Abstract
Context:
Platelet-rich plasma (PRP) therapy is finding importance in aesthetic medicine.
Aim:
The objective of this study was to study efficacy of PRP therapy in follicular unit extraction (FUE) hair transplant.
Materials and Methods:
It is a single-blind, prospective randomised study on 40 FUE hair transplant subjects, allocated in two groups (PRP and non-PRP) alternately. PRP was injected intra-operatively immediately after creating slits over the recipient area in PRP group; and normal saline in non-PRP group. Two groups were evaluated at 2, 4 and 8 weeks, 3 and 6 months of the procedure.
Statistical Analysis:
It was done using Chi-square test and test of significance was set as P < 0.05.
Results:
In PRP group, all subjects had >75% hair regrowth at 6 months, density of >75% grafts was noticed in 12 patients at 4 weeks meaning reduced fall of transplanted hair during catagen phase. New hair growth started at 8 weeks in 16 patients and redness over recipient area completely disappeared in 19 patients at 3 months of surgery and activity in dormant follicles as fine thread like hair was noticed besides the thick transplanted hair in all subjects. In non-PRP group, four patients had >75% hair regrowth at 6 months; none showed >75% graft density at 4 weeks, and 13 subjects showed dormant follicle activity at 4 months. The number of patients having lengthier hairs was significantly more in PRP group.
Conclusion:
Intra-operative PRP therapy is beneficial in giving faster density, reducing the catagen loss of transplanted hair, recovering the skin faster and activating dormant follicles in FUE transplant subjects.
KEYWORDS: Follicular unit extraction hair transplant, hair, platelet-rich plasma therapy
INTRODUCTION
Alopecia is a hereditary and androgen-dependent progressive thinning of scalp hair. In hair follicles, reduction in the anagen phase of hair cycle leads to the entry of hair earlier into the telogen phase.[1,2] Platelet-rich plasma (PRP) helps in tissue augmentation by activating platelets and releasing large amounts of platelet-derived growth factors (PDGFs) which act on stem cells of the follicles, stimulating the development of new follicles and promoting neovascularisation.[3,4,5] By taking this clinical significance of PRP, this study was planned for evaluation of PRP in growing and healing hair after follicular unit extraction (FUE) hair transplant.
MATERIALS AND METHODS
Selection and description of participants
A total of forty patients were randomised alternately into two groups of twenty patients each which were enrolled on the basis of inclusion and exclusion criteria.
Inclusion criteria
Male patients in the age group of 20–55 years willing to give informed consent
Patients with androgenetic alopecia (AGA) Stage III–VII Hamilton–Norwood classification
Patients who had not taken any form of treatment for AGA, at least in the past 3 months.
Exclusion criteria
Patients on anticoagulant medications-aspirin, warfarin, heparin
Patients with alopecia other than AGA such as alopecia areata, alopecia totalis, telogen effluvium and acquired cicatricial alopecia
Patients with history of bleeding disorders, autoimmune disorders, psoriasis or lichen planus
Patients with infection at the local site
Patients who were known to be HIV, hepatitis B or C positive or otherwise immunocompromised.
Technical information
While conducting study treatment, one arm of the two groups received injectable PRP during the transplantation just after the slitting process, and other arm of the subjects received normal saline in place of PRP.[6]
Preparation with platelet-rich plasma and its application procedure
The process of PRP harvesting was standardised before the start of study and uniformly followed in all the subjects with the same kit and methodology. PRP therapy was given to the enrolled subjects using YCELL BIO PRP kit.[7]
We collected 20 ml of whole blood from antecubital vein. We used two PRP kits in each patient containing 1.5 ml ACD-A anticoagulant solution which was mixed with 10 ml blood [Figure 1] and processed in a centrifuge. First spin was done at 3200 rpm for 4 min followed by 1 min second spin at the same revolutions per minute for a better demarcation of buffy coat. Of 10 ml of blood in each PRP kit, 1.5 ml of PRP along with buffy coat was harvested [Figure 1]. Thus, a total of 3 ml PRP was collected from 20 ml of blood.
