐based concomitant (92.2%) and sequential (93.3%) therapies.165
The advantages of concomitant therapy is the efficacy against dual antibiotic‐resistant strains, and the higher compliance rates compared to the sequential therapy.79, 162, 164, 166 However, it should be noted that the efficacy of concomitant therapy, as with many treatment regimens, depends on the prevalence of H. pylori antimicrobial resistance, which varies by geography.
Hybrid therapy (sequential‐concomitant therapy)
Hsu et al. proposed a hybrid therapy which is a combination of sequential therapy and concomitant therapy.167 The hybrid regimens consist of dual therapy with a PPI and amoxicillin for 7 days, with addition of a concomitant quadruple therapy with a PPI, amoxicillin, clarithromycin and metronidazole for another 7 days. The eradication rate was excellent with 99% in PP and 97% in the ITT analysis, even in dual clarithromycin and metronidazole resistance strains.168 A tendency towards better results seems to be the result of extended use of amoxicillin for 14 days, compared to sequential or concomitant therapy. One randomised clinical trial indicated that the hybrid therapy had equivalent eradication rates to 14‐day concomitant therapy.169 However, further validation of these results is needed in order to confirm the effectiveness of hybrid therapy.169
Salvage therapy (Table 2)
Table 2.
Current salvage therapy regimens (Disclaimer: Local resistance rates can markedly impact the choice of therapy)
| Recommendations & Comments | Regimen | Duration of therapy | |
|---|---|---|---|
| Quadruple therapy133 | Consider in patients who were not initially treated with triple therapy |
|
7 days |
| Levofloxacin triple therapy133, 170 | Needs validation in USA |
|
10 days |
| LOAD171 | Needs further evaluation |
|
7–10 days |
| Rifabutin‐based therapy174 | Adverse effect includes myelotoxicity |
|
10–12 days |
| Furazolidone quadruple therapy175 | Needs validation in USA |
|
7 days |
| High‐dose dual therapy55, 177 | Needs validation in USA |
|
14 days |
| Sitafloxacin therapy177 | Needs validation |
|
7 days |
PPI, proton pump inhibitor; b.d., twice daily; q.d., daily; q.d.s., four times daily; q.a.m., each morning; q.p.m., each night; t.d.s., three times daily.
Quinolone‐based therapy
The use of levofloxacin has been proposed as an antibiotic in salvage therapeutic regimens. After failure to eradicate H. pylori using standard triple therapy which includes the use of amoxicillin and clarithromycin, levofloxacin‐based triple therapy is the most validated regimen used as a second line alternative therapy.170 This quinolone‐based therapy consists of a PPI, levofloxacin and amoxicillin for 10 days. Multiple studies conducted predominantly in Europe and Taiwan reported variable rates of eradication (65–96%), perhaps reflecting varying levels of levofloxacin resistance in the studied populations.170
Another quinolone regimen consists of levofloxacin, omeprazole, nitazoxanide and doxycycline (LOAD) for 7 or 10 days. A study consisting of 653 patients conducted by Basu et al. found that the LOAD regimen led to a higher rate of eradication when compared to the standard triple therapy, regardless of the duration of LOAD therapy (approximately 90% vs. 73%).171 Additionally, the study demonstrated that patients following the LOAD regimen had decreased rates of recurrence at 1‐year follow‐up.171
Levofloxacin‐based therapies have also been considered in empiric third‐line therapeutic regimens after patients have failed to achieve H. pylori eradication with the use of standard first‐ and second‐line therapies. One study proposes the use of levofloxacin in a third‐line quadruple therapy in combination with rabeprazole, bismuth subcitrate and amoxicillin. Despite the presence of multi‐antibiotic‐resistant H. pylori, a 10‐day course of treatment led to an overall 84% eradication rate. It should be noted that patients who were found to be amoxicillin or levofloxacin‐resistant demonstrated lower rates of eradication.172
Selection of quinolone therapy should be based on the results of antibiotic susceptibility tests or geographic resistance patterns, as quinolone‐resistant strains have increased in concert with the use of quinolones for infections of the respiratory and urogenital tracts. Therefore, this regimen is not generally recommended as first‐line treatment.173 Presently this regimen is used as a second‐line treatment in populations where clarithromycin resistance rates are greater than 15%–20% and quinolone resistance rates are less than 10%. It has also been demonstrated that levofloxacin‐based triple regimen could be used as an empiric salvage treatment after the failures of first and second‐line therapy in low quinolone resistance areas.1
Rifabutin‐based therapy
Based on in vitro studies, rifabutin‐based therapy comprising amoxicillin, a PPI, and rifabutin has shown encouraging effects as a salvage treatment for H. pylori eradication. However, duration of treatment with this anti‐tuberculous agent remains unclear (7‐day vs. 10‐day vs. 14‐day). In addition, rare myelotoxicity is an important complication that needs to be overcome prior to rifabutin's widespread application.174 Because of the potential for mycobacterial resistance this regimen should be reserved only for rescue treatment. Rifabutin‐based triple therapy could be used as an empiric third line salvage treatment and is listed in guidelines for areas in which bismuth and tetracycline are not available, although shortages in the USA have been temporary.1, 174
Furazolidone‐based therapy
Furazolidone‐based therapy is a 1‐week therapy with lansoprazole, tripotassiumdicitratobismuthate, tetracycline and furazolidone that has been used as salvage treatment, after second‐line treatment failure. This salvage therapy has achieved 90% eradication rate in one analysis.175 However, the problem with this regimen is the high incidence of side effects and cross resistance with metronidazole.175
High‐dose dual therapy
High‐dose dual therapy has been evaluated in high clarithromycin resistance areas. This regimen consists of a PPI and amoxicillin three times a day for 14 days.176, 177 In one study patients were randomly given one of two regimens: (i) 1000 mg amoxicillin with 500 mg clarithromycin and 30 mg lansoprazole b.d. for 2 weeks (triple therapy group), (ii) 750 mg amoxicillin with 30 mg lansoprazole t.d.s. for 2 weeks.176 The eradication rate was 82.8% for standard triple therapy vs. 78.4% for high dose dual therapy, although the difference was not statistically significant.176 While these results are promising, additional studies to validate and optimise the treatment regimen (including differences between PPI used) are needed before it can be established as a main therapeutic regimen.178
Antibiotic resistance mechanisms
Amoxicillin
Amoxicillin is a moderate‐spectrum, bactericidal, beta‐lactam antibiotic in the penicillin family. The main mechanisms leading to amoxicillin resistance of H. pylori are alterations in penicillin‐binding proteins, decreased membrane permeability of antibiotics into the bacterial cell or combinations of these resistance strategies. Expression of active efflux pumps that excrete drugs and point mutations in the pbp1A gene may contribute to the mechanisms of resistance to beta‐lactams. Other mutations such as pbp2, hefC, hopC and hofH have been identified among H. Pylori‐resistant strains.179
Clarithromycin
Clarithromycin is a bacteriostatic antibiotic that inhibits bacterial protein synthesis by reversibly binding to the 50S ribosomal subunits. The 50S ribosomal subunit is itself composed of 23S ribosomal RNA, 5S ribosomal RNA, and RNA binding proteins. The peptidyl transferase loop of the V domain of 23S ribosomal RNA molecule is the target site of clarithromycin. Resistance to clarithromycin is generally caused by point mutations in the 23S rRNA gene, the most frequent is A2143G (69.8%), followed by A2142G (11.7%) and A2142C (2.6%).79 These mutations prevent the macrolide from binding. Moreover, other mutations such as A2115G, G2141A, C2147G, T2190C, C2195T, A2223G and C2694A have been implicated in the development of clarithromycin resistance, although their precise role in the mechanism of resistance remains unclear.180
Metronidazole
Metronidazole, a bactericidal antibiotic, is a synthetic nitroimidazole. This prodrug is activated by nitroreductases within the cytosol of the microorganism, producing a toxic metabolite. In metronidazole‐resistant strains, null mutations in the rdxA gene have been identified. The genecodes for an oxygen‐insensitive NADPH nitroreductase (RdxA), whose expression is necessary for intracellular activation of the drug, and the mutations causes inactivation of these nitroreductases. Nonetheless, a number of resistant strains have been reported, such as frxA (coding for NADPH flavinoxidoreductase), and fdxB (coding for ferredoxin‐like enzyme), which can also confer resistance to metronidazole.181, 182, 183
Tetracycline
Tetracycline is a bactericidal antibiotic which inhibits protein synthesis by binding to the 30S subunit of ribosomes; this blocks the binding of aminoacyl‐tRNA, resulting in stalled synthesis of nascent peptide chains. Antibiotic resistance to tetracycline is due to an enhanced energy‐dependent efflux of tetracycline‐cation complexes across the cell membrane by membrane‐associated efflux proteins which decreases the intracellular concentration of tetracycline.184 Conversely, sensitivity to tetracycline is increased by deletions in these efflux genes.184
Another mechanism of tetracycline resistance is mediated through ribosomal protection proteins. These protection proteins increase tetracycline resistance either by decreasing the affinity of ribosomes for tetracycline or by releasing the bound antibiotic from the ribosome. Additionally, two other mechanisms have been reported including enzymatic inactivation of tetracycline and point mutations in the 16S rRNA genes that affect the binding site of tetracycline.184
Levofloxacin
Point mutations of gyrA, which codes for DNA gyrase, have been identified in the quinolone‐resistant determination region with the major mutations being found at position in the codons coding for amino acid 87, 88, 91 or 97, conferring resistance of levofloxacin and other quinolones.185, 186 However, studies have shown that sitafloxacin may overcome the resistance conferred by these point mutations.187, 188
Rifabutin
Resistance to rifabutin is generally caused by point mutation in codons 524–545 or codon 585 of the rpoB gene.189, 190 Moreover, cross resistance between rifabutin and rifampin has been reported.191
Techniques for detecting H. pylori antibiotic resistance (Table 3)184, 185, 186, 189, 192, 193
Table 3.
