Abstract
Autologous fat grafting is commonly utilised to reconstruct soft tissue defects caused by ageing, trauma, chronic wounds and cancer resection. The benefits of fat grafting are minimal donor site morbidity and ease of availability through liposuction or lipectomy. Nonetheless, survival and longevity of fat grafts remain poor post-engraftment. Various methods to enhance fat graft survival are currently under investigation and its stem cell constituents are of particular interest. Cell-assisted lipotransfer refers to the addition of adipose-derived stem cell (ASC) rich component of stromal vascular fraction to lipoaspirate, the results of which have proven promising. This article aims to review the role of ASCs in fat grafting and reconstructive surgery.
KEYWORDS: Adipose-derived stem cell, liposuction, mesenchymal stem cell, reconstruction
INTRODUCTION AND HISTORY OF ADIPOSE-DERIVED STEM CELLS
Adipose-derived stem cells (ASCs) came under the spotlight in 2001, when Zuk et al. first demonstrated that autologous adipose tissue could be processed to obtain a fibroblast-like population of cells, also termed processed lipoaspirate (PLA). These PLA cells, or otherwise known as ASCs, could be further induced to undergo differentiation into different cells based on lineage-specific induction factors.[1,2] ASCs have since emerged as a key modality in tissue engineering and regenerative medicine. It may be defined as a multi-lineage stem cell population which is isolated from the stromal vascular fraction (SVF) of adipose tissue, processed from lipoaspirate.[3]
ASCs have the versatility to develop into a variety of mature tissues including but not limited to adipose, cardiac, bone, cartilage, muscle, nerve and skin. They may subsequently be harnessed for therapeutic and reparative intent in diseased or damaged conditions of the body. There are multiple benefits in utilizing ASCs, which include the ease of availability and low donor site morbidity from liposuction, a minimally-invasive procedure. In addition, the ASC population yields a comparatively greater proliferation capacity as compared to bone marrow derived stem cells, while simultaneously providing a more convenient retrieval platform.[4] The aim of this review is to succinctly summarise the current clinical applications, harvesting techniques as well as main characteristics of ASCs, which make it a desirable tool for regenerative medicine and surgery.
ADIPOSE-DERIVED STEM CELL APPLICATION IN THE CLINICAL SETTING
In the clinical setting, ASCs have notably been incorporated into plastic surgical procedures and in particular, autologous fat grafting. Fat grafting is a key technique in soft tissue reconstruction using non-vascularised adipose tissue. It is an ideal substance for grafting because it has low immunogenicity to the host, inexpensive, biocompatible and easily obtainable.[5] In addition, it has a multitude of applications to conditions such as burns and irradiation wounds, trauma, post-cancer resection and ageing.[6] Nevertheless, success is often limited by poor survival and unpredictability of the fat graft. A significant proportion of the engrafted fat undergoes resorption and necrosis due to the non-vascularised nature of the transplant.[7,8] As described by Mashiko and Yoshimura, the grafted adipose tissue is placed under oxidative and ischaemic stress immediately post-transplantation. Sustenance to the tissue is provided via plasmatic diffusion from the surrounding host tissue in the first few days, and death of adipocytes occurs within the first 24 h.[5,7]
Recently, Yoshimura et al. have demonstrated that autologous fat grafting with lipoaspirate enriched with SVF, a rich source of ASCs, improves clinical outcome in breast augmentation and facial lipoatrophy patients.[9,10] This observation has been termed ‘cell-assisted lipotransfer (CAL)’ and proponents believe this may augment the eventual volume and long-term stability of fat grafts. Zhou et al. have also shown in a meta-analysis that the pooled fat survival rate was significantly higher in the CAL group compared to the non-lipotransfer group (60 vs. 45%, P = 0.0096). Furthermore, CAL reduced the incidence of multiple or repeat procedures in the face by 13%.[5] Nevertheless, the use of ASCs in the realm of post-oncologic reconstruction has been faced with much criticism due to the possibility of ASCs contributing to tumour recurrence.