Figure 1.

Platelet-rich plasma kit demonstrating buffy coat with staw coloured platelet-rich plasma in the bottleneck area of the tube
This process of PRP preparation was standardised and followed in each of study subjects as it revealed five to seven times increase in the concentration of platelets from the baseline value. Considering an average platelet count of 200,000 ± 75,000/µL, the final platelet concentration in PRP was expected to be around 10–14,00,000/µL.
PRP was freshly prepared while slits were being created. No activator was used before usage of PRP to avoid any external chemical injury to newly implanted hair follicles. After harvesting and slitting were over, 0.2–0.3 ml PRP was injected through insulin syringe at a gap of 1 cm each to the depth of dermis and subcutis in freshly done slits over recipient area, and the blood oozing from these slits itself acted as stimulus for the degranulation of platelets. In comparison, non-PRP group received normal saline instead of PRP after slitting.
Efficacy evaluation criteria
Efficacy of PRP was evaluated in terms of change in hair density (percentage of number of grafts at a given time <25%, 25–50%, 50–75% and >75%), type of grafts, impact of healing process by appearance and recovery from scalp redness, length of shaft, texture and thickness of hair at the end of 2 weeks, 4 weeks, 8 weeks, 3 months and 6 months.
Each patient was photographed with clinical and video microscopic images, keeping identity confidential and data were evaluated independently.
Pre-operative evaluation
All the enrolled patients were evaluated for biochemistry parameters such as fasting plasma sugar, liver function test, renal function test, HbA1c, complete haemogram including platelet count, along with anti-HIV, anti-hepatitis C virus and anti-HBsAg before the initiation of transplant procedure.
Intra-operative methodology
All the patients were given detailed pre- and post-operative instructions. Only scalp hairs were harvested. Transaction rate during harvesting was 5–8%. While doing implantation, only the grafts with intact roots were implanted.
The follicles were kept moist in chilled normal saline and stored inside refrigerator at 2–8° centigrade till implantation. The follicles were implanted within 6 h of harvesting in both the groups and the roots were not fiddled with while implantation. The density of implantation was kept at 40–45 grafts/cm2 in both groups using multipronged slitter [disposable 18G needles and wire holding forceps as shown in Figure 2].
Figure 2.

Image showing indigenous slitter with disposable 18G needles and wire holding forceps for maintaining uniform density
Statistical analysis was done through Chi-square tests and test of significance were set as P < 0.05 significant and <0.01 as highly significant.
RESULTS
A total of forty male patients meeting the inclusion and exclusion criteria of the study were enrolled and evaluated. All the patients were suffering from male pattern of alopecia [Table 1].
Table 1.
Hamilton-Norwood classification of alopecia

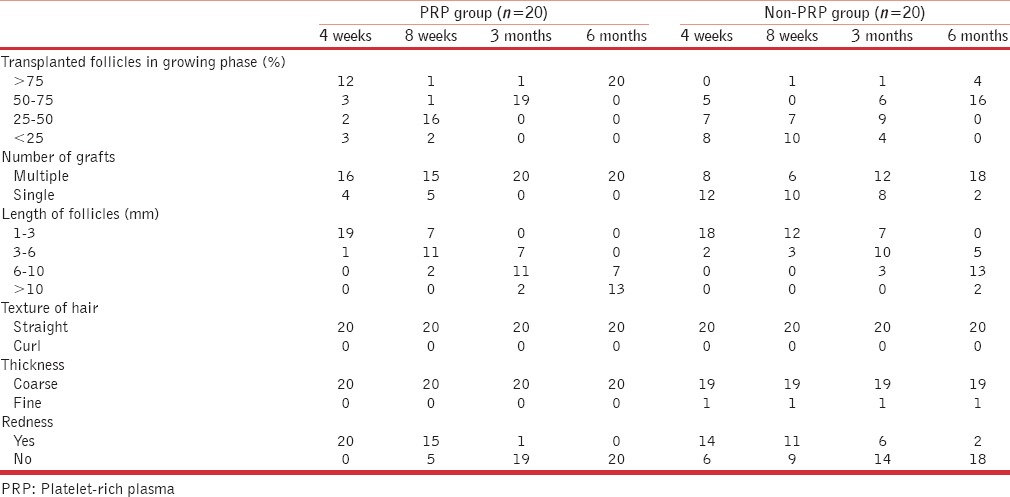
None of the enrolled patients had undergone hair transplant previously or was on any kind of hair regrowth therapy for last 3 months. The impact of PRP treatment on follicle growth, type of grafts, length of hair shafts, activity of dormant follicles and redness on scalp were evaluated [Table 2].