Current techniques for detecting H. pylori antibiotic resistance
| Name of the method | Basis for method | Sensitivity | Specificity | Advantages of the method | Disadvantages of the method |
|---|---|---|---|---|---|
| Agar Dilution Method | Based on phenotypic methods failure of second‐line therapies | – | – | Adaptable for the testing of large numbers of strains |
Technically demanding Time consuming |
| Epsilometer Test (E‐ test) Method184 | Based on phenotypic methods | 45% | 98% |
Adaptable for testing of small numbers of strains Less technically demanding |
Time consuming |
| PCR‐based methods185 | Based on detection of point mutations | 98% | 92% |
High‐sensitivity Rapid detection of microorganisms |
Affected by DNA contamination |
| FISH‐Based Method186 | Fluorescent‐labelled DNA probes to identify DNA sequences on chromosomes | 97% | 94% |
Time‐saving Accurate Cost‐effective |
Degradation of the probe by proteases Inability to penetrate the bacterial cell wall (fresh tissue) |
| PNA‐FISH‐Based Method189 |
Fluorescently‐labelled PNA probes to identify DNA sequences on chromosomes |
80% | 93.8% |
Ability to penetrate the bacterial cell wall Resistant to nucleases and proteases |
Not widely available for standardisation |
| Line Probe Test192, 193 | DNA‐based test to identify multiple variants simultaneously. Commercialised as kits for laboratories. | 100% | 86.2% | Fast, standardised test that examines both clarithromycin and fluoroquinolones resistance | Not available in the USA |
Endoscopic‐guided antibiotic susceptibility testing has been suggested not only for treatment after failure of second‐line therapies but also for determining antibiotic susceptibility prior to the administration of a first‐line clarithromycin‐containing therapy, especially in areas of high clarithromycin resistance.194, 195, 196 Currently, several techniques exist for evaluating antibiotic resistance in H. pylori, but differ in the timing and character of the requisite analytic specimen.
Culture‐based techniques
In vitro susceptibility testing of H. pylori using agar dilution method are practical for testing large numbers of strains; it is not suitable for the testing of small numbers of strains on an ongoing basis.194, 195, 196, 197 The Epsilometer test (E‐test) method involves the use of test strips applied to an inoculated agar plate in order to determine the antibiotic's minimum inhibitory concentration.198 One study found the E‐test produced reproducible results in determining the sensitivity of H. pylori isolates to ampicillin, clarithromycin and metronidazole.198, 199 From an international perspective, E‐test appears to be a suitable method for determining H. pylori antibiotic sensitivity.199, 200, 201 However, the availability of the E‐test strips for one of the key antibiotics of interest, clarithromycin, is currently not globally available for clinical use.
One of the main drawbacks of both the agar dilution and E‐test is that they only test a single H. pylori strain. In areas of high H. pylori prevalence and increased likelihood of patients being infected with multiple H. pylori strains, these two testing modalities may fail to provide complete antimicrobial resistance data.
Molecular techniques
The gold standard methods of antibiotic resistance are based on phenotypic methods performed by the agar dilution method.194, 202 These methods, however, can take up to 2 weeks to be completed. Detection of point mutations using molecular methods was developed in part to shorten the turn‐around time. In addition, molecular techniques can often use either fresh or formalin‐fixed samples.
Real‐time PCR has been used to successfully determine H. pylori susceptibility to clarithromycin.203, 204 Additionally, PCR using formalin‐fixed paraffin‐embedded samples has been shown to reliably detect the H. pylori 23S rRNA mutations associated with clarithromycin resistance.86 Another advantage of PCR is the potential to gather complete antimicrobial resistance data in patients infected with multiple strains of H. pylori. Although the use of PCR‐based methods provides rapid detection of micro‐organisms, these techniques can be affected by DNA contamination or degradation since the high sensitivity of these methods often result in the detection of dead or nonculturable microorganisms.205
Fluorescence in situ hybridisation (FISH) is a time‐saving, accurate and cost‐effective method for the detection of antibiotic resistance in cultured H. pylori colonies. This method can be used directly on biopsy specimens procured for histopathological and microbiological examination, allowing for rapid detection of H. pylori resistance without requiring DNA preparation.201, 206 The results can theoretically be available within 3 hours after an endoscopy by utilising frozen tissue sections.205 The limitations of this method include the degradation of the probe by proteases and nucleases present in the sample and poor accessibility of the microbial cell wall for the probes.
Recently, peptide nucleic acid (PNA) probes using FISH have been used for the detection of several bacteria in lieu of the typical DNA molecular probes.175, 207 PNA molecules are DNA mimics with high affinity for DNA or RNA complementary sequences.208, 209 PNA probes are normally relatively small (13–18 nucleotides), increasing their ability to penetrate the bacterial cell wall. Moreover, the PNA molecules are more resistant to nucleases and proteases than DNA molecules.
Conclusions and future directions
Helicobacter pylori infection remains a very common worldwide condition with strong geographic variations and the prevalence of antibiotic resistance appears to be rapidly increasing. This is particularly evident in countries such as Japan, Korea and China, in which antibiotic resistant strains of H. pylori have been studied most extensively (Figure 2). Indeed, many countries have crossed the 15–20% threshold for antibiotic resistance over the past 20 years (Figure 2). During this period of increasing antibiotic resistance, the eradication rate of empiric therapy for H. pylori has dropped below the 80–90% target, and failure rates range from 29% in the USA to 40% in Western Europe and Japan.25, 210, 211 Treatment failure is multifactorial and requires further study, but is likely due to poor compliance and antibiotic resistance. In addition, reinfection rates are unclear and may influence measured treatment failures.212, 213 Variable antibiotic susceptibility of different H. pylori strains found within the antrum of the stomach vs. the corpus may also be contributing to treatment failure.25, 127 The cagA status of bacterial strains is also a risk factor for treatment failure as H. pylori eradication was more successful in patients harbouring cagA+ strains compared with those infected with cagA− strains.212, 214 This finding may explain why cure rates are higher in patients with peptic ulcer disease than those with non‐ulcer disease.212, 215 Additionally, patients who fail first‐line therapy have been shown to develop secondary resistance to the recommended antibiotics, further promoting the selection of antibiotic‐resistant H. pylori strains.216
Theoretically, the widespread general usage of antibiotics by the public may be further contributing to the development and increased prevalence of antibiotic‐resistant strains of H. pylori. Globally, antibiotic consumption increased by 36% between 2000 and 2010, mainly in developing countries. Perhaps even more importantly, one of the largest absolute increases in consumption was observed for broad‐spectrum antibiotics. This overall increase in antibiotic consumption also includes a global increase in macrolide consumption by approximately 20% over this time period.217 As antibiotic resistance has been correlated with antibiotic usage, the continual increase in usage of broad‐spectrum antibiotics in recent years suggests that rates of H. pylori antibiotic resistance might be increasing in parallel.59, 150 Importantly, the prior usage of macrolides and, specifically, clarithromycin has been shown to directly impact the development of antibiotic‐resistant strains of H. pylori.22, 64, 65
Because of the clinical importance of decreased eradication rates, regional variation in antibiotic resistance rates and the marked increase over time of antibiotic resistance rates in the past 15 years, there is a critical need to determine the current rates of local antibiotic resistance. Such a determination would not only facilitate the selection of appropriate antibiotic treatment regimens but also serve as a potential basis for transitioning to individualised analysis of antibiotic resistance prior to definitive treatment. Therefore, a paradigm shift in therapy towards patient‐specific tailoring of effective antibiotic treatment strategies may lead to reduced treatment failures and stem the tide of increasing H. pylori antibiotic resistance in various populations throughout the world.