CHARACTERISTICS OF ADIPOSE-DERIVED STEM CELLS
ASCs are characterised by the abilities of self-regeneration, asymmetric division and pluripotency.[11] Further differentiation of SVF into various cellular populations has been reported by Li et al., based on CD31/CD34 and CD146 status: Mature endothelial (CD31+/CD34−) cells, endothelial stem (CD31+/CD34+) cells, ASCs (CD31−/CD34+) and pericytes (CD146+/CD31−/CD34−).[12] These cellular markers of ASCs and their subpopulations, coupled with their location adjacent to the vasculature, contribute towards their characteristics as described previously. The components of SVF are summarised in Figure 1. In addition, ASCs do not express major histocompatibility complex-II and thus have potential immunomodulatory functions which are beneficial to the recipient site post-transplantation.[13]
Figure 1.
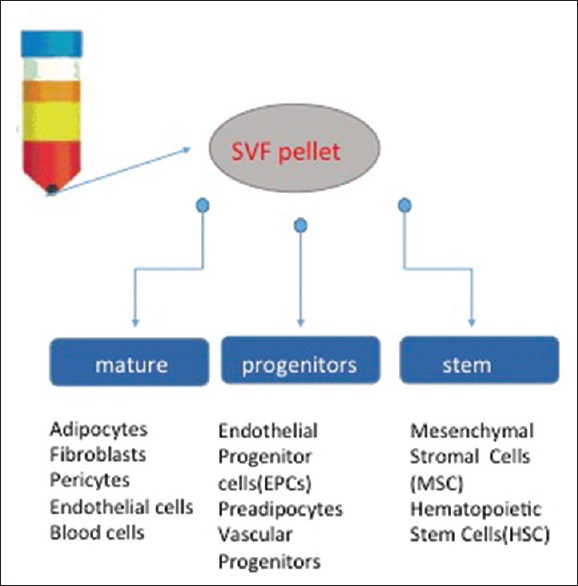
Components of stromal vascular fraction
CLINICAL AND EXPERIMENTAL TRIALS
Several pilot human clinical trials have since been conducted to investigate the potential of ASCs. Interestingly, ASCs have been shown to restore diseased states secondary to Crohn's disease, urinary incontinence as well as calvarial defects with success albeit in small numbers.[14,15,16] Within the experimental realm, ASCs have been adopted in a multitude of animal models for the treatment of diseases and injuries. These include myocardial infarction, acute kidney injury, stroke, wound healing and peripheral nerve regeneration with promising early results.[17] Regardless of the context where ASC therapy and/or fat grafting is utilised, a significant number of delivered cells are lost because of necrosis and apoptosis at the recipient site. This may be attributed to various factors such as trauma, hypoxia, ischemia, oxidative stress and inflammation.[18] Novel strategies have thus been employed to enhance cytoprotection of the ASCs at the target site, which includes ischaemic preconditioning, pharmacological therapy, refinement of liposuction techniques to minimise trauma, as well as the addition of growth factors.[5,19,20] Future experimental work will be centred on safe and Food and Drug Administration - approved agents, which may be administered in conjunction with ASC transplantation to maximise the therapeutic efficacy at the target site.
