Abstract
Background and Aims:
Lactate levels predict outcomes after hepatectomy. We compared metabolic effects of lactated versus lactate free solutions in living donor hepatectomy.
Methods:
Consecutive right lobe donors (n = 53) were alternatively allotted to lactated Ringer's solution and normal saline (Group L-control) or acetated crystalloid (Sterofundin B Braun® Group S -study group) in an observational prospective randomised study. The primary outcome measure was lactate level, and secondary outcomes were base excess, bicarbonate, glucose and chloride intra- and post-operatively. Mann–Whitney and Chi-square tests were used for analysis.
Results:
The intraoperative, post-operative lactate levels and the time for normalisation were comparable. Group L had significantly lower intraoperative bicarbonate levels (mmol/L) at 6 and 8 h (20.0 ± 2.14 vs. 21.3 ± 1.6, P = 0.0471; 18.68 ± 2.04 vs. 20.39 ± 17, P = 0.002), base excess at 4 and 6 h (mmol/L) (−3.64 ± 2.73 vs. −3.0 ± 1.52, P = 0.031; −6.64 ± 2.76 vs. −4.35 ± 1.7 P = 0.006). The intraoperative chloride levels (mmol/L) were higher in group L at 4 and 8 h (108 ± 5.9 vs. 105.99 ± 2.76, P = 0.0471; 109.51 ± 3.86 vs. 106.93 ± 3.09, P = 0.002). Intraoperative glucose (mg/dL) at 6 h was higher in group L, 160.55 ± 31.52 vs. 145.5 ± 24.29, P = 0.043. The highest post-operative chloride (mmol/L) was higher in Group L (112.3 ± 3.86 vs. 109.81 ± 3.72, P = 0.034). Post-operative base excess and bicarbonate showed an improved profile in Group S (−7.37 ± 2.99 vs. −5.06 ± 1.71 P = 0.001 and 17.79 ± 2.23 vs. 19.68 ± 1.51 P = 0.005).
Conclusion:
Acetated fluids were associated with higher levels of bicarbonate, lesser base deficit, glucose and chloride but no difference in lactate levels in comparison with Ringer's lactate and normal saline in living donor hepatectomy.
Key words: Acetate, balanced salt solution, hepatectomy, Ringer's lactate
INTRODUCTION
Living donor liver transplantation is emerging as the most viable option for the management of patients with end-stage liver disease in India and other countries where deceased donors are in short supply.[1,2] Ensuring best outcomes for the donor is necessary in the background of living donation. High lactate levels have been associated with poor outcomes in the critically ill patients. The levels of lactate invariably rise in the donor following hepatectomy both due to a small remnant liver and time taken for regeneration of the residual liver.[3] Metabolism of lactate can be affected during hepatectomy particularly if intermittent clamping or inflow occlusions are performed. Lactate levels have also been used to predict outcomes after hepatectomy.[4] The impact of solutions containing lactate on the capacity for liver metabolism and measured serum lactate levels are unclear. Acetate containing solutions have been introduced as an alternative to lactated solutions as the metabolism of acetate is less dependent upon hepatic function.[5] Solutions with acetate have shown improved survival and better metabolic profile in patients undergoing abdominal surgery.[6]
A recent meta-analysis of studies using low and high chloride containing solutions in the perioperative setting have shown a significant association with acute kidney injury associated with a hyperchloremic acidosis probably related to renal vasoconstriction and an increased morbidity.[7] The consequence of a reduction in the base levels with administration of normal saline and thereby increasing acidosis becomes relevant in the background of hepatic resections.
We aimed at comparing Ringer's lactate and normal saline with acetated fluids on the metabolic profile in living liver donors following right lobe hepatectomy.
