Abstract
An internet search was made looking for articles about chemical and physical modalities that are known to induce collagen and elastin formation. Textbooks, independent articles, journals and books on pathology, biochemistry, aesthetic medicine and cosmetic and plastic surgery were used as references. Here, we take a look at various studies, in vitro and in vivo, that lend credence to the products and procedures used in clinical practice to induce neocollagenesis and neoelastinogenesis.
KEYWORDS: Cutaneous ageing, cutaneous scarring, neocollagenesis, neoelastinogenesis, wrinkles
INTRODUCTION
Collagen and elastin are the main fibres that form the extracellular matrix. Both are formed by fibroblasts. Collagen is responsible for tensile strength and elastin provides elasticity to the skin. Production and density of both decreases as a function of age, and results in sagging and wrinkling. Wounding alters the amount and quality of these fibres. Thus, neocollagenesis and neoelastinogenesis hold the key to managing aesthetic conditions such as cutaneous ageing and scarring.
TOPICALS AND NEUTRACEUTICALS
A healthy diet is one of the prerequisites of healthy skin. Proteins, vitamins, minerals, trace elements and antioxidants obtained from our diet are the routinely available basic ingredients for formation and maintenance of skin fibres. Vitamin and protein supplements in the form of neutraceuticals enhance the availability of substrates for synthesis of new fibres. Antioxidants (Vitamins A, C and E, trace elements such as selenium, copper and zinc, polyphenols, flavonoids, glutathione, peroxidases and superoxide desmutases) prevent degradation of tissues caused by free radicals.
Retinoids
Retinoids are compounds that are chemically related to Vitamin A. Among the retinoids; tretinoin possibly is the most potent and certainly the most widely investigated retinoid for photoaging therapy. Biochemical changes brought about by retinoids include an increase in the expressions of Type 1 and Type 3 procollagen, reticulin fibres, tropoelastin, fibrillin-1 and glycosaminoglycans (GAGs).[1] Tretinoin results in complete blockade of interstitial collagenase and gelatinases synthesis and reduces matrix metalloproteinase 1 (MMP1). Metalloproteinases degrade amorphous collagen. Thus, retinoids prevent collagen degradation.[2] Histologically, tretinoin results in marked increase in epidermal cell layer thickness and a highly undulating dermo-epidermal junction through the development of rete pegs and produces uniformity in keratinocyte density while it decreases melanocyte vacuolisation. Ultrastructurally, an increase in anchoring fibrils is noted at the level of the dermo-epidermal junction. In the dermis, development of several new microvasculatures (angiogenesis) and production of new elastic material and GAGs is observed.[3]
Irritant reactions limit the acceptance of retinoids by patients. This problem is more prominent with tretinoin and tazarotene whereas other retinoids mainly represented by retinaldehyde and retinol are considerably less irritating. To minimize these side effects, various novel drug delivery systems have been developed.[4] Seletinoid G represents the fourth generation of retinoids that might find an important place in the treatment of intrinsic/photoaging. Topical application of seletinoid G under occlusion induced no skin irritation in contrast to tretinoin which caused severe erythema. Thus, seletinoid G appears to be as effective as tretinoin in the treatment of intrinsic/photoaging with the added advantage of the absence of skin irritation.[5] A new drug delivery system, solid lipid nanoparticles for the delivery of retinol based tretinoin gels showed drastic improvement in the tolerability of tretinoin.[6]
Retinol [Figure 1] could be as effective as retinoic acid in producing ‘retinoid-mediated histological changes’ like epidermal thickening and keratinocyte proliferation, but with much less irritancy.[7]
Figure 1.
