Abstract
Background:
Alopecia is one of the most important hair follicle (HF) disorders, which is divided into scarring (cicatricial) and nonscarring (noncicatricial) types.
Objective:
The aim of this study is to investigate the expression of stem cell (SC) markers such as cytokeratin (CK) 17 and CK19 in scarring and nonscarring alopecia.
Materials and Methods:
Thirty patients with scalp alopecia (15 with scarring alopecia and 15 without) together with ten healthy volunteers were included in this study. Biopsies were taken from all participants and stained for CK17 and CK19 using immunohistochemistry.
Results:
There was a statistically significant difference between the nonscarring group and the control group with regard to CK17 expression in the outer layers of the HFs (P = 0.00) and CK19 staining of the inner layers of the HFs (P = 0.008). There was a statistically significant difference between the scarring and the control groups regarding CK17 expression in the outer (P = 0.00) and the inner layers (P = 0.00) of the HFs and CK19 expression in the inner layers of the HFs (P = 0.00). CK17 expression in the outer layers (P = 0.02) and the inner layers of the HFs (P = 0.00) together with CK19 expression in the inner layers of the HFs (P = 0.00) showed statistically significant differences between scarring and nonscarring alopecia groups.
Conclusions:
The presence of SC markers (CK17 and CK19) in the HFs was affected in both scarring and nonscarring alopecia, but the defect in scarring alopecia is more evident than that of nonscarring alopecia. The persistence of SC markers in some types of scarring alopecia could give a hope for the recovery of these lesions. Further studies are recommended to clarify the benefit from using HF SCs in the treatment of alopecia.
KEYWORDS: Alopecia, cytokeratin, scarring, stem cell
INTRODUCTION
Alopecia is one of the most important hair follicle (HF) disorders, which is divided into scarring (cicatricial) and nonscarring (noncicatricial) types. Nonscarring alopecias tend to have preserved follicular ostia without clinically visible inflammation in most presentations, although histologic inflammation may be present.[1] Scarring alopecias have loss of follicular ostia or atrophy. Histologic confirmation is the best method to confirm the presence of a fibrosing/scarring process with loss of HFs.[1]
Stem cells (SCs) are defined as cells with the capacity for unlimited self-renewal and also the ability to generate daughter cells that undergo terminal differentiation.[2] The bulge region of the HF represents the best characterized epidermal SCs population described to date. Moreover, there is an evidence of the presence of SCs populations in the interfollicular epidermis, the sebaceous gland, and the dermis.[3] It has been appreciated for many years that the epidermis contains SCs that are responsible for replacing the differentiated cells of the interfollicular epidermis, HFs, and sebaceous glands.[4] HFs are self-renewing structures that cycle and reconstitute themselves throughout life because they contain keratinocyte SCs.[5]
The HF SCs therefore appear to be responsible for regenerating the HF in each hair cycle.[6] HF SC markers include keratins, integrins, transcription factors, hematopoietic progenitor cells, and others.[7]
In the epidermis, keratinocytes in the basal cell layer express keratin 5 and 14 while keratinocytes in the suprabasal layer express keratin 1 and 10 and the HF expresses keratin 6, 16, and 17.[8] CK17 has been localized as a prominent component of the suprabasal cell layers of the outer follicular root sheath.[9] The Type I keratin 17 (K17) shows a peculiar localization in human epithelial appendages including HFs, which undergo a growth cycle throughout adult life.[10] The onset of Type I K17 synthesis marks the adoption of an appendageal fate within embryonic ectoderm and its expression persists in specific cell types within mature hair, glands, and nail.[11]
Other keratins are found to be restricted to the bulge region. These keratins include cytokeratin 19 (CK19), which have been reported to be found in the outermost layer of the outer root sheath (ORS) proximal and distal to the bulge.[12,13] CK19 may also be important in the commitment of SCs to an epidermal cell fate and differentiation.[14] CK19-expressing keratinocytes are also found in the epidermal stratum of normal skin. CK19 is an important marker to routinely monitor epidermal homeostasis and (at least indirectly) the self-renewing potential of engineered skin.[15] SC markers may provide a bank of SCs of potential uses in cutaneous regenerative medicine. For example, bulge SCs can be used to produce bioengineered HFs to treat alopecias.[16]
The aim of this study is to investigate the expression of SC markers such as CK17 and CK19 in scarring and nonscarring alopecia compared to normal HFs.