METHODS
This was a prospective observational single-blinded study conducted at a gastro surgical division of a tertiary care referral hospital. This study aimed at comparing two accepted methods of fluid administration in living donor hepatectomy. After Ethics Committee approval and informed consent, a total of 67 patients undergoing hepatectomy over a 1 year period were screened for this study. Four patients were excluded as they were left lobe donors. Sixty-three patients belonging to American Society of Anesthesiologists Physical Status 1 and 2, undergoing elective donor right lobe hepatectomy were alternately allotted to receive either acetated solution or a combination of Ringer's lactate with normal saline after informed consent. In ten patients, perioperative use of hydroxyethyl starches containing acetate led to exclusion (6 in Group L and 4 in Group S). A total of 53 patients were included for the study. Patients belonging to Group L received Ringer's lactate until hepatic resection commenced and normal saline solution after commencement of resection until the end of surgery (n = 26). Patients in Group S received balanced salt solution containing acetate as the infusion fluid during surgery (sterofundin; B Braun®) (n = 27). The primary outcome was the levels of lactate intra- and post-operatively. The secondary outcomes were base excess, bicarbonate, glucose and chloride intra- and post-operatively.
After placement of a thoracic epidural catheter and an arterial line, patients were induced by a standard anaesthesia protocol with propofol, fentanyl and midazolam and intubated with atracurium. Anaesthesia was maintained with oxygen, air and isoflurane at 0.8–1.0 minimum alveolar concentration. A restrictive fluid strategy was practised during hepatic resection in both groups (4 mL/kg/h), and the target was a central venous pressure (CVP) of <5 mm Hg. The mean arterial pressure (MAP) was maintained above 65 mm Hg and the systolic blood pressure above 90 mm Hg throughout surgery by administration of vasopressors (noradrenaline) in doses Between 0.02 and 0.2 μg/kg/min. Fluid protocol from the start of surgery until resection commenced was between 8 and 10 mL/kg/h and at the completion of resection, a more liberal strategy was used to target a CVP of 8–10 mm Hg. Blood losses more than 20% of blood volume or a measured haemoglobin <8.0 g/dL was an indication for transfusion of packed cells.
Patients who were haemodynamically stable, on minimal vasopressors, not in metabolic acidosis and with adequate respiratory efforts were considered fit for extubation.
At the end of surgery, all patients were shifted to the Intensive Care Unit (ICU) where both groups received acetated balanced salt solution at a rate of 100ml/h. An observer blinded to the intraoperative protocols noted the post-operative metabolic parameters from the arterial line retained until 48 h postoperatively.
Lactate >5.5 mmol/L was considered an indication for dobutamine infusion and time to normalisation of lactates (<2.5 mmol/L) were noted. Blood gases were measured at baseline and 2 h intervals during surgery and at 6 h intervals in the post-operative ICU for 48 h. Levels of lactate and electrolytes were calculated solely from arterial blood gases.
The levels of intraoperative lactate, base excess, bicarbonate, chloride and glucose were analysed with Mann–Whitney U-test. The post-operative highest lactate, chloride, bicarbonate and base excess and the demographic variables were analysed with the Chi-square test. P < 0.05 was considered statistically significant.
We did not find any studies comparing acetated solutions with a combination of Ringer's lactate and normal saline. Using the first ten cases in each group as a pilot study and lactate levels at the end of surgery as the primary end point, the mean lactate calculated was 3.21 ± 1.43 mmol/L in Group L, whereas it was 3.54 ± 1.98 mmol/L in Group S. Using 95% confidence interval, the calculated sample size was 210 in each group. On account of limited numbers of patients in the donor hepatectomy group, we included 53 patients over a 1 year duration.
RESULTS
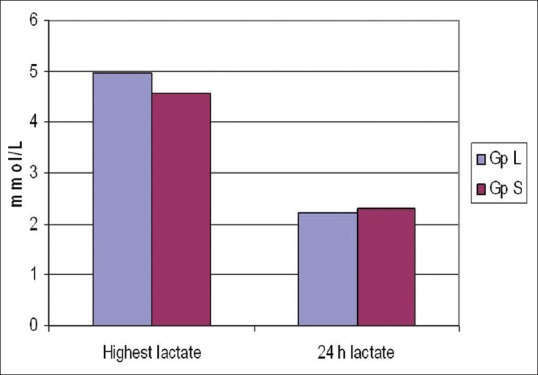
The age and weight of patients and fluids in both groups were comparable. The duration of surgery was longer in Group L (9.96 h vs. 9.06 h P = 0.014 [Table 1]). The lactate levels at baseline and 2, 4 and 6 h during surgery were not significantly different [Table 2 and Figure 1].