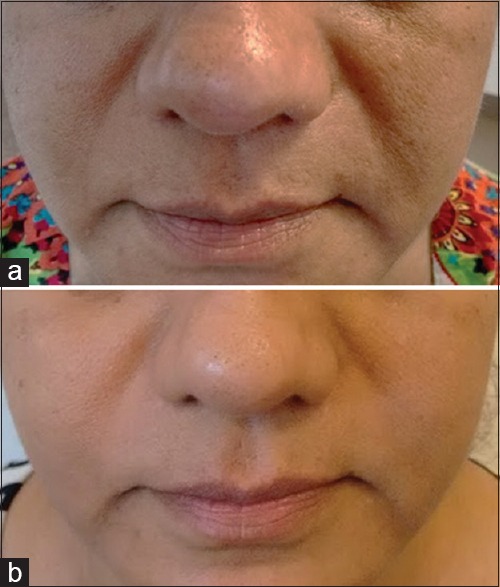
(a) Topical retinoids for antiaging- before treatment. (b) Topical retinoids for antiaging - after 2 months of application
Vitamin C
Vitamin C (ascorbic acid) is a cofactor in the hydroxylation of lysine and proline required for making the triple helix structure of collagen. It is needed in the cross-linking process that converts soluble tropoelastin to insoluble elastin. Vitamin C induces a dose-dependent increase in collagen Type I deposits by normal human fibroblasts.[8]
In a study by Fitzpatrick and Rostan a formulation of Vitamin C - 10% ascorbic acid (water soluble) and 7% tetrahexyldecyl ascorbate (lipid soluble) in an anhydrous polysilicone gel base resulted in clinically visible and statistically significant improvement in wrinkling when used topically for 12 weeks. Biopsies showed increased Grenz zone collagen, as well as increased staining for microRNA (mRNA) for Type I collagen.[9]
Proteins, peptides and amino acids
Both collagen and elastin are proteins. Collagen is rich in the amino acids glycine, proline and hydroxyproline. Elastin is mainly composed of glycine, valine and lysine.
Net collagen accumulation depends not only on increased synthesis but also on decreased degradation.[10]
Hydrolysed collagen made from native peptides found in animals consists of small peptides with low molecular weight, enriched in specific amino acids: Glycine, proline and hydroxyproline. Due to its low molecular weight, hydrolysed collagen is highly digestible, absorbed and distributed in the different tissues of the human body. In the dermis, hydrolysed collagen has a dual action mechanism - (a) free amino acids provide building blocks for the formation of collagen and elastin fibres, (b) collagen oligopetides act as ligands, binding to receptors present on the fibroblasts’ membrane and stimulate the production of new collagen, elastin and hyaluronic acid (HA).[11]
Oestrogens and geneistein
Oestrogens have a profound influence on skin. The relative hypoestrogenism that accompanies menopause exacerbates the deleterious effects of both intrinsic and environmental aging. Oestrogens increase collagen content and skin thickness and improve skin moisture. Although systemic hormone replacement therapy (HRT) has been used for many years, recent trials have reported a significant increased risk of breast cancer and other pathologies with this treatment. For this reason, systemic HRT cannot be recommended today to treat skin aging. Currently, intensive research is being conducted to develop new drugs called selective oestrogen receptor modulators (SERMs).[12]
Geneistein (a soy isoflavone, known for its phytoestrogenic properties) is the best known natural SERM. As oestrogen receptor agonist, genistein stimulates the production of new collagen. It inhibits tyrosine protein kinase and MMPs, thus stimulating de novo production and inhibiting degradation of collagen and elastin.[13] Topically applied genistein cannot exert significant physiological effects on the skin unless encapsulated in liposomes. Liposomal genistein has shown to increase skin thickness and firmness which is principally determined by collagen.[14]
ENERGY BASED TECHNOLOGIES
Various energy based technologies like radio-frequency (RF), infra-red and deep ultrasound are available that trigger neocollagenesis and help to firm, tighten, lift and smoothen skin [Figure 2]. Application of monopolar RF creates an electro-magnetic field (EMF) with alternating polarity which stimulates the movement of charged particles and sets up an electric current. The current conducts most effectively through hydrophilic structures such as dermal collagen framework and underlying fibrous septae and much less effectively through subcutaneous fat. Tissue resistance to the flow of current generates localised heat within the collagen-based structures.[15] The threshold of collagen denaturation is approximately 60-65°C. Temperatures below 60° have minimal effect and above 70° show insignificant additional benefits.[16]
Figure 2.