Table 2.
Master chart with variables and results
Follicle growth
Follicle growth means percentage of grafts in actively growing phase at the time of inspection. Clinical images at 2 weeks post-transplant served as 100% reference density as the transplanted follicles were actively growing at 2 weeks. This is succeeded by catagen or dystrophic phase where there is fall of transplanted follicles leading to reduced density followed by gradual restoration of density over next few months. Various densities at interval of 4 weeks, 8 weeks, 3 months and 6 months were evaluated and compared clinically and using photographs with this 2 week baseline image and assessed as <25%, 25–50%, 50–75% and >75% accordingly. In PRP treated group, after 1 month 60% of the patients (n = 12) showed >75% density in comparison to none in non-PRP group. The difference was statistically highly significant with P < 0.001. After 6 months of hair transplant, all the patients (n = 20) had more than 75% follicle growth, wherein non-PRP group only 20% (n = 4) of patients showed more than 75% growth and rest 80% of the patients had follicle growth in the range on 50–75% [Figures 3 and 4]. PRP-treated group showed much denser and lengthier follicle growth as compared to non-PRP treated group, and difference was again statistically highly significant [Figures 5 and 6]. Activity in dormant follicles as fine vellus hair along the thick transplanted hair was seen as early as 3 months in PRP group in all twenty subjects as compared to non-PRP group where this activity was seen in 13 subjects at 4 months onwards.
Figure 3.
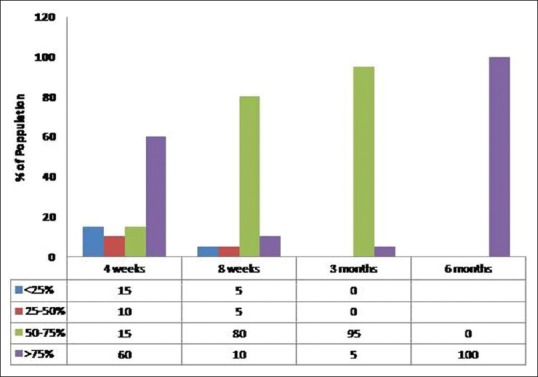
Follicle growth in platelet-rich plasma group (percentage of patients vs. number of weeks of treatment)
Figure 4.
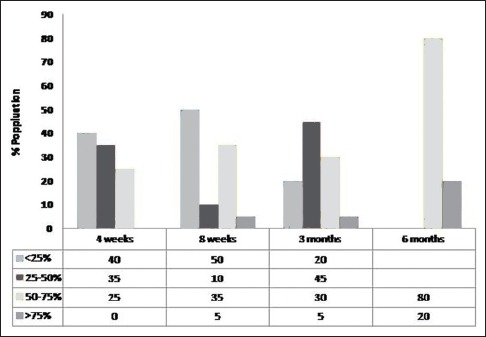
Follicle growth in non-platelet-rich plasma group (percentage of patients vs. number of weeks of treatment)
Figure 5.