MATERIALS AND METHODS
This case-controlled study was carried out on thirty patients presented with alopecia and ten healthy individuals as a control group. They were age- and sex-matched. The patients were selected from the outpatient clinic of Dermatology and Plastic Surgery Departments, Menoufia University Hospital in the period between May 2011 and May 2012. Patients were divided according to the type of alopecia into two groups: (a) Nonscarring alopecia (15 patients of alopecia areata) and (b) scarring alopecia (15 patients) (five patients postburn, five patients with old scarring discoid lupus erythematous, and five patients with old scarring lichen planopilaris). All cases of congenital alopecias were excluded. Punch biopsies were taken from the scalp of thirty alopecia patients as well as ten biopsies from scalps of healthy control subjects (from patients were doing plastic surgery in scalp). Informed consent was taken from all participants. This work had been approved by Ethics Committee of Menoufia University.
Three sections (4 u thickness) from each block were prepared. One section was stained with hematoxylin and eosin and the other two sections were stained with immunohistochemical (IHC) stain for detection of CK17 (Lab Vision Corporation, USA, Clone E3, ready-to-use) and CK19 (Cell Marque, USA, A53-B/A2.26, ready-to-use).
Sections stained with hematoxylin and eosin were examined by light microscope to evaluate and verify the histopathological features such as:
The condition of the HFs was evaluated whether they are intact, distorted, or fibrosed
The inflammatory infiltrate was evaluated for type, density, and localization
The fibrosis was evaluated for the presence and its severity.
Immunohistochemical staining procedure
The method used for immunostaining was streptavidin-biotin amplified system. Paraffin-embedded tissue sections were deparaffinized in xylene, rehydrated in a graded series of ethanol, and then incubated with 3% hydrogen peroxide. Slides were rinsed in phosphate-buffered saline (PBS) and then exposed to heat-induced epitope retrieval in citrate buffer solution (pH 6) for 20 min. After cooling, the slides were incubated overnight at room temperature with mouse monoclonal antibodies against CK17 and CK19. Detection of immunoreactivity was carried out using the ultravision detection system, ready-to-use anti-polyvalent horseradish peroxidase/diaminobenzidine (NeoMarkers, LabVision, California, USA). Finally, the reaction was visualized by an appropriate substrate/chromogen (diaminobenzidine) reagent. Counterstain was carried out using Mayer's hematoxylin. Cancer colon and squamous cell carcinoma served as positive tissue controls for CK19 for CK17, respectively. The staining procedure included negative controls obtained by substitution of primary antibodies with PBS.
Immunohistochemical interpretation of cytokeratin 17 and cytokeratin 19 immunostaining
Cytoplasmic expression in any number of cells is required to assign CK17 and CK19-positive expression. The studied cases were assessed as follows:
At the HFs:
The presence or absence of both markers (CK17 and CK19) was evaluated
The site of the positivity either in the outer layers or in the inner layers of HFs or in both
Intensity of the markers was assessed subjectively by the examiner as mild, moderate, and marked intensity.[17]
Statistical analysis
Statistical analysis was performed using Statistical Package for Social Sciences, SPSS 16.0 statistical software (SPSS Inc., Chicago, IL, USA). Chi-square and Fisher exact tests were used in comparison between qualitative variables. P ≤0.05 was considered statistically significant.
RESULTS
Hematoxylin and eosin staining results
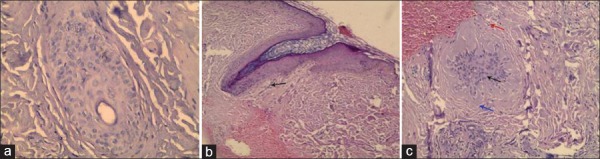
Control group: A transverse biopsy from a scalp of apparently healthy individual showed normal intact HFs. There is no inflammatory infiltrate and no fibrosis [Figure 1a]
Nonscarring group: A longitudinal biopsy from nonscarring alopecia showed intact HF with inflammatory infiltrate (mainly lymphocytes) [Figure 1b]
Scarring group: A transverse biopsy from scarring alopecia showed distorted HF with mild lymphocytic inflammatory infiltrate (as in scarring alopecia of discoid lupus and lichen planopilaris), mild to severe fibrosis and epidermal atrophy. Marked fibrosis was so prominent and clear, not in need for special stains [Figure 1c].
Figure 1.