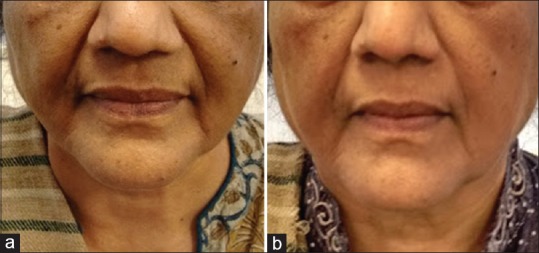
(a) Skin tightening with radiofrequency and ultrasound - before treatment. (b) Skin tightening with radiofrequency and ultrasound - after two sittings at 10 days interval
Monopolar RF leads to neocollagenesis in the papillary dermis, and upper, mid, and deep reticular dermis, and neoelastinogenesis in the papillary dermis, and upper- and mid-reticular dermis. High-intensity focused ultrasound (HIFU) leads to neocollagenesis in the mid and deep reticular dermis and neoelastogenesis in the deep reticular dermis. Among these treatment methods, HIFU shows the highest level of neocollagenesis and neoelastogenesis in the deep reticular dermis.[17]
Collagen rejuvenates over a month or so after treatment. Increased small collagen fiber formation-evidence of neocollagenesis, has been noted at 30 days post heat treatment. On tracking tissue changes after heating to denaturation range, neocollagenesis, neoelastinogenesis and deposition of new HA at 10 weeks post treatment were found.[18]
Nonablative RF is a safe and effective method to achieve tissue tightening of the face to correct excessive sagging from photo aging. Patients had visible results as early as 1 week and generally within 3 months after the procedure without wounding or scarring. Fractional RF (FRF) treatment generates an RF thermal zone (RFTZ) pattern in the reticular dermis that consists of zones of denatured collagen separated by zones of spared dermis. A vigorous wound healing response is initiated post-treatment, with a progressive increase in inflammatory cell infiltration from day two through 10 weeks. An active dermal remodelling process driven by the collagen chaperone HSP47 leads to complete replacement of RFTZs with new collagen by 10 weeks post-treatment. RFTZs are observed through day 28 post-treatment but are replaced by new dermal tissue by 10 weeks. Reticular dermal volume, cellularity, HA and elastin content increase. Furthermore, using immunohistochemical and polymerase chain reaction (PCR) studies, evidence of profound neoelastinogenesis following RF treatment of human skin is demonstrated. The combination of neoelastinogenesis and neocollagenesis induced by treatment with the FRF system may provide a reliable treatment option for skin laxity and/or rhytids.[18,19]
Broad-spectrum (1100–1800 nm) infrared light in multi-second cycles heats water in the dermis. With this band, bulk heating of the dermis is maximised at one to three mm, and absorption by melanin and haemoglobin is low. The epidermis is protected by a cooling head. Collagen fibril denaturation has been shown by electron microscopy. Multiple passes at low energy levels are associated with bulk dermal heating and collagen contraction.[20]
LASER AND LIGHT THERAPIES
Stimulation of new collagen production by laser treatments has been attributed to the release of inflammatory mediators from vascular epithelial cells. When stimulated by treatment with a combination of 532 nm and 1064 nm laser devices, collagen remodelling continues for 6–12 months and increases with the number and intensity of treatments, as well as elapsed time.[21,22]
Cell growth and cellular viability increase in fibroblasts 24 and 48 h after intense pulsed light irradiation. The levels of Type 1 and 3 procollagen mRNA expression in fibroblasts increase.[23] There is rearrangement of both collagen and elastin within the stroma.[24]
Twenty-two patients completed a 1-year study by Rosenberg et al. in which biopsy specimens from the upper lip were taken preoperatively and 6 weeks, 6 months and 1 year after CO2 laser resurfacing. Trichrome stains and Verhoeff-van Gieson stains were used to demonstrate tissue collagen and tissue elastin. Neocollagenesis beginning at 6 weeks and progressively increasing at 6 months and 1 year was clearly demonstrated. Neoelastinogenesis showed significant increases at 6 months and 1 year. It is hypothesised that the additive effects of initial collagen shrinkage and the long-term effect of neocollagenesis and neoelastinogenesis are the significant factors contributing to the long-lasting results of CO2 laser skin resurfacing.[25]
MICRONEEDLING/COLLAGEN INDUCTION THERAPY