Authorship
Guarantor of the article: Mark A. Valasek, MD, PhD.
Author contributions: Irene Thung, Hermineh Aramin, Vera Vavinskaya, Samir Gupta, Jason Y. Park, Sheila E. Crowe, Mark A. Valasek have made a substantial contribution to research design, the analysis and interpretation of data, drafted and revised the manuscript and approved the submitted and final version.
All authors approved the final version of the manuscript.
Acknowledgements
We would like to thank Dr. John W. Thomas for his suggestions and proofreading of this article.
Declaration of personal interests: (i) JP has served as an advisory board member for Fujirebio and Targeted Diagnostics and Therapeutics. MV has served as a consultant for AbSci and Celgene. SC has served as an author for UpToDate. No research funding has been received related to this study. (ii) JP is an employee of UT Southwestern Medical Center. HA, IT, VV, MV, SG, and SC are employees of UC San Diego Health System. (iii) None related to this study. (iv) None related to this study.
Declaration of funding interests: None.
This uncommissioned review article was subject to full peer‐review.
References
- 1. Malfertheiner P, Megraud F, O'Morain CA, et al Management of Helicobacter pylori infection–the Maastricht IV/Florence consensus report. Gut 2012; 61: 646–64. [DOI] [PubMed] [Google Scholar]
- 2. Williams MP, Pounder RE. Helicobacter pylori: from the benign to the malignant. Am J Gastroenterol 1999; 94(11 Suppl.): S11–6. [DOI] [PubMed] [Google Scholar]
- 3. Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest 2004; 113: 321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dinis‐Ribeiro M, Areia M, de Vries AC, et al Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa. Endoscopy 2012; 44: 74–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984; 1: 1311–5. [DOI] [PubMed] [Google Scholar]
- 6. Vakil N, Megraud F. Eradication therapy for Helicobacter pylori . Gastroenterology 2007; 133: 985–1001. [DOI] [PubMed] [Google Scholar]
- 7. Fukase K, Kato M, Kikuchi S, et al Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open‐label, randomised controlled trial. Lancet 2008; 372: 392–7. [DOI] [PubMed] [Google Scholar]
- 8. Banić M, Franceschi F, Babić Z, Gasbarrini A. Extragastric manifestations of Helicobacter pylori infection. Helicobacter 2012; 17(Suppl. 1): 49–55. [DOI] [PubMed] [Google Scholar]
- 9. Chen B‐F, Xu X, Deng Y, et al Relationship between Helicobacter pylori infection and serum interleukin‐18 in patients with carotid atherosclerosis. Helicobacter 2013; 18: 124–8. [DOI] [PubMed] [Google Scholar]
- 10. Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev 2000; 22: 283–97. [DOI] [PubMed] [Google Scholar]
- 11. Vale FF, Vítor JMB. Transmission pathway of Helicobacter pylori: does food play a role in rural and urban areas? Int J Food Microbiol 2010; 138: 1–12. [DOI] [PubMed] [Google Scholar]
- 12. Khalifa MM, Sharaf RR, Aziz RK. Helicobacter pylori: a poor man's gut pathogen? Gut Pathog 2010; 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bassily S, Frenck RW, Mohareb EW, et al Seroprevalence of Helicobacter pylori among Egyptian newborns and their mothers: a preliminary report. Am J Trop Med Hyg 1999; 61: 37–40. [DOI] [PubMed] [Google Scholar]
- 14. Yang Y‐J, Sheu B‐S, Lee S‐C, Yang H‐B, Wu J‐J. Children of Helicobacter pylori‐infected dyspeptic mothers are predisposed to H. pylori acquisition with subsequent iron deficiency and growth retardation. Helicobacter 2005; 10: 249–55. [DOI] [PubMed] [Google Scholar]
- 15. Kivi M, Johansson ALV, Reilly M, Tindberg Y. Helicobacter pylori status in family members as risk factors for infection in children. Epidemiol Infect 2005; 133: 645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weyermann M, Rothenbacher D, Brenner H. Acquisition of Helicobacter pylori infection in early childhood: independent contributions of infected mothers, fathers, and siblings. Am J Gastroenterol 2009; 104: 182–9. [DOI] [PubMed] [Google Scholar]
- 17. Opekun AR, Gilger MA, Denyes SM, et al Helicobacter pylori infection in children of Texas. J Pediatr Gastroenterol Nutr 2000; 31: 405–10. [DOI] [PubMed] [Google Scholar]
- 18. Gisbert JP, Pajares JM. Review article: Helicobacter pylori “rescue” regimen when proton pump inhibitor‐based triple therapies fail. Aliment Pharmacol Ther 2002; 16: 1047–57. [DOI] [PubMed] [Google Scholar]
- 19. Glupczynski Y, Mégraud F, Lopez‐Brea M, Andersen LP. European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori . Eur J Clin Microbiol Infect Dis 2001; 20: 820–3. [DOI] [PubMed] [Google Scholar]
- 20. Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol 2008; 5: 321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gisbert JP, Pajares JM. Helicobacter pylori “rescue” therapy after failure of two eradication treatments. Helicobacter 2005; 10: 363–72. [DOI] [PubMed] [Google Scholar]
- 22. Megraud F, Coenen S, Versporten A, et al Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013; 62: 34–42. [DOI] [PubMed] [Google Scholar]
- 23. Selgrad M, Malfertheiner P. Treatment of Helicobacter pylori . Curr Opin Gastroenterol 2011; 27: 565–70. [DOI] [PubMed] [Google Scholar]
- 24. Boyanova L, Mitov I. Geographic map and evolution of primary Helicobacter pylori resistance to antibacterial agents. Expert Rev Anti Infect Ther 2010; 8: 59–70. [DOI] [PubMed] [Google Scholar]
- 25. Sugano K, Tack J, Kuipers EJ, et al Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015; 64: 1353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zullo A, Hassan C, Lorenzetti R, Winn S, Morini S. A clinical practice viewpoint: to culture or not to culture Helicobacter pylori? Dig Liver Dis 2003; 35: 357–61. [DOI] [PubMed] [Google Scholar]
- 27. De Boer WA, Tytgat GN. Regular review: treatment of Helicobacter pylori infection. BMJ 2000; 320: 31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Calvet X, Gené E, Sanfeliu I. Therapeutic strategies in Helicobacter pylori infection. Med Clin (Barc) 2001; 116: 239. [DOI] [PubMed] [Google Scholar]
- 29. Hunt RH, Xiao SD, Megraud F, et al Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointestin Liver Dis 2011; 20: 299–304. [PubMed] [Google Scholar]
- 30. Bastos J, Peleteiro B, Barros R, et al Sociodemographic determinants of prevalence and incidence of Helicobacter pylori infection in Portuguese adults. Helicobacter 2013; 18: 413–22. [DOI] [PubMed] [Google Scholar]
- 31. Bruce MG, Maaroos HI. Epidemiology of Helicobacter pylori infection. Helicobacter 2008; 13(Suppl. 1): 1–6. [DOI] [PubMed] [Google Scholar]
- 32. Fujimoto Y, Furusyo N, Toyoda K, Takeoka H, Sawayama Y, Hayashi J. Intrafamilial transmission of Helicobacter pylori among the population of endemic areas in Japan. Helicobacter 2007; 12: 170–6. [DOI] [PubMed] [Google Scholar]
- 33. Ford AC, Axon ATR. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter 2010; 15(Suppl. 1): 1–6. [DOI] [PubMed] [Google Scholar]
- 34. Sánchez Ceballos F, Taxonera Samsó C, García Alonso C, Alba López C, Sainz de Los Terreros Soler L, Díaz‐Rubio M. [Prevalence of Helicobacter pylori infection in the healthy population of Madrid (Spain)]. Rev Esp Enferm Dig 2007; 99: 497–501. [DOI] [PubMed] [Google Scholar]
- 35. Zhang M, Zhou Y‐Z, Li X‐Y, et al Seroepidemiology of Helicobacter pylori infection in elderly people in the Beijing region, China. World J Gastroenterol 2014; 20: 3635–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fujisawa T, Kumagai T, Akamatsu T, Kiyosawa K, Matsunaga Y. Changes in seroepidemiological pattern of Helicobacter pylori and hepatitis A virus over the last 20 years in Japan. Am J Gastroenterol 1999; 94: 2094–9. [DOI] [PubMed] [Google Scholar]
- 37. Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol 2014; 20: 4483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ma JL, You WC, Gail MH, et al Helicobacter pylori infection and mode of transmission in a population at high risk of stomach cancer. Int J Epidemiol 1998; 27: 570–3. [DOI] [PubMed] [Google Scholar]