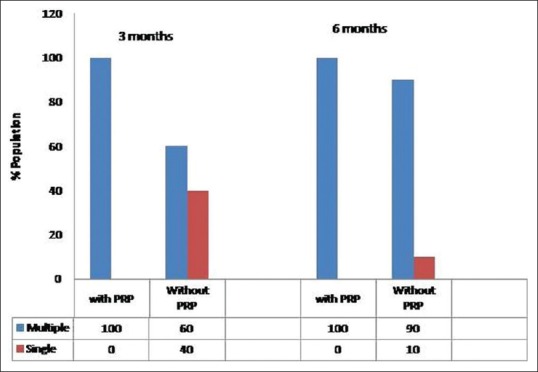
Effect of PRP on type of grafts (single or multiple)
Figure 6.
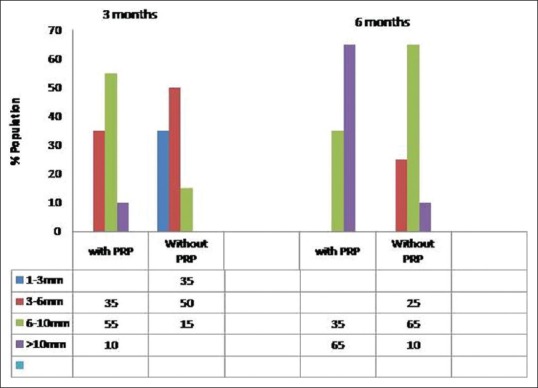
Effect of PRP on length of shaft
Type of grafts
In PRP-treated group, the number of patients having multiple grafts was significantly higher as compared to non-PRP treated group.
After 3 months of hair transplant, in PRP group, all the twenty patients had multiple grafts erupting out in areas compared to the ones at 2 weeks baseline images. Whereas in non-PRP group, only 60% of patients had multiple grafts which increased to 80% at 6 months when compared with 2 weeks baseline images. The difference at 3 months was statistically highly significant (P = 0.001) [Figure 5].
Length of shafts
The length of hair mentioned is not the length of longest hair but of the erupting hair recorded in video microscopic images. The cut-off value of 10 mm was selected as this is the maximum length supported by video microscopic images. While more than 10 mm length is being mentioned, many of these would be much more than 10 mm in actual length as is visible in clinical images.
After 6 months of hair transplant, in PRP-treated group 65% (n = 13) of the patient had more than 10 mm hair shaft length. While in non-PRP treated group, only 10% (n = 2) of the patient had more than 10 mm shaft length [Figure 6]. Remaining 35% (n = 7) in PRP group showed the follicle length of 6–10 mm whereas in non-PRP group 65% (n = 13) showed 6–10 mm length at 6 month. The difference was statistically highly significant (P < 0.001). Transplanted hair grow at a slower pace as compared to normal hair (1.25 cm/month for normal hair) and it is a common observation that many clients do not need a frequent hair trimming over transplanted area as compared to donor area which requires regular trimming.
Impact on scalp redness
At 4 weeks, all the patients in PRP group showed scalp redness over recipient area in comparison to 70% in non-PRP group. In PRP-treated group, after 3 months of hair transplant, only 5% of patients had scalp redness whereas, in non-PRP treated group, 30% of patient had scalp redness [Figure 7].
Figure 7.