In 2006, Dr. Laaff, a dermatopathologist, evaluated twenty blinded biopsies from a study performed by Dr. M. Schwarz, a plastic surgeon, into the effects of the derma roller device on collagen and elastin formation. Biopsies were taken from various parts of the body of ten patients undergoing elective cosmetic surgery. A first ‘control’ biopsy was taken adjacent to the site that was needled with the derma roller. Six to eight weeks later, another biopsy was taken from the needled skin. New collagen and elastin fibre formation was obvious and quite dramatic. On average, an increase of new fibres of 206% was observed.[26] Compared to wounds caused by trauma or surgery, in wounds caused by microneedling, the haemostasis, inflammation, proliferation and remodelling phases are significantly curtailed and healing never ends in a scar formation. Microneedling of normal skin leads to a polarised EMF in the inter-cellular electrolyte. The EMF stimulates DNA-expression of the surrounding cells. This epigenetic DNA-information by electro-taxis leads to an enhanced motility of epithelial and endothelial cells in the wounded area and subsequently to gene expression of growth factors that facilitate healing. After microneedling of a scar, new capillaries and fibroblasts migrate to the scar tissue. Under the influence of transforming growth factor-B3 (TGF-B3),[10] new Type 3 collagen fibres integrate into the existing skin matrix without any trace of fibrotic tissue. MMPs degrade excess fibrotic tissue. New collagen is deposited from a depth of 0.6 mm upwards and towards the basal membrane, in the most cases when needles with a length of 1.5 mm are used.[27]
Skin improvement is evident 3–4 weeks after a microneedle session. However, collagen maturation needs time, especially to transform into more tensile collagen Type 1.[28,29]
FILLERS
HA fillers were designed to provide temporary correction to enhance static contour defects. It is now theorised that these HA fillers may have more permanent and longer lasting effects.[30] Wang et al. explored the mechanisms behind potential neocollagenesis from injection of cross-linked HA fillers.[31] They were able to prove that fibroblasts take on a more morphologically stretched shape and a more active phenotype as a result of HA fillers. HA injection, however, was not shown to have a direct stimulatory effect on fibroblasts. Instead, it was demonstrated and theorised that existing collagen fibers are stretched by HA injection, which imposes mechanical tension on surrounding fibroblasts, therefore, stimulating them to increase production of Types 1 and 3 collagen. Procollagen, MMP and MMP tissue inhibitors of metalloproteinase (TIMP-1) were also measured by ELISA and quantitative PCR. They also found a statistically significant increase in procollagen, TIMP-1 and gene expression of procollagen 1 and 3, 1 month after injection. Procollagen levels remained elevated at three and 6 months of subsequent injections.[32]
DERMAL THREADS
Polydioxanone (PDS) is a colourless, crystalline, biodegradable synthetic polymer. It has been used as a suture material for many years. All sutures, regardless of composition, are foreign materials and elicit a foreign body reaction in the body.[10] Absorption of suture is by simple hydrolysis within 180 days from implantation day. PDS sutures cause minimal tissue reaction. They stimulate the activity of fibroblasts, thus activating the mechanism of neocollagenesis. It has been histologically confirmed that within the 1st month a delicate fibrous capsule forms around the implanted threads. It coats the threads as a small soft connective tissue cover, creating artificial ligaments that strengthen the effect achieved after the thread introduction. As the result, there has been noted a double effect of absorbable threads usage – consistent mechanical lift effect and tissue revitalisation.[33,34]
PLATELET RICH PLASMA
Autologous platelet rich plasma (PRP) has received a lot of attention recently for its healing and rejuvenating properties and is used to treat scars, [Figure 3] wrinkles or hair loss. Platelets on degranulation release growth factors and cytokines like platelet derived growth factor, TGF-B, fibroblast growth factor-2, vascular endothelial growth factor, epidermal growth factor, etc.[10] PRP has been investigated for its effects on the remodelling of the extracellular matrix, a process that requires activation of dermal fibroblasts, which is essential for rejuvenation of aged skin.