(a) A transverse biopsy from a scalp of apparently healthy individual showed normal intact hair follicle. There is no inflammatory infiltrate and no fibrosis (H and E, ×400). (b) A longitudinal scalp biopsy from nonscarring alopecia showed intact hair follicle with inflammatory infiltrate (mainly lymphocytes) black arrow (H and E, ×200). (c) A transverse scalp biopsy from scarring alopecia showed distorted hair follicle (black arrow) with mild inflammatory infiltrate (red arrow) and mild fibrosis (blue arrow) (H and E, ×400)
Immunohistochemical staining results
Immunohistochemical staining of cytokeratin 17
-
Control group
- At the outer layers of the HFs: CK17 was found to be positive in all control cases with moderate intensity in eight cases (80%) and marked intensity in two cases (20%) [Figure 2a]
-
Nonscarring group
- At the outer layers of the HFs: CK17 was negative in four patients (26.7%), positive with mild intensity in seven patients (46.6%), and positive with moderate intensity in four patients (26.7%) [Figure 2b]
-
Scarring group
- At the outer layers of the HFs: CK17 was negative in 12 patients (80%), positive with mild intensity in two patients (13.3%), and positive with marked intensity in one patient (6.7%) [Figure 2c]
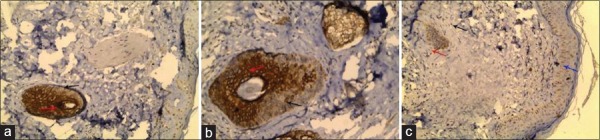
Figure 2.
(a) A transverse scalp biopsy from apparently healthy individual showed positive cytokeratin 17 immunostaining with marked intensity in the inner layers (red arrow) of the hair follicle and with moderate intensity in the outer layers (black arrow) of hair follicle (IHC, ×200). (b) A transverse scalp biopsy from nonscarring alopecia showed positive cytokeratin 17 immunostaining with marked intensity in the inner layers (red arrow) of hair follicle and with mild intensity in the outer layers (black arrow) of hair follicle (IHC, ×200). (c) A transverse scalp biopsy of scarring alopecia showed distorted hair follicle and positive cytokeratin 17 immunostaining with very mild intensity in the inner layers (red arrow) of hair follicle while the outer layers (black arrow) of hair follicle were negative and also there are scattered positive cells with mild intensity in the basal layer of epidermis (IHC, ×200)
Table 1.
Comparison among control and alopecia groups regarding cytokeratin 17 expression
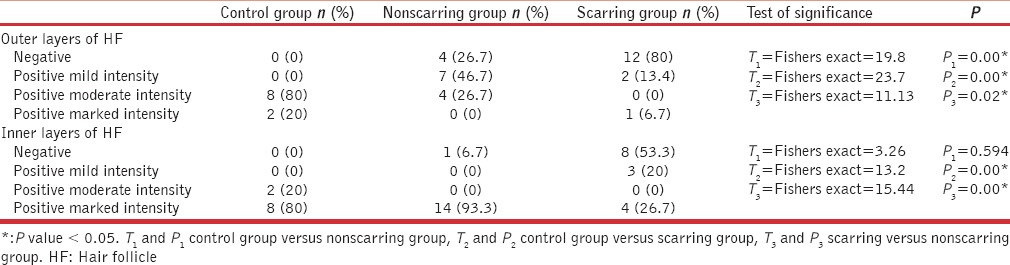
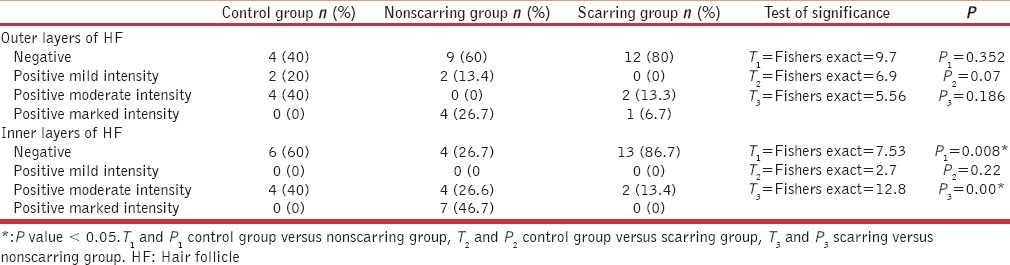
Table 2.