- 39. Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol 2010; 25: 479–86. [DOI] [PubMed] [Google Scholar]
- 40. Lin H‐Y, Chuang C‐K, Lee H‐C, Chiu N‐C, Lin S‐P, Yeung C‐Y. A seroepidemiologic study of Helicobacter pylori and hepatitis A virus infection in primary school students in Taipei. J Microbiol Immunol Infect 2005; 38: 176–82. [PubMed] [Google Scholar]
- 41. Yim JY, Kim N, Choi SH, et al Seroprevalence of Helicobacter pylori in South Korea. Helicobacter 2007; 12: 333–40. [DOI] [PubMed] [Google Scholar]
- 42. Lim SH, Kwon J‐W, Kim N, et al Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol 2013; 13: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martín‐de‐Argila C, Boixeda D, Cantón R, et al Helicobacter pylori infection in a healthy population in Spain. Eur J Gastroenterol Hepatol 1996; 8: 1165–8. [DOI] [PubMed] [Google Scholar]
- 44. Macenlle García R, Gayoso Diz P, Sueiro Benavides RA, Fernández Seara J. Prevalence of Helicobacter pylori infection in the general adult population of the province of Ourense. Rev Esp Enferm Dig 2006; 98: 241–8. [DOI] [PubMed] [Google Scholar]
- 45. Grande M, Cadeddu F, Villa M, et al Helicobacter pylori and gastroesophageal reflux disease. World J Surg Oncol 2008; 6: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scarpa M, Angriman I, Prando D, et al Helicobacter pylori and gastroesophageal reflux disease: a cross sectional study. Hepatogast‐roenterology 2011; 58: 69–75. [PubMed] [Google Scholar]
- 47. Ierardi E, Giorgio F, Losurdo G, Di Leo A, Principi M. How antibiotic resistances could change Helicobacter pylori treatment: a matter of geography? World J Gastroenterol 2013; 19: 8168–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Storskrubb T, Aro P, Ronkainen J, et al Antimicrobial susceptibility of Helicobacter pylori strains in a random adult Swedish population. Helicobacter 2006; 11: 224–30. [DOI] [PubMed] [Google Scholar]
- 49. Sörberg M, Nyrén O, Granström M. Unexpected decrease with age of Helicobacter pylori seroprevalence among Swedish blood donors. J Clin Microbiol 2003; 41: 4038–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boyanova L, Koumanova R, Jelev C, Petrov S. Helicobacters in Bulgarian children. J R Soc Med 1997; 90: 588–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Us D, Hasçelik G. Seroprevalence of Helicobacter pylori infection in an asymptomatic Turkish population. J Infect 1998; 37: 148–50. [DOI] [PubMed] [Google Scholar]
- 52. Ozaydin N, Turkyilmaz SA, Cali S. Prevalence and risk factors of Helicobacter pylori in Turkey: a nationally‐representative, cross‐sectional, screening with the 13C‐Urea breath test. BMC Public Health 2013; 13: 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol 2012; 175: 54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pérez‐Pérez GI, Bhat N, Gaensbauer J, et al Country‐specific constancy by age in cagA+ proportion of Helicobacter pylori infections. Int J Cancer 1997; 72: 453–6. [DOI] [PubMed] [Google Scholar]
- 55. Bernstein CN, McKeown I, Embil JM, et al Seroprevalence of Helicobacter pylori, incidence of gastric cancer, and peptic ulcer‐associated hospitalizations in a Canadian Indian population. Dig Dis Sci 1999; 44: 668–74. [DOI] [PubMed] [Google Scholar]
- 56. Chen Y, Blaser MJ. Association between gastric Helicobacter pylori colonization and glycated hemoglobin levels. J Infect Dis 2012; 205: 1195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meyer JM, Silliman NP, Wang W, et al Risk factors for Helicobacter pylori resistance in the United States: the surveillance of H. pylori antimicrobial resistance partnership (SHARP) study, 1993‐1999. Ann Intern Med 2002; 136: 13–24. [DOI] [PubMed] [Google Scholar]
- 58. Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology 2015; 148: 719–31.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Graham DY, Shiotani A. Which therapy for Helicobacter pylori infection? Gastroenterology 2012; 143: 10–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chuah S‐K, Tsay F‐W, Hsu P‐I, Wu D‐C. A new look at anti‐Helicobacter pylori therapy. World J Gastroenterol 2011; 17: 3971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010; 59: 1143–53. [DOI] [PubMed] [Google Scholar]
- 62. Kobayashi I, Murakami K, Kato M, et al Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol 2007; 45: 4006–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Duck WM, Sobel J, Pruckler JM, et al Antimicrobial resistance incidence and risk factors among Helicobacter pylori‐infected persons, United States. Emerg Infect Dis 2004; 10: 1088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Perez Aldana L, Kato M, Nakagawa S, et al The relationship between consumption of antimicrobial agents and the prevalence of primary Helicobacter pylori resistance. Helicobacter 2002; 7: 306–9. [DOI] [PubMed] [Google Scholar]
- 65. McMahon BJ, Hennessy TW, Bensler JM, et al The relationship among previous antimicrobial use, antimicrobial resistance, and treatment outcomes for Helicobacter pylori infections. Ann Intern Med 2003; 139: 463–9. [DOI] [PubMed] [Google Scholar]
- 66. Bruce MG, Bruden DL, McMahon BJ, et al Alaska sentinel surveillance for antimicrobial resistance in Helicobacter pylori isolates from Alaska native persons, 1999‐2003. Helicobacter 2006; 11: 581–8. [DOI] [PubMed] [Google Scholar]
- 67. Carothers JJ, Bruce MG, Hennessy TW, et al The relationship between previous fluoroquinolone use and levofloxacin resistance in Helicobacter pylori infection. Clin Infect Dis 2007; 44: e5–8. [DOI] [PubMed] [Google Scholar]
- 68. McNulty CAM, Lasseter G, Shaw I, et al Is Helicobacter pylori antibiotic resistance surveillance needed and how can it be delivered? Aliment Pharmacol Ther 2012; 35: 1221–30. [DOI] [PubMed] [Google Scholar]
- 69. Cars O, Mölstad S, Melander A. Variation in antibiotic use in the European Union. Lancet (London, England) 2001; 357: 1851–3. [DOI] [PubMed] [Google Scholar]
- 70. Horiki N, Omata F, Uemura M, et al Annual change of primary resistance to clarithromycin among Helicobacter pylori isolates from 1996 through 2008 in Japan. Helicobacter 2009; 14: 86–90. [DOI] [PubMed] [Google Scholar]
- 71. Okamura T, Suga T, Nagaya T, et al Antimicrobial resistance and characteristics of eradication therapy of Helicobacter pylori in Japan: a multi‐generational comparison. Helicobacter 2014; 19: 214–20. [DOI] [PubMed] [Google Scholar]
- 72. Gao W, Cheng H, Hu F, et al The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter 2010; 15: 460–6. [DOI] [PubMed] [Google Scholar]
- 73. Zhang Y‐X, Zhou L‐Y, Song Z‐Q, Zhang J‐Z, He L‐H, Ding Y. Primary antibiotic resistance of Helicobacter pylori strains isolated from patients with dyspeptic symptoms in Beijing: a prospective serial study. World J Gastroenterol 2015; 21: 2786–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. De Francesco V, Giorgio F, Hassan C, et al Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis 2010; 19: 409–14. [PubMed] [Google Scholar]
- 75. Tsay F‐W, Wu D‐C, Kao S‐S, et al Reverse sequential therapy achieves a similar eradication rate as standard sequential therapy for Helicobacter pylori eradication: a randomized controlled trial. Helicobacter 2015; 20: 71–7. [DOI] [PubMed] [Google Scholar]
- 76. Kim JJ, Reddy R, Lee M, et al Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates from Korea. J Antimicrob Chemother 2001; 47: 459–61. [DOI] [PubMed] [Google Scholar]
- 77. Hwang TJ, Kim N, Bin Kim H, et al Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol 2010; 44: 536–43. [DOI] [PubMed] [Google Scholar]