Figure 3.
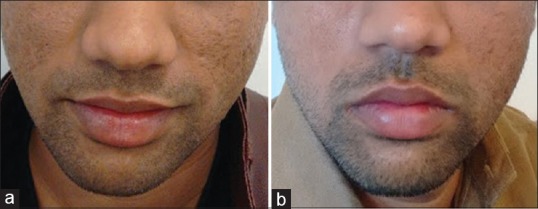
(a) Acne scars - before platelet rich plasma. (b) Acne scars - after 2 months of single session of platelet rich plasma with microneedling
Levels of procollagen Type 1 carboxy-terminal peptide are high in cells grown in the presence of 5% PRP. In addition, PRP increases the expression of Type 1 collagen, MMP-1 protein, and mRNA in human dermal fibroblasts.[35]
In a clinical trial by Redaelli et al. involving monthly injections of four ml PRP in 23 volunteers of average age 47 years for facial rejuvenation, almost 30% improvement was seen in deep wrinkles. Furthermore, every patient noted improvement in skin texture, homogenicity and small wrinkles.[36] In a split-face trial using PRP after fractional CO2 resurfacing, PRP enhanced recovery and clinical improvement of acne scars.[37]
DISCUSSION
Skin shows the first visible signs of ageing of the body as the ageing of internal organs are hidden from sight. Scars can cause tremendous psychological distress even in children. In a market that is flooded with products that claim to treat scars and prevent or even reverse ageing, it is important to confirm the bioavailability and efficacy of the ingredients at the level of the dermis and to use formulations that provide maximum benefits with minimal adverse effects. Newer peptides are introduced to us every day in cosmeceuticals, with every new formulation containing new combinations and claiming amazing, almost unrealistic results. Matrikines (Avarta, Dr. Reddy’s) are peptide fragments derived from proteolysis of extracellular matrix. They exert feedback control over connective tissue renewal. When combined with palmitic acid, their lipophilicity and hence skin penetration, is enhanced.[38] Signal peptides such as Palmitoyl pentapeptides may assist in neocollagenesis. Copper peptides have a dual role as collagen stimulators and antioxidants for wound healing. Peptides may be synergistic with energy based devices to sustain the neocollagenesis initiated by them. Growth factors are also being used for skin repair and regeneration. They also pose risks because, unregulated, they may mediate carcinogenic transformation of skin cells. This new field of cosmeceuticals is exciting but still in its infancy.[39]
Outpatient noninvasive or minimally invasive clinical procedures look promising and effective in the short-term. Neocollagenesis is easily induced, but that does not necessarily translate into mature collagen formation as procollagen has a very short half-life and is easily degraded. Collagen maturation is a process that requires years.[40] Long-term studies are needed to know if these energy-based treatments affect the content of mature collagen in the dermis.