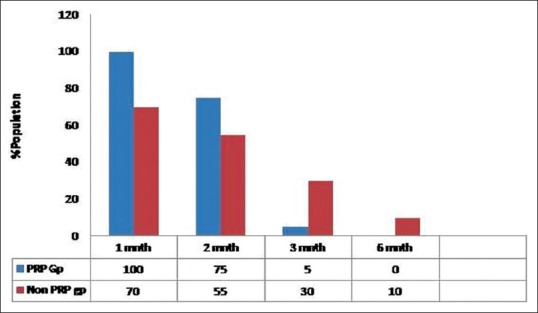
Impact of PRP treatment on scalp redness
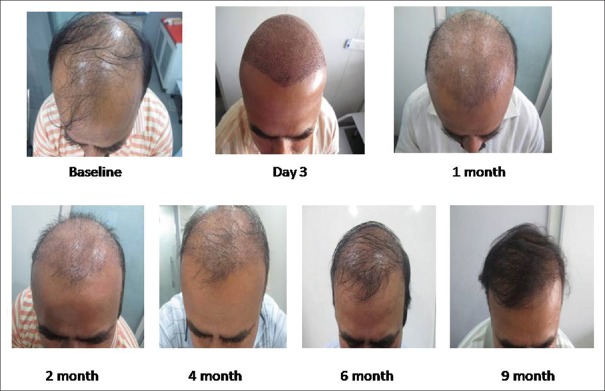
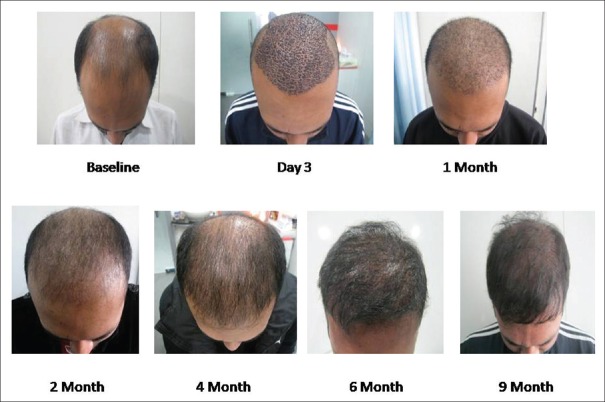
After 6 months, in PRP-treated group, no patient had scalp redness whereas, in the case of non-PRP group, 10% of patients still reported scalp redness. Comparative images over 9 months post transplant have been shown non PRP and PRP group in Figures 8 and 9, respectively.
Figure 8.
Hair growth without platelet-rich plasma treatment with comparative images over a period of 9 months. At 1 month interval almost 50% catagen fall of transplanted hair is noticed which is more severe in 2 months image, followed by visible new growth at 4 months image. Density is still less at 6 months interval, and final density is shown as in 9 months image
Figure 9.
Hair growth with platelet-rich plasma treatment with comparative images over a period of 9 months. At 1 month interval more than 75% transplanted hair is in growing phase. At 2 months, there is relative thinning due to delayed catagen and breakage of hair strands above surface of skin. Images at 4 and 6 months show more density and length of transplanted hair as compared to non-platelet-rich plasma
DISCUSSION
Male androgenic alopecia is one of the most prevalent dermal disorders in males.[7] It is also referred to as male pattern baldness as it happens in specific pattern and it impacts adolescents as well as adults. Polygenic heredity is considered to be its cause; in addition male, hormone testosterone plays a major role. Hormone binds to androgenic receptor on hair follicle leading to early entry to telogen phase and inducing hair fall.[8] PRP is rich in many growth factors and proteins. It is very rich in PDGF, epidermal growth factor, transforming growth factor, fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF) and various pro and anti-inflammatory cytokines such as interleukin 4 (IL-4), IL-8, IL-13, IL-17, tumour necrosis factor alpha and interferon alpha.[8] These growth factors may induce an internal signal-transduction pathway, unlocking the expression of a normal gene sequence of a cell thereby initiating and improving tissue regeneration process.[5,6,7,8]