- 78. Lee JH, Shin J‐H, Roe IH, et al Impact of clarithromycin resistance on eradication of Helicobacter pylori in infected adults. Antimicrob Agents Chemother 2005; 49: 1600–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mégraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 2004; 53: 1374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Agudo S, Pérez‐Pérez G, Alarcón T, López‐Brea M. High prevalence of clarithromycin‐resistant Helicobacter pylori strains and risk factors associated with resistance in Madrid, Spain. J Clin Microbiol 2010; 48: 3703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Saracino IM, Zullo A, Holton J, et al High prevalence of primary antibiotic resistance in Helicobacter pylori isolates in Italy. J Gastrointestin Liver Dis 2012; 21: 363–5. [PubMed] [Google Scholar]
- 82. Boyanova L, Nikolov R, Gergova G, et al Two‐decade trends in primary Helicobacter pylori resistance to antibiotics in Bulgaria. Diagn Microbiol Infect Dis 2010; 67: 319–26. [DOI] [PubMed] [Google Scholar]
- 83. Boyanova L, Davidkov L, Gergova G, et al Helicobacter pylori susceptibility to fosfomycin, rifampin, and 5 usual antibiotics for H. pylori eradication. Diagn Microbiol Infect Dis 2014; 79: 358–61. [DOI] [PubMed] [Google Scholar]
- 84. Osato MS, Reddy R, Reddy SG, Penland RL, Malaty HM, Graham DY. Pattern of primary resistance of Helicobacter pylori to metronidazole or clarithromycin in the United States. Arch Intern Med 2001; 161: 1217–20. [DOI] [PubMed] [Google Scholar]
- 85. Shiota S, Reddy R, Alsarraj A, El‐Serag HB, Graham DY. Antibiotic resistance of Helicobacter pylori among male United States Veterans. Clin Gastroenterol Hepatol 2015; 13: 1616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mitui M, Patel A, Leos NK, Doern CD, Park JY. Novel Helicobacter pylori sequencing test identifies high rate of clarithromycin resistance. J Pediatr Gastroenterol Nutr 2014; 59: 6–9. [DOI] [PubMed] [Google Scholar]
- 87. An B, Moon BS, Kim H, et al Antibiotic resistance in Helicobacter pylori strains and its effect on H. pylori eradication rates in a single center in Korea. Ann Lab Med 2013; 33: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chang W‐L, Sheu B‐S, Cheng H‐C, Yang Y‐J, Yang H‐B, Wu J‐J. Resistance to metronidazole, clarithromycin and levofloxacin of Helicobacter pylori before and after clarithromycin‐based therapy in Taiwan. J Gastroenterol Hepatol 2009; 24: 1230–5. [DOI] [PubMed] [Google Scholar]
- 89. Chung J‐W, Lee GH, Jeong J‐Y, et al Resistance of Helicobacter pylori strains to antibiotics in Korea with a focus on fluoroquinolone resistance. J Gastroenterol Hepatol 2012; 27: 493–7. [DOI] [PubMed] [Google Scholar]
- 90. Cuadrado‐Lavin A, Salcines‐Caviedes JR, Carrascosa MF, et al Antimicrobial susceptibility of Helicobacter pylori to six antibiotics currently used in Spain. J Antimicrob Chemother 2011; 67: 170–3. [DOI] [PubMed] [Google Scholar]
- 91. Ferrero M, Ducóns JA, Sicilia B, Santolaria S, Sierra E, Gomollón F. Factors affecting the variation in antibiotic resistance of Helicobacter pylori over a 3‐year period. Int J Antimicrob Agents 2000; 16: 245–8. [DOI] [PubMed] [Google Scholar]
- 92. Gotoh A, Kawakami Y, Akahane T, et al Susceptibility of Helicobacter pylori isolates against agents commonly administered for eradication therapy and the efficacy of chemotherapy. Microbiol Immunol 1997; 41: 7–12. [DOI] [PubMed] [Google Scholar]
- 93. Hiyama T, Tanaka S, Masuda H, et al Prevalence of Helicobacter pylori resistance to clarithromycin and metronidazole determined by 23S ribosomal RNA and rdxA gene analyses in Hiroshima, Japan. J Gastroenterol Hepatol 2003; 18: 1202–7. [DOI] [PubMed] [Google Scholar]
- 94. Kato M, Yamaoka Y, Kim JJ, et al Regional differences in metronidazole resistance and increasing clarithromycin resistance among Helicobacter pylori isolates from Japan. Antimicrob Agents Chemother 2000; 44: 2214–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kim N, Kim JM, Kim CH, et al Institutional difference of antibiotic resistance of Helicobacter pylori strains in Korea. J Clin Gastroenterol 2006; 40: 683–7. [DOI] [PubMed] [Google Scholar]
- 96. Liou J‐M, Chang C‐Y, Chen M‐J, et al The primary resistance of Helicobacter pylori in Taiwan after the National Policy to Restrict Antibiotic Consumption and Its Relation to Virulence Factors—A Nationwide Study, Schildgen O, editor.PLoS ONE 2015; 10: e0124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. López‐Brea M, Domingo D, Sanchez I, Alarcon T. Evolution of resistance to metronidazole and clarithromycin in Helicobacter pylori clinical isolates from Spain. J Antimicrob Chemother 1997; 40: 279–81. [DOI] [PubMed] [Google Scholar]
- 98. López‐Brea M, Martínez MJ, Domingo D, Alarcón T. A 9 year study of clarithromycin and metronidazole resistance in Helicobacter pylori from Spanish children. J Antimicrob Chemother 2001; 48: 295–7. [DOI] [PubMed] [Google Scholar]
- 99. Perna F, Gatta L, Figura N, et al Susceptibility of Helicobacter pylori to metronidazole. Am J Gastroenterol 2003; 98: 2157–61. [DOI] [PubMed] [Google Scholar]
- 100. Pilotto A, Rassu M, Leandro G, Franceschi M, Di Mario F. Prevalence of Helicobacter pylori resistance to antibiotics in Northeast Italy: a multicentre study. GISU. Interdisciplinary Group for the Study of Ulcer. Dig Liver Dis 2000; 32: 763–8. [DOI] [PubMed] [Google Scholar]
- 101. Poon S‐K, Lai C‐H, Chang C‐S, et al Prevalence of antimicrobial resistance in Helicobacter pylori isolates in Taiwan in relation to consumption of antimicrobial agents. Int J Antimicrob Agents 2009; 34: 162–5. [DOI] [PubMed] [Google Scholar]
- 102. Realdi G, Dore MP, Piana A, et al Pretreatment antibiotic resistance in Helicobacter pylori infection: results of three randomized controlled studies. Helicobacter 1999; 4: 106–12. [DOI] [PubMed] [Google Scholar]
- 103. Boyanova L, Stancheva I, Spassova Z, Katzarov N, Mitov I, Koumanova R. Primary and combined resistance to four antimicrobial agents in Helicobacter pylori in Sofia, Bulgaria. J Med Microbiol 2000; 49: 415–8. [DOI] [PubMed] [Google Scholar]
- 104. Boyanova L, Gergova G, Nikolov R, et al Prevalence and evolution of Helicobacter pylori resistance to 6 antibacterial agents over 12 years and correlation between susceptibility testing methods. Diagn Microbiol Infect Dis 2008; 60: 409–15. [DOI] [PubMed] [Google Scholar]
- 105. Ozcay F, Kocak N, Temizel INS, et al Helicobacter pylori infection in Turkish children: comparison of diagnostic tests, evaluation of eradication rate, and changes in symptoms after eradication. Helicobacter 2004; 9: 242–8. [DOI] [PubMed] [Google Scholar]
- 106. Caliskan R, Tokman HB, Erzin Y, et al Antimicrobial resistance of Helicobacter pylori strains to five antibiotics, including levofloxacin, in Northwestern Turkey. Rev Soc Bras Med Trop 2015; 48: 278–84. [DOI] [PubMed] [Google Scholar]
- 107. Megraud F. Surveillance de la résistance de Helicobacter pylori aux antibiotiques In: Desenclos JC, Vaillant V, Bonmarin I, eds. Surveillance Nationale des Maladies Infectieuses 1998–2000. St Maurice, France: Inst Veill Sanit, 2003; 327–9. [Google Scholar]
- 108. Wolle K, Leodolter A, Malfertheiner P, König W. Antibiotic susceptibility of Helicobacter pylori in Germany: stable primary resistance from 1995 to 2000. J Med Microbiol 2002; 51: 705–9. [DOI] [PubMed] [Google Scholar]
- 109. Toracchio S, Marzio L. Primary and secondary antibiotic resistance of Helicobacter pylori strains isolated in central Italy during the years 1998‐2002. Dig Liver Dis 2003; 35: 541–5. [DOI] [PubMed] [Google Scholar]
- 110. Torres J, Camorlinga‐Ponce M, Pérez‐Pérez G, et al Increasing multidrug resistance in Helicobacter pylori strains isolated from children and adults in Mexico. J Clin Microbiol 2001; 39: 2677–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bago J, Halle ZB, Strinić D, et al The impact of primary antibiotic resistance on the efficacy of ranitidine bismuth citrate‐ vs. omeprazole‐based one‐week triple therapies in H. pylori eradication–a randomised controlled trial. Wien Klin Wochenschr 2002; 114: 448–53. [PubMed] [Google Scholar]