Table 1.
Demographic data, fluids, vasopressors and graft details
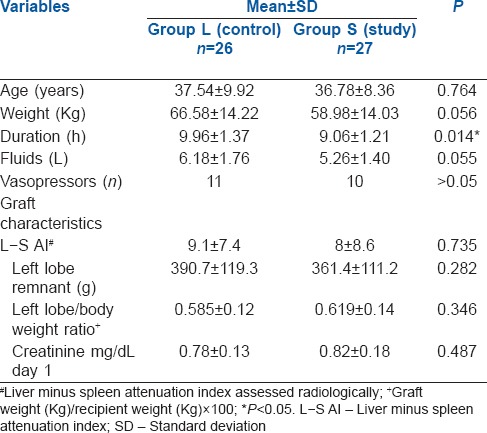
Table 2.
Intra-operative lactate, base excess and bicarbonate
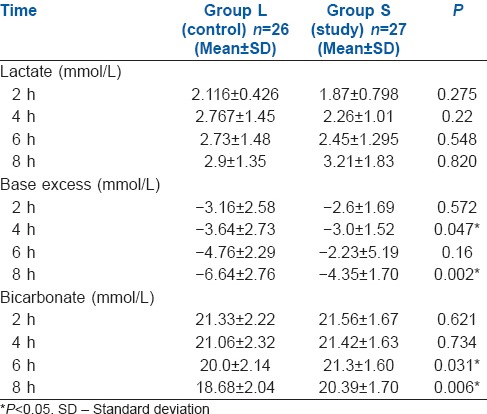
Figure 1.
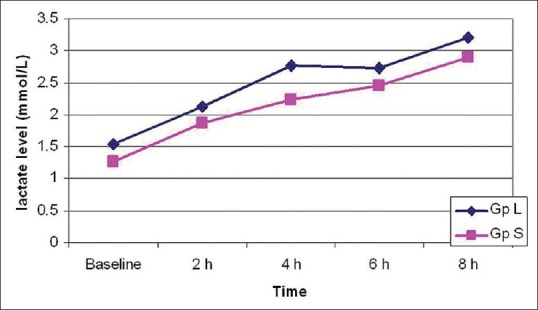
Intra-operative lactate
The levels of chloride intraoperatively were significantly higher in Group L at 4 and 8 h and the level of glucose was also higher in Group L at 6 h [Table 3]. The lowest value of bicarbonate postoperatively was significantly lower in Group L as compared to Group S. Correspondingly, the lowest value of base excess postoperatively was more in Group L [Table 4]. The post-operative highest values of lactate and the time to normalisation were comparable between the two groups [Table 4 and Figure 2].
Table 3.
Intra-operative glucose and chloride
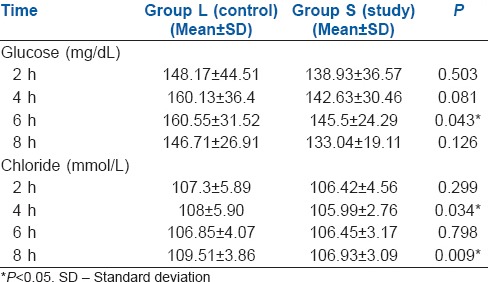
Table 4.
Post-operative variable
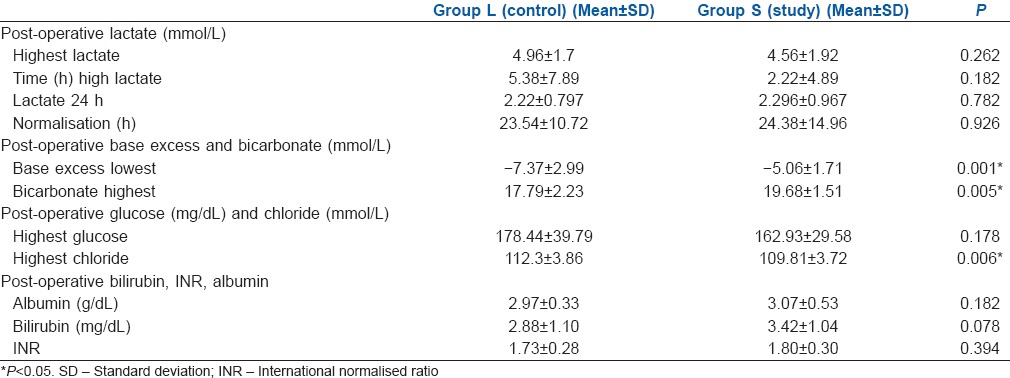
Figure 2.