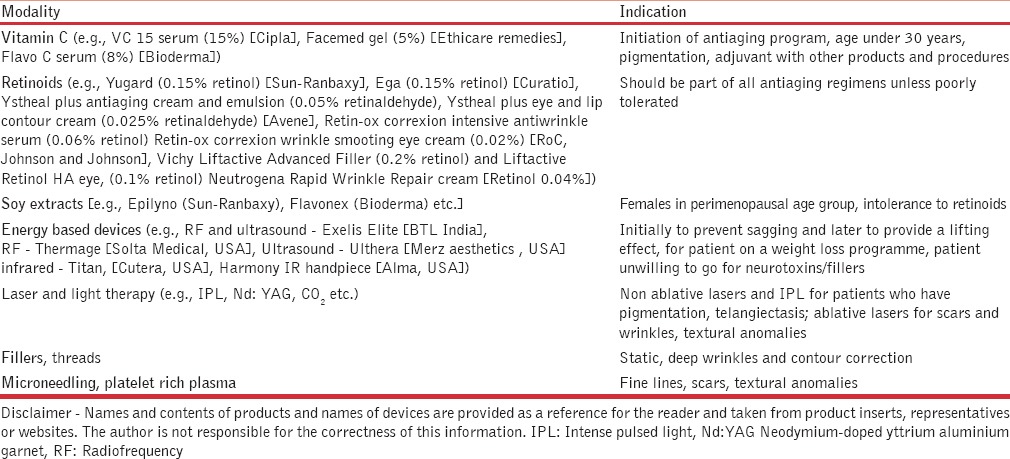
The clinician may be faced with a dilemma as to which modality for neocollagenesis and neoelastinogenesis needs to be used in what patient and when [Table 1]. Apart from a healthy lifestyle and neutraceuticals, Table 2 gives a guideline by the author to help select the modality. Different approaches can be used even in the same patient at different times depending on the prevailing skin condition.
Table 1.
Summary of products and procedures currently used in clinical practice to induce neocollagenesis and neoelastinogenesis and their mechanism of action

Table 2.
Guideline by the author to help select modality to induce neocollagenesis and neoelastinogenesis
Options are growing by the day, so one must carefully screen patients to provide them the therapy ideally suited to their skin which is backed by evidence-based research.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kligman AM, Grove GL, Hirose R, Leyden JJ. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986;15(4 Pt 2):836–59. doi: 10.1016/s0190-9622(86)70242-9. [DOI] [PubMed] [Google Scholar]
- 2.Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–9. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 3.Kligman AM, Dogadkina D, Lavker RM. Effects of topical tretinoin on non-sun-exposed protected skin of the elderly. J Am Acad Dermatol. 1993;29:25–33. doi: 10.1016/0190-9622(93)70147-l. [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: An overview of clinical efficacy and safety. Clin Interv Aging. 2006;1:327–48. doi: 10.2147/ciia.2006.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim MS, Lee S, Rho HS, Kim DH, Chang IS, Chung JH. The effects of a novel synthetic retinoid, seletinoid G, on the expression of extracellular matrix proteins in aged human skin in vivo. Clin Chim Acta. 2005;362:161–9. doi: 10.1016/j.cccn.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Jenning V, Gohla SH. Encapsulation of retinoids in solid lipid nanoparticles (SLN) J Microencapsul. 2001;18:149–58. doi: 10.1080/02652040010000361. [DOI] [PubMed] [Google Scholar]
- 7.Duell EA, Derguini F, Kang S, Elder JT, Voorhees JJ. Extraction of human epidermis treated with retinol yields retro-retinoids in addition to free retinol and retinyl esters. J Invest Dermatol. 1996;107:178–82. doi: 10.1111/1523-1747.ep12329576. [DOI] [PubMed] [Google Scholar]
- 8.Boyera N, Galey I, Bernard BA. Effect of Vitamin C and its derivatives on collagen synthesis and cross-linking by normal human fibroblasts. Int J Cosmet Sci. 1998;20:151–8. doi: 10.1046/j.1467-2494.1998.171747.x. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick RE, Rostan EF. Double-blind, half-face study comparing topical Vitamin C and vehicle for rejuvenation of photodamage. Dermatol Surg. 2002;28:231–6. doi: 10.1046/j.1524-4725.2002.01129.x. [DOI] [PubMed] [Google Scholar]
- 10.Kumar V, Cotran R, Robbins S. Basic Pathology. 6th ed. Philadelphia: Saunders; 1999. [Google Scholar]
- 11.Sibilla S, Godfrey M, Brewer S, Budh-Raja A, Genovese L. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: Scientific background and clinical studies. Open Nutraceuticals J. 2015;8:29–42. [Google Scholar]