- 112. Fallone CA. Epidemiology of the antibiotic resistance of Helicobacter pylori in Canada. Can J Gastroenterol 2000; 14: 879–82. [DOI] [PubMed] [Google Scholar]
- 113. Cuchí Burgos E, Forné Bardera M, Quintana Riera S, Lite Lite J, Garau Alemany J. [Evolution of the sensitivity of 235 strains of Helicobacter pylori from 1995 to 1998 and impact of antibiotic treatment]. Enferm Infecc Microbiol Clin 2002; 20: 157–60. [DOI] [PubMed] [Google Scholar]
- 114. Parsons HK, Carter MJ, Sanders DS, Winstanley T, Lobo AJ. Helicobacter pylori antimicrobial resistance in the United Kingdom: the effect of age, sex and socio‐economic status. Aliment Pharmacol Ther 2001; 15: 1473–8. [DOI] [PubMed] [Google Scholar]
- 115. Ling TKW, Leung WK, Lee CC, et al The antimicrobial susceptibility of Helicobacter pylori in Hong Kong (1997‐2001). Helicobacter 2002; 7: 327–8. [DOI] [PubMed] [Google Scholar]
- 116. Cabrita J, Oleastro M, Matos R, et al Features and trends in Helicobacter pylori antibiotic resistance in Lisbon area, Portugal (1990‐1999). J Antimicrob Chemother 2000; 46: 1029–31. [DOI] [PubMed] [Google Scholar]
- 117. Kim JM. Distribution of fluoroquinolone MICs in Helicobacter pylori strains from Korean patients. J Antimicrob Chemother 2005; 56: 965–7. [DOI] [PubMed] [Google Scholar]
- 118. Lee CC, Lee VWY, Chan FKL, Ling TKW. Levofloxacin‐Resistant Helicobacter pylori in Hong Kong. Chemotherapy 2008; 54: 50–3. [DOI] [PubMed] [Google Scholar]
- 119. Liao J, Zheng Q, Liang X, et al Effect of fluoroquinolone resistance on 14‐day levofloxacin triple and triple plus bismuth quadruple therapy. Helicobacter 2013; 18: 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Miyachi H, Miki I, Aoyama N, et al Primary levofloxacin resistance and gyrA/B mutations among Helicobacter pylori in Japan. Helicobacter 2006; 11: 243–9. [DOI] [PubMed] [Google Scholar]
- 121. Sun Q‐J, Liang X, Zheng Q, et al Resistance of Helicobacter pylori to antibiotics from 2000 to 2009 in Shanghai. World J Gastroenterol 2010; 16: 5118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Debets‐Ossenkopp YJ, Herscheid AJ, Pot RG, Kuipers EJ, Kusters JG, Vandenbroucke‐Grauls CM. Prevalence of Helicobacter pylori resistance to metronidazole, clarithromycin, amoxycillin, tetracycline and trovafloxacin in The Netherlands. J Antimicrob Chemother 1999; 43: 511–5. [DOI] [PubMed] [Google Scholar]
- 123. Tankovic J, Lascols C, Sculo Q, Petit J‐C, Soussy C‐J. Single and double mutations in gyrA but not in gyrB are associated with low‐ and high‐level fluoroquinolone resistance in Helicobacter pylori . Antimicrob Agents Chemother 2003; 47: 3942–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Boyanova L, Mentis A, Gubina M, et al The status of antimicrobial resistance of Helicobacter pylori in eastern Europe. Clin Microbiol Infect 2002; 8: 388–96. [DOI] [PubMed] [Google Scholar]
- 125. Sugimoto M, Sahara S, Ichikawa H, Kagami T, Uotani T, Furuta T. High Helicobacter pylori cure rate with sitafloxacin‐based triple therapy. Aliment Pharmacol Ther 2015; 42: 477–83. [DOI] [PubMed] [Google Scholar]
- 126. Song M, Ang TL. Second and third line treatment options for Helicobacter pylori eradication. World J Gastroenterol 2014; 20: 1517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Selgrad M, Tammer I, Langner C, et al Different antibiotic susceptibility between antrum and corpus of the stomach, a possible reason for treatment failure of Helicobacter pylori infection. World J Gastroenterol 2014; 20: 16245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Godoy APO, Ribeiro ML, Benvengo YHB, et al Analysis of antimicrobial susceptibility and virulence factors in Helicobacter pylori clinical isolates. BMC Gastroenterol 2003; 3: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Yoon K‐H, Park SW, Lee SW, Kim BJ, Kim JG. Clarithromycin‐based standard triple therapy can still be effective for Helicobacter pylori eradication in some parts of the Korea. J Korean Med Sci 2014; 29: 1240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Gerrits MM, Schuijffel D, van Zwet AA, Kuipers EJ, Vandenbroucke‐Grauls CMJE, Kusters JG. Alterations in penicillin‐binding protein 1A confer resistance to beta‐lactam antibiotics in Helicobacter pylori . Antimicrob Agents Chemother 2002; 46: 2229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Stenström B, Mendis A, Marshall B. Helicobacter pylori–the latest in diagnosis and treatment. Aust Fam Physician 2008; 37: 608–12. [PubMed] [Google Scholar]
- 132. Biological agents . Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 2012; 100(Pt B): 1–441. [PMC free article] [PubMed] [Google Scholar]
- 133. Chey WD, Wong BCY. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007; 102: 1808–25. [DOI] [PubMed] [Google Scholar]
- 134. Talley NJ, Vakil N. Guidelines for the management of dyspepsia. Am J Gastroenterol 2005; 100: 2324–37. [DOI] [PubMed] [Google Scholar]
- 135. Manes G, Menchise A, de Nucci C, Balzano A. Empirical prescribing for dyspepsia: randomised controlled trial of test and treat versus omeprazole treatment. BMJ 2003; 326: 1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Rosenstock S, Jørgensen T, Andersen L, Bonnevie O. Seroconversion and seroreversion in IgG antibodies to Helicobacter pylori: a serology based prospective cohort study. J Epidemiol Community Health 2000; 54: 444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Oderda G, Vaira D, Holton J. Seroconversion for Helicobacter pylori . J Clin Pathol 1994; 47: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Andersen LP, Rosenstock SJ, Bonnevie O, Jørgensen T. Seroprevalence of immunoglobulin G, M, and A antibodies to Helicobacter pylori in an unselected Danish population. Am J Epidemiol 1996; 143: 1157–64. [DOI] [PubMed] [Google Scholar]
- 139. She RC, Wilson AR, Litwin CM. Evaluation of Helicobacter pylori immunoglobulin G (IgG), IgA, and IgM serologic testing compared to stool antigen testing. Clin Vaccine Immunol 2009; 16: 1253–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Atherton JC. Non‐endoscopic tests in the diagnosis of Helicobacter pylori infection. Aliment Pharmacol Ther 1997; 11(Suppl. 1): 11–20. [DOI] [PubMed] [Google Scholar]
- 141. Domínguez J, Forné M, Blanco S, et al Comparison of a monoclonal with a polyclonal antibody‐based enzyme immunoassay stool test in diagnosing Helicobacter pylori infection before and after eradication therapy. Aliment Pharmacol Ther 2006; 23: 1735–40. [DOI] [PubMed] [Google Scholar]
- 142. Yaxley J, Chakravarty B. Helicobacter pylori eradication ‐ an update on the latest therapies. Aust Fam Physician 2014; 43: 301–5. [PubMed] [Google Scholar]
- 143. Lopes AI, Vale FF, Oleastro M. Helicobacter pylori infection ‐ recent developments in diagnosis. World J Gastroenterol 2014; 20: 9299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Malfertheiner P, Enrique Domínguez‐Muñoz J, Heckenmüller H, Neubrand M, Fischer HP, Sauerbruch T. Modified rapid urease test for detection of Helicobacter pylori infection. Eur J Gastroenterol Hepatol 1996; 8: 53–6. [DOI] [PubMed] [Google Scholar]
- 145. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996; 20: 1161–81. [DOI] [PubMed] [Google Scholar]
- 146. Gisbert JP, González L, Calvet X, et al Proton pump inhibitor, clarithromycin and either amoxycillin or nitroimidazole: a meta‐analysis of eradication of Helicobacter pylori . Aliment Pharmacol Ther 2000; 14: 1319–28. [DOI] [PubMed] [Google Scholar]
- 147. Bang CS, Baik GH. Attempts to enhance the eradication rate of Helicobacter pylori infection. World J Gastroenterol 2014; 20: 5252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Malfertheiner P, Mégraud F, O'Morain C, et al Current concepts in the management of Helicobacter pylori infection–the Maastricht 2‐2000 Consensus Report. Aliment Pharmacol Ther 2002; 16: 167–80. [DOI] [PubMed] [Google Scholar]