Post-operative lactate
However, the levels of bicarbonate were significantly higher (P < 0.05) in Group S at 6 and 8 h intraoperatively [Table 2]. Similarly, the values of base excess were lesser (more negative) in group L in comparison with group S at 4 and 8 h intraoperatively.
The highest value of chloride postoperatively was significantly higher in Group L versus Group S. However, the highest glucose value postoperatively was similar between the groups [Table 4].
All patients were extubated on the table and were discharged uneventfully from the ICU. Two patients in Group L needed blood transfusion and received one unit blood each. Five in Group L and three in Group S received 100 mL 20% albumin during surgery. Eleven patients in Group L (11/26) received noradrenaline during surgery compared to 10 (10/27) in Group S. Seven patients in each group received dobutamine postoperatively for normalisation of lactate levels.
The liver minus spleen attenuation index, computerised tomographic estimate of remnant left lobe and the ratio of left lobe to the patient's weight were comparable in both groups. None of the patients had renal dysfunction and post-operative creatinine level was comparable between the two groups [Table 1].
DISCUSSION
There is limited literature evaluating the impact of perioperative fluids on the lactate levels and metabolic profiles in living donor hepatectomy.[4] We sought to compare the metabolic profiles of Ringer's lactate and normal saline versus acetated balanced salt solution in the perioperative management of donor hepatectomy.
We found that the levels of lactate at baseline and 2, 4, 6 and 8 h of resection or in the post-operative period were not significantly different between the two groups. Perioperative lactic acidosis in the donor adds concerns on the residual liver functions and morbidity in donor outcomes. The levels of lactate have been used to predict recovery in hepatic surgeries, sepsis and other abdominal surgeries.[8,9,10]
Lactate is converted to pyruvate and generates adenosine triphosphate (ATP) by oxidative phosphorylation in the mitochondria. A portion of the lactate is also metabolised by the Cori cycle to generate glucose with the consumption of ATP.[11] Although the liver has a large functional reserve and can metabolise endogenous lactate after major hepatectomy, the effects of exogenous lactate are not clear.[12] Lactate levels can rise if the volume of liver is inadequate as in situations following major hepatectomy[3] and has been shown to have a close association with sequential organ failure assessment scores in intensive care patients.[13]
For this reason, the use of lactate-containing solutions is usually avoided during hepatic resection as it raises concerns on the accuracy of levels of lactate as a prognostic indicator. The levels of lactate remained similar despite administration of Ringer's lactate in our group probably because we had only administered Ringer's lactate until hepatic resection was commenced and because the residual liver volumes were adequate to complete the metabolism of lactate. We had used a value of lactate of 5.5 mmol/L above which dobutamine was added as an inotrope. There were no differences between the two groups in the need for dobutamine and levels of lactate had normalised by 24 h postoperatively.
Although the lactate levels were similar, the levels of bicarbonate and base excess showed an improved profile in Group S. This could have been due to the administration of normal saline in Group L versus a balanced salt solution in Group S. An observational study from a database of patients undergoing abdominal surgery have concluded that the use of an acetate base balanced crystalloid was associated with reduced mortality and post-operative complications in comparison to normal saline.[6] Here, there were no differences in outcomes of patients in either group and the length of ICU stay was comparable.
Normal saline is considered unphysiological and associated with renal injury due to hyperchloremic acidosis.[14,15,16] This study showed no difference between the two groups in renal outcomes, average creatinine between the two groups postoperatively was normal and similar [Table 4].