ISOLATION AND HARVESTING OF ADIPOSE STEM CELLS
The lower abdomen and inner thighs are not only less cosmetically sensitive areas but also the most common sites of fat harvest due to the ease of surgical access and the higher concentration of ASCs that can be obtained compared with other donor sites. Various factors such as age, body mass index and specific donor sites may influence adipocyte survival. In particular for younger (<45-year-old) patients, Geissler et al. have demonstrated that adipocyte viability was greater in the lower abdomen as compared to the flanks.[19]
Under anaesthesia, tumescent solution containing lidocaine (0.01–0.04%, to maintain adipocyte viability) and 1:1,000,000 epinephrine is infiltrated into the donor site to provide pain relief and haemostasis, respectively, and to facilitate easy harvest of adipose tissue.[20] The amount of tumescent solution to be infiltrated should be approximately 1:1 with the volume of fat to be aspirated. To maintain the viability and maximise the cellular yield of ASCs, syringe aspiration (10 mL Luer-Lok syringe with harvesting cannula), which is less traumatic than conventional liposuction, is the preferred technique for the harvest of fat grafts.[21] This method is usually sufficient when smaller volumes of fat (<100 mL) are required; for larger volumes, the use of devices to streamline the procedure is of obvious appeal, but lower aspiration pressures are recommended to minimise trauma and improve the yield of ASCs.[22]
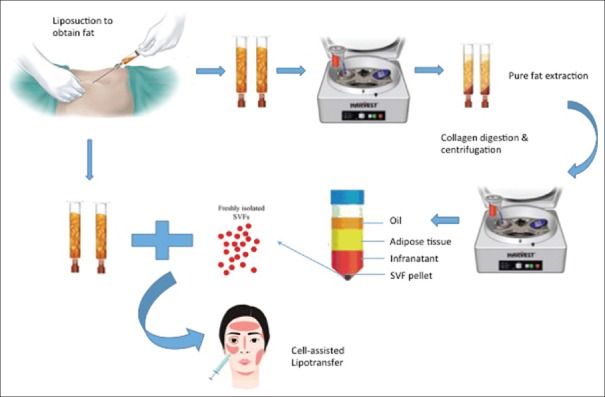
Once fat has been harvested, it is processed to obtain SVF from the fat graft. While much debate remains with regard to the optimal method of processing between washing, sedimentation and centrifugation; evidence from Kurita et al. suggests that use of the latter (at 1200G/3000 rpm for 3 min will not only allow concentration of adipocytes, ASCs and growth factors but also improve fat graft survival).[23] Conversely, centrifugation of adipose tissue at higher G forces or durations may prove detrimental instead.[24] Once centrifugation is complete, three layers are obtained from the lipoaspirate – the top, oily layer can be decanted while the bottom, fluid layer drained; the middle layer, which is now free of cellular debris and other inflammatory materials, can be transferred to 1 mL syringes for administration. A summary of the entire procedure is shown in Figure 2. The described procedure is otherwise commonly known as structural fat grafting or lipoinjection, which was first popularised by Coleman.[25] As described earlier, Yoshimura et al. have developed the CAL technique which has been demonstrated to be superior to the aforementioned lipoinjection method by combining liposuction harvested fat with SVF to increase the overall efficacy of fat transfer, which can be found in both the fatty and fluid portions of the lipoaspirate product.
Figure 2.
Isolation and preparation of stromal vascular fraction in cell-assisted lipotransfer
FAT INJECTION TECHNIQUES
Resorption rates after fat grafting are generally reported to be between 20% and 80%.[5,26] Therefore, to maximise survival, multiple passes in different tissue planes are required to optimise plasmatic imbibition and neovascularisation of the transplanted fat grafts by increasing the surface area of contact with the host environment. Injection of the fat graft should be met with minimal resistance, and a small volume (~0.1 mL) is administered upon entry with the remaining volume given during withdrawal to minimise trauma.[27] Coleman and Ross et al. advocate the use of 1–2 mL Luer-Lok syringes with 17-gauge cannula and smaller graft volumes affording fine control around aesthetically sensitive areas such as eyelids.[25,26] A larger bore cannula may be employed for less cosmetically sensitive area such as the buttock. In CAL, the fat graft is augmented with SVF, which contains ASCs, CD45+ myeloid-derived cells, endothelial (progenitor) cells and pericytes. This combination effect is purported to improve graft retention through improved angiogenesis and adipocyte regeneration.
Post-administration, extreme care is required to prevent unnecessary trauma to the grafted area, which can threaten the viability of the fat graft. Thus, patients should be cautioned against massage and tight bandages due to the overlying pressure effect on the fat grafts, but gentle taping over the grafted areas for comfort is allowed. A multiple choice questionnaire testing understanding of key concepts is appended at the end of this article.
CONCLUSIONS
With the advent of ASC therapy, autologous fat transfer holds much promise for the future, especially in the realm of soft tissue reconstruction and aesthetic surgery. There remains certain skepticism over the safety of the use of ASCs for post-oncological defects, which needs to be addressed in an ethical and well-conducted human clinical trial. Nevertheless, the heralded success of CAL in the field of breast augmentation and facial ageing is a testament to the potential of ASCs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
MULTIPLE CHOICE QUESTIONS
-
1. Cell - assisted lipotransfer refers to the supplementation of lipoaspirate with:
- a. Adipose-derived stem cells
- Endothelial cells
- Stromal vascular fraction
- Stromal cells.