- 149. Graham DY, Lee Y‐C, Wu M‐S. Rational Helicobacter pylori therapy: evidence‐based medicine rather than medicine‐based evidence. Clin Gastroenterol Hepatol 2014; 12: 177–86.e3; Discussion e12–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Malfertheiner P, Megraud F, O'Morain C, et al Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007; 56: 772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Paoluzi P, Iacopini F, Crispino P, et al 2‐week triple therapy for Helicobacter pylori infection is better than 1‐week in clinical practice: a large prospective single‐center randomized study. Helicobacter 2006; 11: 562–8. [DOI] [PubMed] [Google Scholar]
- 152. McColl KEL. Clinical practice. Helicobacter pylori infection. N Engl J Med 2010; 362: 1597–604. [DOI] [PubMed] [Google Scholar]
- 153. Fischbach L, Evans EL. Meta‐analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first‐line therapies for Helicobacter pylori . Aliment Pharmacol Ther 2007; 26: 343–57. [DOI] [PubMed] [Google Scholar]
- 154. Sun Q, Liang X, Zheng Q, et al High efficacy of 14‐day triple therapy‐based, bismuth‐containing quadruple therapy for initial Helicobacter pylori eradication. Helicobacter 2010; 15: 233–8. [DOI] [PubMed] [Google Scholar]
- 155. Garcia N, Calvet X, Gené E, Campo R, Brullet E. Limited usefulness of a seven‐day twice‐a‐day quadruple therapy. Eur J Gastroenterol Hepatol 2000; 12: 1315–8. [DOI] [PubMed] [Google Scholar]
- 156. Perri F, Festa V, Merla A, Quitadamo M, Clemente R, Andriulli A. Amoxicillin/tetracycline combinations are inadequate as alternative therapies for Helicobacter pylori infection. Helicobacter 2002; 7: 99–104. [DOI] [PubMed] [Google Scholar]
- 157. Wang Z, Wu S. Doxycycline‐based quadruple regimen versus routine quadruple regimen for rescue eradication of Helicobacter pylori: an open‐label control study in Chinese patients. Singapore Med J 2012; 53: 273–6. [PubMed] [Google Scholar]
- 158. Akyildiz M, Akay S, Musoglu A, Tuncyurek M, Aydin A. The efficacy of ranitidine bismuth citrate, amoxicillin and doxycycline or tetracycline regimens as a first line treatment for Helicobacter pylori eradication. Eur J Intern Med 2009; 20: 53–7. [DOI] [PubMed] [Google Scholar]
- 159. Malfertheiner P, Bazzoli F, Delchier J‐C, et al Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin‐based triple therapy: a randomised, open‐label, non‐inferiority, phase 3 trial. Lancet 2011; 377: 905–13. [DOI] [PubMed] [Google Scholar]
- 160. Romano M, Cuomo A, Gravina AG, et al Empirical levofloxacin‐containing versus clarithromycin‐containing sequential therapy for Helicobacter pylori eradication: a randomised trial. Gut 2010; 59: 1465–70. [DOI] [PubMed] [Google Scholar]
- 161. Webber MA, Piddock LJV. The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother 2003; 51: 9–11. [DOI] [PubMed] [Google Scholar]
- 162. Essa AS, Kramer JR, Graham DY, Treiber G. Meta‐analysis: four‐drug, three‐antibiotic, non‐bismuth‐containing “concomitant therapy” versus triple therapy for Helicobacter pylori eradication. Helicobacter 2009; 14: 109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Georgopoulos S, Papastergiou V, Xirouchakis E, et al Nonbismuth quadruple “concomitant” therapy versus standard triple therapy, both of the duration of 10 days, for first‐line H. pylori eradication: a randomized trial. J Clin Gastroenterol 2013; 47: 228–32. [DOI] [PubMed] [Google Scholar]
- 164. Wu D‐C, Hsu P‐I, Wu J‐Y, et al Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol 2010; 8: 36–41.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Federico A, Nardone G, Gravina AG, et al Efficacy of 5‐day levofloxacin‐containing concomitant therapy in eradication of Helicobacter pylori infection. Gastroenterology 2012; 143: 55–61. e1; quize e13–4. [DOI] [PubMed] [Google Scholar]
- 166. Kongchayanun C, Vilaichone R, Pornthisarn B, Amornsawadwattana S, Mahachai V. Pilot studies to identify the optimum duration of concomitant Helicobacter pylori eradication therapy in Thailand. Helicobacter 2012; 17: 282–5. [DOI] [PubMed] [Google Scholar]
- 167. Hsu P‐I, Wu D‐C, Wu J‐Y, Graham DY. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter 2011; 16: 139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Molina‐Infante J, Romano M, Fernandez‐Bermejo M, Federico A, Gravina A, Pozzati L. 14‐day, high‐dose acid suppression, non‐bismuth quadruple therapies (“hybrid” vs. “concomitant”) for Helicobacter pylori infection: a randomized trial. Gut 2012; 61 (Suppl. 3): A47. [Google Scholar]
- 169. Molina‐Infante J, Romano M, Fernandez‐Bermejo M, et al Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology 2013; 145: 121–8.e1. [DOI] [PubMed] [Google Scholar]
- 170. Berning M, Krasz S, Miehlke S. Should quinolones come first in Helicobacter pylori therapy? Therap Adv Gastroenterol 2011; 4: 103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Basu PP, Rayapudi K, Pacana T, Shah NJ, Krishnaswamy N, Flynn M. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori . Am J Gastroenterol 2011; 106: 1970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Hsu PI, Wu DC, Chen A, et al Quadruple rescue therapy for Helicobacter pylori infection after two treatment failures. Eur J Clin Invest 2008; 38: 404–9. [DOI] [PubMed] [Google Scholar]
- 173. Nishizawa T, Suzuki H, Hibi T. Quinolone‐based third‐line therapy for Helicobacter pylori eradication. J Clin Biochem Nutr 2009; 44: 119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther 2012; 35: 209–21. [DOI] [PubMed] [Google Scholar]
- 175. Almeida C, Azevedo NF, Iversen C, Fanning S, Keevil CW, Vieira MJ. Development and application of a novel peptide nucleic acid probe for the specific detection of Cronobacter genomospecies (Enterobacter sakazakii) in powdered infant formula. Appl Environ Microbiol 2009; 75: 2925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Kim SY, Jung SW, Kim JH, et al Effectiveness of three times daily lansoprazole/amoxicillin dual therapy for Helicobacter pylori infection in Korea. Br J Clin Pharmacol 2012; 73: 140–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Sugimoto M, Furuta T, Shirai N, et al Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter 2007; 12: 317–23. [DOI] [PubMed] [Google Scholar]
- 178. Kim YK, Kim JS, Kim B‐W. Recent trends of Helicobacter pylori eradication therapy in Korea. Korean J Helicobacter Up Gastrointest Res 2012; 12: 219. [Google Scholar]
- 179. Qureshi NN, Gallaher B, Schiller NL. Evolution of amoxicillin resistance of Helicobacter pylori in vitro: characterization of resistance mechanisms. Microb Drug Resist 2014; 20: 509–16. [DOI] [PubMed] [Google Scholar]
- 180. Klesiewicz K, Nowak P, Karczewska E, et al PCR‐RFLP detection of point mutations A2143G and A2142G in 23S rRNA gene conferring resistance to clarithromycin in Helicobacter pylori strains. Acta Biochim Pol 2014; 61: 311–5. [PubMed] [Google Scholar]
- 181. Goodwin A, Kersulyte D, Sisson G, Veldhuyzen van Zanten SJ, Berg DE, Hoffman PS. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen‐insensitive NADPH nitroreductase. Mol Microbiol 1998; 28: 383–93. [DOI] [PubMed] [Google Scholar]
- 182. Jenks PJ, Edwards DI. Metronidazole resistance in Helicobacter pylori . Int J Antimicrob Agents 2002; 19: 1–7. [DOI] [PubMed] [Google Scholar]
- 183. Mirzaei N, Poursina F, Moghim S, Rahimi E, Safaei HG. The mutation of the rdxA gene in metronidazole‐resistant Helicobacter pylori clinical isolates. Adv Biomed Res 2014; 3: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Gerrits MM, de Zoete MR, Arents NLA, Kuipers EJ, Kusters JG. 16S rRNA mutation‐mediated tetracycline resistance in Helicobacter pylori . Antimicrob Agents Chemother 2002; 46: 2996–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Cattoir V, Nectoux J, Lascols C, et al Update on fluoroquinolone resistance in Helicobacter pylori: new mutations leading to resistance and first description of a gyrA polymorphism associated with hypersusceptibility. Int J Antimicrob Agents 2007; 29: 389–96. [DOI] [PubMed] [Google Scholar]