- 12.Verdier-Sévrain S, Bonté F, Gilchrest B. Biology of estrogens in skin: Implications for skin aging. Exp Dermatol. 2006;15:83–94. doi: 10.1111/j.1600-0625.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- 13.Yoon HK, Chen K, Baylink DJ, Lau KH. Differential effects of two protein kinase inhibitors, tryphostin and genistein, on human bone cell proliferation. Tissue Int. 1998;63:243–9. doi: 10.1007/s002239900521. [DOI] [PubMed] [Google Scholar]
- 14.Schmid D, Belser E, Meister S. Use of soy isoflavones for stimulation of skin collagen synthesis. Switzerland: Mibelle Biochemistry; 2008. [Google Scholar]
- 15.Abraham MT, Vic Ross E. Current concepts in nonablative radiofrequency rejuvenation of the lower face and neck. Facial Plast Surg. 2005;21:65–73. doi: 10.1055/s-2005-871765. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi K, Thabit G, Massa KL, Bogdanske JJ, Cooley AJ, Orwin JF, et al. The effect of thermal heating on the length and histologic properties of the glenohumeral joint capsule. Am J Sports Med. 1997;25:107–12. doi: 10.1177/036354659702500121. [DOI] [PubMed] [Google Scholar]
- 17.Suh DH, Choi JH, Lee SJ, Jeong KH, Song KY, Shin MK. Comparative histometric analysis of the effects of high-intensity focused ultrasound and radiofrequency on skin. J Cosmet Laser Ther. 2015;17:230–6. doi: 10.3109/14764172.2015.1022189. [DOI] [PubMed] [Google Scholar]
- 18.Hantash BM, Ubeid AA, Chang H, Kafi R, Renton B. Bipolar fractional radiofrequency treatment induces neoelastogenesis and neocollagenesis. Lasers Surg Med. 2009;41:1–9. doi: 10.1002/lsm.20731. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz-Esparza J, Gomez JB. The medical face lift: A noninvasive, nonsurgical approach to tissue tightening in facial skin using nonablative radiofrequency. Dermatol Surg. 2003;29:325–32. doi: 10.1046/j.1524-4725.2003.29080.x. [DOI] [PubMed] [Google Scholar]
- 20.Bunin LS, Carniol PJ. Cervical facial skin tightening with an infrared device. Facial Plast Surg Clin North Am. 2007;15:179–84. doi: 10.1016/j.fsc.2007.01.006. vi. [DOI] [PubMed] [Google Scholar]
- 21.Lee MW. Combination 532-nm and 1064-nm lasers for noninvasive skin rejuvenation and toning. Arch Dermatol. 2003;139:1265–76. doi: 10.1001/archderm.139.10.1265. Erratum in: Arch Dermatol 2004;140:625. [DOI] [PubMed] [Google Scholar]
- 22.Bjerring P, Clement M, Heickendorff L, Egevist H, Kiernan M. Selective non-ablative wrinkle reduction by laser. J Cutan Laser Ther. 2000;2:9–15. doi: 10.1080/14628830050516542. [DOI] [PubMed] [Google Scholar]
- 23.Wu D, Zhou B, Xu Y, Yin Z, Luo D. Impact of intense pulsed light irradiation on cultured primary fibroblasts and a vascular endothelial cell line. Exp Ther Med. 2012;4:669–74. doi: 10.3892/etm.2012.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Y, Zhao J, Gold MH. Skin rejuvenation in Asian skin: The analysis of clinical effects and basic mechanisms of intense pulsed light. J Drugs Dermatol. 2008;7:273–9. [PubMed] [Google Scholar]