Data present in this manuscript clearly suggests that PRP treatment during the hair transplant plays a significant role in hair re-growth, and it remarkably improves density and quality of hair growth. As shown in Figures 3 and 4, all patients in PRP-treated group had more than 75% follicle growth whereas in non-PRP treated group only 20% of the patient showed more than 75% follicle growth. The catagen loss in transplanted hair reduced significantly at 1 month interval though there was a delayed fall but not as severe as in non-PRP group.[5] The fall was noticed as breakage of hair strand just above skin surface rather than actual shedding of transplanted hair in these cases. This can be explained by the effect of growth factors on the newly transplanted hair. Furthermore, the new growth of follicles was noticed as early as 2 months in video microscopic images [Figure 10]. In contrast in the study by Uebel et al., new anagen hair started after 4 months of transplant. Only the length of newly growing follicles was studied in video microscopic images, so maximum length attained may not be commented upon. Since most of the clients that showed more than 10 mm length, even the trailing anagen hair got activated at around 4–5 months to sport a length of 10 mm or more. On the other hand in non-PRP group, 65% (No. 13) clients showed the length of follicles 6–10 mm at 6 months meaning most of these trailing follicles entered anagen phase more recently [Table 2]. The difference could be attributed to injected PRP therapy in our study compared to submersion of grafts in PRP in the above-mentioned study. The injected therapy could be better as there is less wastage, added benefit of faster recovery of skin, activity of dormant follicles and faster entry into the new anagen hair. Figure 10 shows video microscope image at 2 months showing new anagen hair in centre and activity of dormant follicles in the periphery. It may be inferred that spontaneous apoptosis of 15–30% hairs mentioned by Uebel et al. can be reduced by injected PRP significantly with PRP induced new vessel formation and effect of growth factors on stem cells of bulge region and mesenchymal cells in dermal papillae. The combination of growth factors plays pivotal role in tissue repair, and regeneration and presence of plasma proteins act as a scaffold in epithelial migration. The effect may result into anagen hair growth as early as 2 months.
Figure 10.
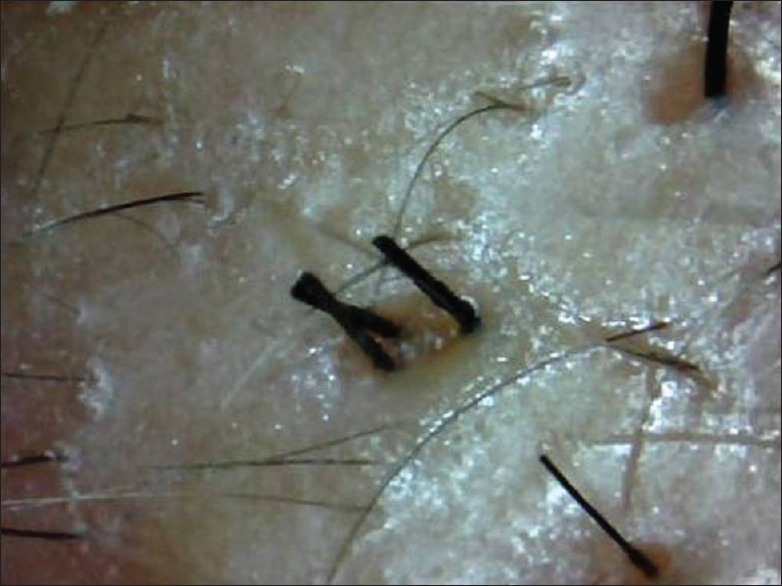
Digital microscopic image showing new anagen hair at 2 months post transplant
The quality of hair was better in PRP-treated group as compared to non-PRP group. In PRP group, multiple erupting roots from the graft as early as 3 months besides lengthier shafts clearly suggest the role of growth factors in PRP in stimulating as well as nourishing transplanted follicular unit grafts [Figures 8 and 9]. However, we did not compare the exact diameter in the two groups, which is a limitation in this study.