-
What is the recommended rate of centrifugation to concentrate adipocytes and to improve fat graft survival based on Kurita's work?
- 800G for 3 min
- 1000G for 3 min
- 1200G for 3 min
- 1200G for 5 min.
-
Which procedure is currently shown to be safe and efficacious for cell-assisted lipotransfer as demonstrated by Yoshimura et al.?
- Post-oncological breast reconstruction
- Cosmetic breast augmentation
- Burns reconstruction
- Radiation injury reversal.
-
Which of the following factors does NOT contribute to cell death of adipose-derived stem cells and fat grafts post-transplantation?
- Trauma
- Ischemia
- Oxidative stress
- Plasmatic imbibition.
-
Which area in the body has been demonstrated to have the greatest adipocyte viability in young patients?
- Lower abdomen
- Flanks
- Inner thigh
- Breast.
-
How many layers of lipoaspirate are obtained following centrifugation?
- 1
- 2
- 3
- 4.
-
What is the recommended tumescence solution based on recent work by Keck et al., which improves viability of pre-adipocytes?
- Marcaine
- Lidocaine
- Lidocaine+MARCAINE
- Lidocaine+Epinephrine.
-
What is the recommended ratio of tumescence solution to volume of fat to be injected?
- 1:1
- 1:2
- 1:3
- 1:4.
-
The Luer-Lok syringe application is suitable for volumes of fat up to:
- 50 mL
- 100 mL
- 150 mL
- 200 mL.
-
Adipose-derived stem cells have been shown by Li et al. to have greater proliferation capacity as compared to:
- Umbilical cord stem cells
- Amniotic tissue-derived stem cells
- Bone-marrow-derived stem cells
- Muscle-derived mesenchymal stem cells.
Answers:
1. C
2. C
3. B
4. D
5. A
6. C
7. D
8. A
9. B
10. C
REFERENCES
- 1.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abiko H, Fujiwara S, Ohashi K, Hiatari R, Mashiko T, Sakamoto N, et al. Rho guanine nucleotide exchange factors involved in cyclic-stretch-induced reorientation of vascular endothelial cells. J Cell Sci. 2015;128:1683–95. doi: 10.1242/jcs.157503. [DOI] [PubMed] [Google Scholar]
- 3.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 4.Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, et al. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6:55. doi: 10.1186/s13287-015-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y, Wang J, Li H, Liang X, Bae J, Huang X, et al. Efficacy and safety of cell-assisted lipotransfer: A systematic review and meta-analysis. Plast Reconstr Surg. 2016;137:44e–57e. doi: 10.1097/PRS.0000000000001981. [DOI] [PubMed] [Google Scholar]
- 6.Pu LL, Yoshimura K, Coleman SR. Fat grafting: Current concept, clinical application, and regenerative potential, part 2. Preface. Clin Plast Surg. 2015;42:xiii–xiv. doi: 10.1016/j.cps.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Mashiko T, Yoshimura K. How does fat survive and remodel after grafting? Clin Plast Surg. 2015;42:181–90. doi: 10.1016/j.cps.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Pu LL, Yoshimura K, Coleman SR. Fat grafting: Current concept, clinical application, and regenerative potential, part 1. Clin Plast Surg. 2015;42:ix–x. doi: 10.1016/j.cps.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48–55. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshimura K, Sato K, Aoi N, Kurita M, Inoue K, Suga H, et al. Cell-assisted lipotransfer for facial lipoatrophy: Efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;34:1178–85. doi: 10.1111/j.1524-4725.2008.34256.x. [DOI] [PubMed] [Google Scholar]
- 11.Minteer DM, Marra KG, Rubin JP. Adipose stem cells: Biology, safety, regulation, and regenerative potential. Clin Plast Surg. 2015;42:169–79. doi: 10.1016/j.cps.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Zimmerlin L, Marra KG, Donnenberg VS, Donnenberg AD, Rubin JP. Adipogenic potential of adipose stem cell subpopulations. Plast Reconstr Surg. 2011;128:663–72. doi: 10.1097/PRS.0b013e318221db33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brayfield CA, Marra KG, Rubin JP. Adipose tissue regeneration. Curr Stem Cell Res Ther. 2010;5:116–21. doi: 10.2174/157488810791268582. [DOI] [PubMed] [Google Scholar]