- 25.Rosenberg GJ, Brito MA, Jr, Aportella R, Kapoor S. Long-term histologic effects of the CO2 laser. Plast Reconstr Surg. 1999;104:2239–44. doi: 10.1097/00006534-199912000-00046. [DOI] [PubMed] [Google Scholar]
- 26.Liebl H. Abstract reflections about collagen induction therapy – A hypothesis for the mechanism of action of collagen induction therapy (CIT) using micro-needles. (1st ed) 2006 Feb; [Google Scholar]
- 27.Beltraminelli H, Dietrich N, Hunziker T. Fractional transepidermal delivery: A histological analysis. Dermatology. 2011;223:321–4. doi: 10.1159/000334165. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz M, Laaff H. A prospective controlled assessment of microneedling with the Dermaroller device. Plast Reconstr Surg. 2011;127:146e–8e. doi: 10.1097/PRS.0b013e3182131e0f. [DOI] [PubMed] [Google Scholar]
- 29.Liebl H, Kloth LC. Skin cell proliferation stimulated by microneedles. J Am Coll Clin Wound Spec. 2012;4:2–6. doi: 10.1016/j.jccw.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koger C, Cohen J. The lasting effects of fillers through neocollagenesis. Dermatologist. 2014:22. [Google Scholar]
- 31.Wang F, Garza LA, Kang S, Varani J, Orringer JS, Fisher GJ, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143:155–63. doi: 10.1001/archderm.143.2.155. [DOI] [PubMed] [Google Scholar]
- 32.Turlier V, Delalleau A, Casas C, Rouquier A, Bianchi P, Alvarez S, et al. Association between collagen production and mechanical stretching in dermal extracellular matrix: in vivo effect of cross-linked hyaluronic acid filler. A randomised, placebo-controlled study. J Dermatol Sci. 2013;69:187–94. doi: 10.1016/j.jdermsci.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Skuba ND, Adamyan AA, Sulamanidze MA, Husnutdinova ZR. Morphological foundation of face skin reinforcement by Aptos threads. Ann Plast Reconstr Esthet Surg. 2002;3:19–26. [Google Scholar]
- 34.Sulamanidze M, Sulamanidze G. APTOS suture lifting methods: 10 years of experience. Clin Plast Surg. 2009;36:281–306. doi: 10.1016/j.cps.2008.12.003. viii. [DOI] [PubMed] [Google Scholar]
- 35.Kim DH, Je YJ, Kim CD, Lee YH, Seo YJ, Lee JH, et al. Can platelet-rich plasma be used for skin rejuvenation. Evaluation of effects of platelet-rich plasma on human dermal fibroblast? Ann Dermatol. 2011;23:424–31. doi: 10.5021/ad.2011.23.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redaelli A, Romano D, Marcianó A. Face and neck revitalization with platelet-rich plasma (PRP): Clinical outcome in a series of 23 consecutively treated patients. J Drugs Dermatol. 2010;9:466–72. [PubMed] [Google Scholar]
- 37.Lee JW, Kim BJ, Kim MN, Mun SK. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: A simultaneous split-face trial. Dermatol Surg. 2011;37:931–8. doi: 10.1111/j.1524-4725.2011.01999.x. [DOI] [PubMed] [Google Scholar]
- 38.Reddys. Product Monograph, Avarta. 2016 [Google Scholar]
- 39.Narurkar VA. Cosmeceutical Science in Clinical Practice. Florida: CRC Press; 2010. [Google Scholar]
- 40.Kruglikov IL. Neocollagenesis in non-invasive aesthetic treatments. J Cosmet Dermatol Sci Appl. 2013;3:1A001. [Google Scholar]