Comparison among control and alopecia groups regarding cytokeratin 19 expression
Immunohistochemical staining of cytokeratin 19
-
Control group
- At the outer layers of the HFs: CK19 was negative in four cases (40%), positive with mild intensity in two cases (20%), and positive with moderate intensity in four cases (40%) [Figure 3a]
-
Nonscarring group
- At the outer layers of the HFs: CK19 was negative in nine patients (60%), positive with mild intensity in two patients (13.3%), and positive with marked intensity in four patients (26.7%) [Figure 3b]
-
Scarring group
- At the outer layers of the HFs: CK19 was negative in 12 patients (80%), positive with moderate intensity in two patients (13.3%), and positive with marked intensity in one patient (6.7%) [Figure 3c]
Figure 3.
(a) A transverse scalp biopsy from apparently healthy individual showed negative cytokeratin 19 immunostaining in the inner layers (red arrow), outer layers (black arrow) of hair follicle, and epidermis (blue arrow) (IHC, ×200). (b) A transverse scalp biopsy of nonscarring alopecia stained with CK19 showed one large differentiated hair follicle which was negative in the outer layers (black arrow) and inner layers (red arrow) of hair follicle and another small primitive hair follicle that showed positivity with marked intensity in the inner layers (yellow arrow) of hair follicle while the outer layers (green arrow) were negative (IHC, ×200). (c) A transverse scalp biopsy of scarring alopecia showed distorted hair follicle and positive cytokeratin 19 immunostaining with mild intensity in the outer layers (black arrow) of distorted hair follicle while the inner layers (red arrow) were negative (IHC, ×400)
Comparison between the control group in one hand and the scarring and nonscarring alopecia groups on the other hand regarding cytokeratin 17 and cytokeratin 19 expression
There was a statistically significant difference between control and nonscarring alopecia with regards to the presence of CK17 in the outer layers of the HFs (P = 0.00) but they did not differ regarding its expression in inner layers (P = 0.594) [Table 1]. There was a statistically significant difference between control and scarring alopecia with regards to the presence of CK17 in the outer layers and the inner layers of the HFs (P = 0.00 for both) [Table 1].
There was a statistically significant difference between nonscarring group and the control group with regards to the presence of CK19 in the inner layers of the HFs (P = 0.008) and not in the outer layers of the HFs (P = 0.352) [Table 2]. There was no statistically significant difference between the scarring group and the control group considering the presence of CK19 in the outer (P = 0.07) and inner layers (P = 0.22) of the HFs [Table 2].
Differences between scarring and nonscarring alopecias regarding cytokeratin 17 and cytokeratin 19 expression and localization
There was a statistically significant difference between scarring nonscarring groups regarding CK17 expression since the presence of CK17 in the outer layers (P = 0.02), and the inner layers (P = 0.00) of the HFs were in favor of nonscarring group [Table 1].
On the other hand, the expression of CK19 in the inner layers of HFs was in favor of nonscarring compared to scarring groups (P = 0.00). The two groups did not differ with regards to CK19 expression by the outer layers of HFs (P = 0.186) [Table 2].
DISCUSSION
Alopecias can be classified into scarring and nonscarring types.[18] The localization of HF SCs to the bulge area may explain why some types of inflammatory alopecias cause permanent follicle loss (such as postburn alopecia), while others (such as alopecia areata) are reversible.[19] In scarring alopecias, inflammation and fibrosis involve the superficial portion of the follicle, including the bulge area, suggesting that SCs, which are necessary for follicle regeneration are damaged.[20]
The aim of the present study is to investigate the IHC expression of SC markers (CK17 and CK19) in scarring and nonscarring alopecias and compare them with each other and with normal scalp skin.
In the current study, CK17 was detected in the outer and inner layers of HFs of all the control subjects. Panteleyev et al.[21] reported that CK17 expression in the mature HFs is limited to the ORS. In the current study, CK17 is predominantly expressed in the outer and inner layers of HFs in the lesional skin of patients with nonscarring alopecia. According to our results, CK17 expression by outer layers of HFs was diminished in nonscarring alopecia compared to normal HFs while its expression by inner layers of HFs did not show any statistical differences compared to normal HFs. According to Van Baar et al.,[22] they found that all patients with alopecia areata showed weak staining of CK17 in the ORS together with positive staining of medulla and cortex. They showed no gross differences regarding CK17 expression pattern in the HFs between alopecia areata and normal scalp. The difference between our results and Van Baar's study may be due to the difference in methodology and numbers of the patients in both studies.