- 186. Moore RA, Beckthold B, Wong S, Kureishi A, Bryan LE. Nucleotide sequence of the gyrA gene and characterization of ciprofloxacin‐resistant mutants of Helicobacter pylori . Antimicrob Agents Chemother 1995; 39: 107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Yamamoto T, Takano T, Higuchi W, et al Helicobacter pylori eradication by sitafloxacin‐lansoprazole combination and sitafloxacin pharmacokinetics in Mongolian gerbils and its in vitro activity and resistance development. Antimicrob Agents Chemother 2011; 55: 4261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Hirata Y, Ohmae T, Yanai A, et al Sitafloxacin resistance in Helicobacter pylori isolates and sitafloxacin‐based triple therapy as a third‐line regimen in Japan. Int J Antimicrob Agents 2012; 39: 352–5. [DOI] [PubMed] [Google Scholar]
- 189. Heep M, Beck D, Bayerdörffer E, Lehn N. Rifampin and rifabutin resistance mechanism in Helicobacter pylori . Antimicrob Agents Chemother 1999; 43: 1497–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Heep M, Rieger U, Beck D, Lehn N. Mutations in the beginning of the rpoB gene can induce resistance to rifamycins in both Helicobacter pylori and Mycobacterium tuberculosis . Antimicrob Agents Chemother 2000; 44: 1075–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Suzuki S, Suzuki H, Nishizawa T, et al Past rifampicin dosing determines rifabutin resistance of Helicobacter pylori . Digestion 2009; 79: 1–4. [DOI] [PubMed] [Google Scholar]
- 192. Van der Ende A, van Doorn LJ, Rooijakkers S, Feller M, Tytgat GN, Dankert J. Clarithromycin‐susceptible and ‐resistant Helicobacter pylori isolates with identical randomly amplified polymorphic DNA‐PCR genotypes cultured from single gastric biopsy specimens prior to antibiotic therapy. J Clin Microbiol 2001; 39: 2648–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Miendje Deyi VY, Burette A, Bentatou Z, et al Practical use of GenoType® HelicoDR, a molecular test for Helicobacter pylori detection and susceptibility testing. Diagn Microbiol Infect Dis 2011; 70: 557–60. [DOI] [PubMed] [Google Scholar]
- 194. Grignon B, Tankovic J, Mégraud F, et al Validation of diffusion methods for macrolide susceptibility testing of Helicobacter pylori. Microb Drug Resist 2002; 8: 61–6. [DOI] [PubMed] [Google Scholar]
- 195. Hartzen SH, Andersen LP, Bremmelgaard A, et al Antimicrobial susceptibility testing of 230 Helicobacter pylori strains: importance of medium, inoculum, and incubation time. Antimicrob Agents Chemother 1997; 41: 2634–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Henriksen TH, Lia A, Schøyen R, Thoresen T, Berstad A. Assessment of optimal atmospheric conditions for growth of Helicobacter pylori . Eur J Clin Microbiol Infect Dis 2000; 19: 718–20. [DOI] [PubMed] [Google Scholar]
- 197. National Committee for Clinical Laboratory Standards . Performance Standards for Antimicrobial Susceptibility Testing and Approved Standard M7‐A5. Informational Supplement M100‐S10. Wayne, PA: National Committee for Clinical Laboratory Standards, 2000. [Google Scholar]
- 198. Hachem CY, Clarridge JE, Reddy R, et al Antimicrobial susceptibility testing of Helicobacter pylori. Comparison of E‐test, broth microdilution, and disk diffusion for ampicillin, clarithromycin, and metronidazole. Diagn Microbiol Infect Dis 1996; 24: 37–41. [DOI] [PubMed] [Google Scholar]
- 199. Destura RV, Labio ED, Barrett LJ, et al Laboratory diagnosis and susceptibility profile of Helicobacter pylori infection in the Philippines. Ann Clin Microbiol Antimicrob 2004; 3: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Glupczynski Y, Broutet N, Cantagrel A, et al Comparison of the E test and agar dilution method for antimicrobial susceptibility testing of Helicobacter pylori . Eur J Clin Microbiol Infect Dis 2002; 21: 549–52. [DOI] [PubMed] [Google Scholar]
- 201. Yilmaz O, Demiray E. Clinical role and importance of fluorescence in situ hybridization method in diagnosis of H pylori infection and determination of clarithromycin resistance in H pylori eradication therapy. World J Gastroenterol 2007; 13: 671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Burucoa C, Garnier M, Silvain C, Fauchère J‐L. Quadruplex real‐time PCR assay using allele‐specific scorpion primers for detection of mutations conferring clarithromycin resistance to Helicobacter pylori . J Clin Microbiol 2008; 46: 2320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Van Doorn LJ, Glupczynski Y, Kusters JG, et al Accurate prediction of macrolide resistance in Helicobacter pylori by a PCR line probe assay for detection of mutations in the 23S rRNA gene: multicenter validation study. Antimicrob Agents Chemother 2001; 45: 1500–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Schabereiter‐Gurtner C, Hirschl AM, Dragosics B, et al Novel real‐time PCR assay for detection of Helicobacter pylori infection and simultaneous clarithromycin susceptibility testing of stool and biopsy specimens. J Clin Microbiol 2004; 42: 4512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Mégraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev 2007; 20: 280–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Rüssmann H, Adler K, Haas R, Gebert B, Koletzko S, Heesemann J. Rapid and accurate determination of genotypic clarithromycin resistance in cultured Helicobacter pylori by fluorescent in situ hybridization. J Clin Microbiol 2001; 39: 4142–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Perry‐O'Keefe H, Stender H, Broomer A, Oliveira K, Coull J, Hyldig‐Nielsen JJ. Filter‐based PNA in situ hybridization for rapid detection, identification and enumeration of specific micro‐organisms. J Appl Microbiol 2001; 90: 180–9. [DOI] [PubMed] [Google Scholar]
- 208. Stender H, Fiandaca M, Hyldig‐Nielsen JJ, Coull J. PNA for rapid microbiology. J Microbiol Methods 2002; 48: 1–17. [DOI] [PubMed] [Google Scholar]
- 209. Cerqueira L, Azevedo NF, Almeida C, Jardim T, Keevil CW, Vieira MJ. DNA mimics for the rapid identification of microorganisms by fluorescence in situ hybridization (FISH). Int J Mol Sci 2008; 9: 1944–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta‐analysis of sequential therapy. BMJ 2013; 347: f4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Fujioka T, Aoyama N, Sakai K, et al A large‐scale nationwide multicenter prospective observational study of triple therapy using rabeprazole, amoxicillin, and clarithromycin for Helicobacter pylori eradication in Japan. J Gastroenterol 2011; 47: 276–83. [DOI] [PubMed] [Google Scholar]
- 212. Huang JQ, Hunt RH. Treatment after failure: the problem of “non‐responders”. Gut 1999; 45(Suppl. 1): I40–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Bruce MG, Bruden DL, Morris JM, et al Reinfection after successful eradication of Helicobacter pylori in three different populations in Alaska. Epidemiol Infect 2015; 143: 1236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Marais A, Monteiro L, Lamouliatte H, Samoyeau R, Megraud F. Cag negative status of Helicobacter pylori is a risk factor for failure of PPI‐based triple therapies in non‐ulcer dyspepsia. Gastroenterology 1998; 114: A214. [Google Scholar]
- 215. Van Doorn LJ, Schneeberger PM, Nouhan N, Plaisier AP, Quint WG, de Boer WA. Importance of Helicobacter pylori cagA and vacA status for the efficacy of antibiotic treatment. Gut 2000; 46: 321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Selgrad M, Malfertheiner P. New strategies for Helicobacter pylori eradication. Curr Opin Pharmacol 2008; 8: 593–7. [DOI] [PubMed] [Google Scholar]
- 217. Van Boeckel TP, Gandra S, Ashok A, et al Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14: 742–50. [DOI] [PubMed] [Google Scholar]