PRP is the source of various growth and regulatory factors involved in cells growth and differentiation.[9,10,11] PRP not only induces growth but also improves cell survival by its anti-apoptotic activity. Activated PRP stimulates growth and differentiation of stem cells in hair follicle bulge along with activation of mesenchymal cells in dermal papilla. It stimulates transcriptional activity of b-catenin responsible for differentiation of stems cells to hair follicle cells. PRP is reported to stimulate Bcl-2 regulatory protein levels, which possess anti-apoptotic activity and prolongs survival of derma papilla cells.[11] This effect was clearly observed in vivo and in vitro study by Rinaldi et al. where they observed prolongation of the anagen and shortening of the catagen and telogen phase due to the effect of PRP released growth factors.[12] PRP is also reported to activate AKT and ERK signalling pathways to protective dermal papilla cells from apoptosis. PRP up-regulates FGF-7 growth factors, which are known to stimulate hair growth.[13] VEGFs and platelet-derived growth factors contribute in increasing peri-follicular vasculature thus, improving the blood supply and nourishment to the transplanted grafts.[14,15,16] This was also evident in our 1-month results where redness was more marked in PRP group due to new vessel formation but after 3 months, most of the patients had recovered from scalp redness whereas in non-PRP group 30% of the patient still reported scalp redness [Figure 11]. PRP has already been reported to augment tissue repair and regeneration process. Activity in dormant hair follicles was significantly high in PRP group which can again be explained by normalisation of scalp milieu with reduced fibrosis and neovascularisation secondary to the action of FGF and VEGF, which facilitated significantly higher activity in PRP group.
Figure 11.
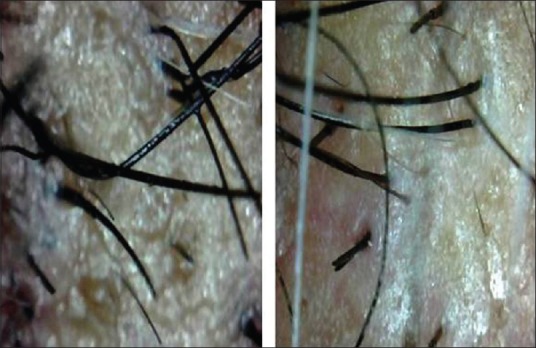
Digital microscopic images of PRP And Non-PRP Group, evidence of persistent redness in Non-PRP Group at 3 Months Post Transplant
Overall, PRP therapy improves the skin milieu of grafted area by cell growth and differentiation, anti-apoptotic activity and neovascularisation making grafted area more receptive and fertile for newly transplanted hair. It also helps in providing conducive environment for dormant hair follicles leading to their activity and appearance of new anagen hair as early as 2 months.
Our study is the first reported study observing various aspects of hair transplant from follicle growth and density, catagen loss of transplanted hair, re-growth of transplanted hair, type of graft, length of shafts, activity of dormant hair follicles and post-surgical healing over a period of 6 months.
Some researchers believe that the comparison between the two groups should be on half and half side of scalp rather than on different subjects. Since this study was based on injected PRP therapy, there will be a possibility of diffusion of plasma into the control side and thus confounding the outcome. Moreover, hair transplant is an aesthetic procedure and we as clinician need to understand the implications of attaining and justifying variable densities on two sides of the scalp. Hence, in the quest of achieving better results for our future clients, whether it is justified to give aesthetically unpleasant result to present ones is again debatable!
The limitation in the study is that exact hair count is not performed as can be done with more sophisticated equipment like cross-sectional trichogram, though we have taken serial video microscopic images. Future studies may be directed to achieve more objective evidence using these equipment.