With regards to CK19, it was immunolocalized to outer layers of normal HFs in 60% of cases while its localization in inner layers was detected in 40% of normal scalp HFs. Similar results were obtained by Geng et al.,[23] who detected the HF SCs by CK19 immunostaining in normal human skin. They found that the HF SCs mainly located in ORS in HF and human anagen HFs containing two distinct reservoirs for CK19-positive cells located in the bulge and bulb of the follicle. Moreover, Narisawa et al.[24] demonstrated that CK19-reactive cells were found either continuously or at intervals in the outermost cell layer of the ORS while a small number of CK19-positive cells were sporadically found in the inner cell layer of the ORS of terminal HFs of the scalp.
In the current study, CK19 was predominantly expressed in the inner layers of HFs in the lesional skin of patients with nonscarring alopecia with a significant difference compared to normal scalp HFs. On the other hand, CK19 expression by outer layers of HFs did not make any significant difference between nonscarring alopecia and normal HFs. According to Van Baar et al.,[22] they found CK19 expression in the HFs at the margins of active lesions in patients suffering from alopecia areata. They also found that CK19 was not consistently expressed in the lower part of the ORS, consisting of only one cell layer. When the ORS is multilayered, positive cell clustering could be seen, which disappeared at the level of papillary dermis. CK19 expression pattern in the HFs of alopecia areata did not differ from that in normal scalp.[22]
In the current study, we also investigated the IHC expression of CK17 and CK19 in the lesional skin of patients with scarring alopecia. CK17 was not expressed in the outer layers of HF in most of patients (80%) and was also not expressed in inner layers of HF in nearly half of cases (53%). CK19 showed predominant negative expression (80%) in outer as well as inner layers (86.7%) of HF in scarring alopecia group. The information about SC markers expression in scarring alopecia is limited and to our knowledge, the present study may be the first to demonstrate the expression of these markers (CK17 and CK19) in this type of alopecia. In the current study, CK17 was predominantly expressed in outer layers (73%) and inner layers (94.3%) of HF in nonscarring alopecia compared to scarring alopecia. This may be explained by the presence of excessive fibrosis in scarring alopecia and could explain the spontaneous recovery that occurs in nonscarring alopecia. Moreover, there was a statistically significant difference between scarring and nonscarring groups regarding CK19 expression in the inner layers of the HFs.
The identification and isolation of bulge SCs by the help of SC markers can be used to produce bioengineered HFs to treat alopecias.[16]
CONCLUSIONS
The presence of SC markers (CK17 and CK19) in the HFs was affected in both scarring and nonscarring alopecia but the defect in scarring alopecia is more evident than that of nonscarring alopecia. The persistence of SCs markers in some types of scarring alopecia could give a hope for the recovery of these lesions. Further studies are recommended to clarify the benefit from using HFs SCs in the treatment of alopecia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hantash BM, Carlos MT, Butler D, Heymann WR, Elston DM, Rao J, et al. [Last updated on 2016 Feb 26]. Available from http://emedicine.medscape.com/article/1073559-overview/Scarring alopecia .
- 2.Watt FM, Hogan BL. Out of Eden: Stem cells and their niches. Science. 2000;287:1427–30. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 3.Bieniek R, Lazar AJ, Photopoulos C, Lyle S. Sebaceous tumours contain a subpopulation of cells expressing the keratin 15 stem cell marker. Br J Dermatol. 2007;156:378–80. doi: 10.1111/j.1365-2133.2006.07623.x. [DOI] [PubMed] [Google Scholar]
- 4.Watt FM, Jensen KB. Epidermal stem cell diversity, quiescence and versatility. Mol Med. 2009;1:260–7. doi: 10.1002/emmm.200900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs E, Merrill BJ, Jamora C, DasGupta R. At the roots of a never-ending cycle. Dev Cell. 2001;1:13–25. doi: 10.1016/s1534-5807(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 6.Amoh Y, Li L, Katsuoka K, Hoffman RM. Multipotent nestin-expressing hair follicle stem cells. J Dermatol. 2009;36:1–9. doi: 10.1111/j.1346-8138.2008.00578.x. [DOI] [PubMed] [Google Scholar]