- 14.Bohnenblust ME, Steigelman MB, Wang Q, Walker JA, Wang HT. An experimental design to study adipocyte stem cells for reconstruction of calvarial defects. J Craniofac Surg. 2009;20:340–6. doi: 10.1097/SCS.0b013e3181992316. [DOI] [PubMed] [Google Scholar]
- 15.Lee WY, Park KJ, Cho YB, Yoon SN, Song KH, Kim do S, et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn's fistula. Stem Cells. 2013;31:2575–81. doi: 10.1002/stem.1357. [DOI] [PubMed] [Google Scholar]
- 16.Tran C, Damaser MS. The potential role of stem cells in the treatment of urinary incontinence. Ther Adv Urol. 2015;7:22–40. doi: 10.1177/1756287214553968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gimble JM, Bunnell BA, Guilak F. Human adipose-derived cells: An update on the transition to clinical translation. Regen Med. 2012;7:225–35. doi: 10.2217/rme.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stubbs SL, Hsiao ST, Peshavariya HM, Lim SY, Dusting GJ, Dilley RJ. Hypoxic preconditioning enhances survival of human adipose-derived stem cells and conditions endothelial cells in vitro. Stem Cells Dev. 2012;21:1887–96. doi: 10.1089/scd.2011.0289. [DOI] [PubMed] [Google Scholar]
- 19.Geissler PJ, Davis K, Roostaeian J, Unger J, Huang J, Rohrich RJ. Improving fat transfer viability: The role of aging, body mass index, and harvest site. Plast Reconstr Surg. 2014;134:227–32. doi: 10.1097/PRS.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 20.Keck M, Zeyda M, Gollinger K, Burjak S, Kamolz LP, Frey M, et al. Local anesthetics have a major impact on viability of preadipocytes and their differentiation into adipocytes. Plast Reconstr Surg. 2010;126:1500–5. doi: 10.1097/PRS.0b013e3181ef8beb. [DOI] [PubMed] [Google Scholar]
- 21.Pu LL, Coleman SR, Cui X, Ferguson RE, Jr, Vasconez HC. Autologous fat grafts harvested and refined by the Coleman technique: A comparative study. Plast Reconstr Surg. 2008;122:932–7. doi: 10.1097/PRS.0b013e3181811ff0. [DOI] [PubMed] [Google Scholar]
- 22.Cheriyan T, Kao HK, Qiao X, Guo L. Low harvest pressure enhances autologous fat graft viability. Plast Reconstr Surg. 2014;133:1365–8. doi: 10.1097/PRS.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 23.Kurita M, Matsumoto D, Shigeura T, Sato K, Gonda K, Harii K, et al. Influences of centrifugation on cells and tissues in liposuction aspirates: Optimized centrifugation for lipotransfer and cell isolation. Plast Reconstr Surg. 2008;121:1033–41. doi: 10.1097/01.prs.0000299384.53131.87. [DOI] [PubMed] [Google Scholar]
- 24.Kim IH, Yang JD, Lee DG, Chung HY, Cho BC. Evaluation of centrifugation technique and effect of epinephrine on fat cell viability in autologous fat injection. Aesthet Surg J. 2009;29:35–9. doi: 10.1016/j.asj.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Coleman SR. Structural fat grafting: More than a permanent filler. Plast Reconstr Surg. 2006;118(3 Suppl):108S–20S. doi: 10.1097/01.prs.0000234610.81672.e7. [DOI] [PubMed] [Google Scholar]
- 26.Ross RJ, Shayan R, Mutimer KL, Ashton MW. Autologous fat grafting: Current state of the art and critical review. Ann Plast Surg. 2014;73:352–7. doi: 10.1097/SAP.0b013e31827aeb51. [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Kirkham JC, McCormack MC, Nicholls AM, Randolph MA, Austen WG., Jr The effect of pressure and shear on autologous fat grafting. Plast Reconstr Surg. 2013;131:1125–36. doi: 10.1097/PRS.0b013e3182879f4a. [DOI] [PubMed] [Google Scholar]