CONCLUSION
This study clearly demonstrated impact of PRP treatment during and after hair transplant and its beneficial effect on quantity and quality of hair re-growth. Intra-operative injectable PRP therapy is beneficial in giving faster density, reducing the catagen loss of transplanted hair, early recovery of the skin, faster appearance of new anagen hair in FUE transplant subjects and also activating existing dormant follicles.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bienová M, Kucerová R, Fiurásková M, Hajdúch M, Kolár Z. Androgenetic alopecia and current methods of treatment. Acta Dermatovenerol Alp Pannonica Adriat. 2005;14:5–8. [PubMed] [Google Scholar]
- 2.Parsley WM, Perez-Meza D. Review of factors affecting the growth and survival of follicular grafts. J Cutan Aesthet Surg. 2010;3:69–75. doi: 10.4103/0974-2077.69014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacci KM, Dardik A. Platelet-rich plasma: Support for its use in wound healing. Yale J Biol Med. 2010;83:1–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhari ND, Sharma YK, Dash K, Deshmukh P. Role of platelet-rich plasma in the management of androgenetic alopecia. Int J Trichology. 2012;4:291–2. doi: 10.4103/0974-7753.111222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uebel CO, da Silva JB, Cantarelli D, Martins P. The role of platelet plasma growth factors in male pattern baldness surgery. Plast Reconstr Surg. 2006;118:1458–66. doi: 10.1097/01.prs.0000239560.29172.33. [DOI] [PubMed] [Google Scholar]
- 6.Moon PG, Kwack MH, Lee JE, Cho YE, Park JH, Hwang D, et al. Proteomic analysis of balding and non-balding mesenchyme-derived dermal papilla cells from androgenetic alopecia patients using on-line two-dimensional reversed phase-reversed phase LC-MS/MS. J Proteomics. 2013;85:174–91. doi: 10.1016/j.jprot.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Jain R, De-Eknamkul W. Potential targets in the discovery of new hair growth promoters for androgenic alopecia. Expert Opin Ther Targets. 2014;18:787–806. doi: 10.1517/14728222.2014.922956. [DOI] [PubMed] [Google Scholar]
- 8.Cervelli V, Garcovich S, Bielli A, Cervelli G, Curcio BC, Scioli MG, et al. The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: Clinical and histomorphometric evaluation. Biomed Res Int 2014. 2014 doi: 10.1155/2014/760709. 760709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett NT, Schultz GS. Growth factors and wound healing: Biochemical properties of growth factors and their receptors. Am J Surg. 1993;165:728–37. doi: 10.1016/s0002-9610(05)80797-4. [DOI] [PubMed] [Google Scholar]
- 10.Steed DL. The role of growth factors in wound healing. Surg Clin North Am. 1997;77:575. doi: 10.1016/s0039-6109(05)70569-7. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Meza D. Wound healing and revascularization of the hair transplant graft: The role of growth factors. Hair Transpl. 1995;1:287. [Google Scholar]
- 12.Rinaldi F, Sorbellini E, Coscera T. The role of platelet rich plasma to control anagen phase: Evaluation in vitro and in vivo hair transplant and hair treatment. Int J Trichology. 2011;3:514–5. [Google Scholar]
- 13.Akiyama M, Smith LT, Holbrook KA. Growth factor and growth factor receptor localization in the hair follicle bulge and associated tissue in human fetus. J Invest Dermatol. 1996;106:391–6. doi: 10.1111/1523-1747.ep12343381. [DOI] [PubMed] [Google Scholar]
- 14.Arshdeep, Kumaran MS. Platelet-rich plasma in dermatology: Boon or a bane? Indian J Dermatol Venereol Leprol. 2014;80:5–14. doi: 10.4103/0378-6323.125467. [DOI] [PubMed] [Google Scholar]
- 15.Kamp H, Geilen CC, Sommer C, Blume-Peytavi U. Regulation of PDGF and PDGF receptor in cultured dermal papilla cells and follicular keratinocytes of the human hair follicle. Exp Dermatol. 2003;12:662–72. doi: 10.1034/j.1600-0625.2003.00089.x. [DOI] [PubMed] [Google Scholar]
- 16.Kang JS, Zheng Z, Choi MJ, Lee SH, Kim DY, Cho SB. The effect of CD34+cell-containing autologous platelet-rich plasma injection on pattern hair loss: A preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72–9. doi: 10.1111/jdv.12062. [DOI] [PubMed] [Google Scholar]