- 7.Ma DR, Yang EN, Lee ST. A review: The location, molecular characterisation and multipotency of hair follicle epidermal stem cells. Ann Acad Med Singapore. 2004;33:784–8. [PubMed] [Google Scholar]
- 8.Cotsarelis G. Gene expression profiling gets to the root of human hair follicle stem cells. J Clin Invest. 2006;116:19–22. doi: 10.1172/JCI27490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulombe PA, Wong P. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat Cell Biol. 2004;6:699–706. doi: 10.1038/ncb0804-699. [DOI] [PubMed] [Google Scholar]
- 10.McGowan KM, Coulombe PA. Onset of keratin 17 expression coincides with the definition of major epithelial lineages during skin development. J Cell Biol. 1998;143:469–86. doi: 10.1083/jcb.143.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGowan KM, Coulombe PA. Keratin 17 expression in the hard epithelial context of the hair and nail, and its relevance for the pachyonychia congenita phenotype. J Invest Dermatol. 2000;114:1101–7. doi: 10.1046/j.1523-1747.2000.00986.x. [DOI] [PubMed] [Google Scholar]
- 12.Lyle S, Christofidou-Solomidou M, Liu Y, Elder DE, Albelda S, Cotsarelis G. Human hair follicle bulge cells are biochemically distinct and possess an epithelial stem cell phenotype. J Investig Dermatol Symp Proc. 1999;4:296–301. doi: 10.1038/sj.jidsp.5640233. [DOI] [PubMed] [Google Scholar]
- 13.Abbas O, Mahalingam M. Epidermal stem cells: Practical perspectives and potential uses. Br J Dermatol. 2009;161:228–36. doi: 10.1111/j.1365-2133.2009.09250.x. [DOI] [PubMed] [Google Scholar]
- 14.Morris RJ. A perspective on keratinocyte stem cells as targets for skin carcinogenesis. Differentiation. 2004;72:381–6. doi: 10.1111/j.1432-0436.2004.07208004.x. [DOI] [PubMed] [Google Scholar]
- 15.Pontiggia L, Biedermann T, Meuli M, Widmer D, Böttcher-Haberzeth S, Schiestl C, et al. Markers to evaluate the quality and self-renewing potential of engineered human skin substitutes in vitro and after transplantation. J Invest Dermatol. 2009;129:480–90. doi: 10.1038/jid.2008.254. [DOI] [PubMed] [Google Scholar]
- 16.Fimiani M, Pianigiani E, Di Simplicio FC, Sbano P, Cuccia A, Pompella G, et al. Other uses of homologous skin grafts and skin bank bioproducts. Clin Dermatol. 2005;23:396–402. doi: 10.1016/j.clindermatol.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 17.Stark HJ, Breitkreutz D, Limat A, Bowden P, Fusenig NE. Keratins of the human hair follicle: “Hyperproliferative” keratins consistently expressed in outer root sheath cells in vivo and in vitro. Differentiation. 1987;35:236–48. doi: 10.1111/j.1432-0436.1987.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 18.Olsen EA, Bergfeld WF, Cotsarelis G, Price VH, Shapiro J, Sinclair R, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103–10. doi: 10.1067/mjd.2003.68. [DOI] [PubMed] [Google Scholar]
- 19.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–7. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 20.Mobini N, Tam S, Kamino H. Possible role of the bulge region in the pathogenesis of inflammatory scarring alopecia: Lichen planopilaris as the prototype. J Cutan Pathol. 2005;32:675–9. doi: 10.1111/j.0303-6987.2005.00399.x. [DOI] [PubMed] [Google Scholar]
- 21.Panteleyev AA, Paus R, Wanner R, Nürnberg W, Eichmüller S, Thiel R, et al. Keratin 17 gene expression during the murine hair cycle. J Invest Dermatol. 1997;108:324–9. doi: 10.1111/1523-1747.ep12286476. [DOI] [PubMed] [Google Scholar]
- 22.Van Baar HM, Van Vlijmen IM, Ramaekers FC, Van Muijen GN, Troyanovsky SM, Perret CM, et al. Cytokeratin expression in alopecia areata hair follicles. Acta Derm Venereol. 1994;74:28–32. [PubMed] [Google Scholar]
- 23.Geng SM, Wang JL, Wang WJ, Tan SS, Peng ZH. Localization and differentiation of hair follicle stem cells. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006;28:360–3. [PubMed] [Google Scholar]
- 24.Narisawa Y, Hashimoto K, Kohda H. Immunohistochemical demonstration of keratin 19 expression in isolated human hair follicles. J Invest Dermatol. 1994;103:191–5. doi: 10.1111/1523-1747.ep12392730. [DOI] [PubMed] [Google Scholar]