Studies of patients in the emergency department have shown a correlation of base excess with elevations in lactate in septic patients.[17] In this study, we did not find a correlation with the lactate levels and base excess. We hypothesised that the mechanism for decreased base levels was independent of lactate implying that this was a direct consequence of fluid administration.
We found that the chloride level was higher in the group receiving a combination of normal saline and Ringer's lactate, and this continued into the post-operative period. This chloride elevation was probably the consequence of a high chloride content in normal saline. However, no renal dysfunction was noted in any of the patients included in this study.
Excessive volume loading is avoided during hepatectomy as venous bleeding may increase. Studies have shown that maintaining a low CVP during hepatectomy reduces the blood loss during hepatectomy and reduces the length of hospital stay without any detrimental renal or hepatic effects.[18] We aimed to keep the CVP between 3 and 5 mm Hg during hepatic resection to reduce or minimise bleeding during hepatectomy. No vascular occlusion was employed, and Cavitron® ultrasonic aspirator and Harmonic® scalpel[19] were used along the plane of resection. Hypovolaemia may contribute to an increase in lactate level during surgical resection. The concurrent use of an epidural analgesia further causes a sympathetic blockade leading to hypotension and hypoperfusion. We aimed to keep the MAP >65 mm Hg throughout the surgery by the administration of noradrenaline as an infusion as the presumed mechanism of hypotension is vasodilation following anaesthesia and epidural anaesthetic-induced sympathetic blockade. We believed that this would avoid hypoperfusion to the liver and the possibilities of an increase in lactate following hypoperfusion. Liberal fluid administration by goal-directed therapy has been shown to reduce lactate levels in high-risk patients undergoing abdominal surgery.[20] However, our fluid strategy for hepatectomy did not affect lactates, and there were no differences in the volume of fluids or need for inotropes between both groups. The blood losses were minimal, and transfusion was administered to two patients in group L following blood loss due to clamp slippage. None of the patients needed blood transfusions postoperatively.
Diabetic patients administered Ringer's lactate can have a higher level of glucose although similar profiles are not seen in normal patients.[21] We had excluded donors with diabetes in this study, yet there was an increase in the glucose level in the group receiving Ringer's lactate at 6 h of surgery although this was not seen in the post-operative period. The increase in gluconeogenesis with exogenous lactate that is present in Ringer's lactate may have accounted for the difference in the blood glucose levels in our study. The levels had normalised in both groups in the post-operative period and did not result in any increased morbidity.
Acetate is an alternative base to lactate in crystalloid solutions. While administration of lactated solutions is avoided in the presence of ongoing lactic acidosis, little information is available on direct comparisons of acetate versus lactate in hepatectomy. Infusions of acetate in normal human volunteers appear to be well tolerated[22] but advantages over lactated solutions in liver surgery are not well documented. Noradrenaline was administered when MAP was <65 mm Hg. The intraoperative use of vasopressors was similar in both groups [Table 1].
We chose donors whose residual liver volume was more than the recommended 30% for liver resections. The average weight of the left lobes assessed by computed tomography (CT) volumetry was 390.7 ± 119.3 g versus 361.4 ± 111.2 g and the ratio of the left lobe versus donor weights were 0.567 and 0.618 in Groups L and S, respectively. The quality of the grafts were assessed by CT and quantified by the liver minus spleen attenuation index. This utilises radiologic attenuation of the hepatic vessels and is an accurate indicator of hepatic steatosis.[23] The values were comparable between the two groups. We believe that both groups had a similar graft profile, and metabolic profile changes were due to administered solutions. We did not find any difference in the post-operative bilirubin, albumin and INR in this study group, unlike an earlier study.[24]
This study involved a small group and an alternative allocation of patients to either group was performed; however, randomisation may have been a superior method of allocation. The donor management intraoperatively was at the discretion of the different anaesthesiologists. Donors who received starch solutions that contain acetate as a base or those in whom the protocol was breached were retrospectively excluded from the study. The administration of fluids intraoperatively was open labelled, but the post-operative readings were by the intensivist who was unaware of the intraoperative management. The recordings were objective recordings of patient's arterial samples that excluded any element of observer bias in this study.
We believe that a combination of Ringer's lactate with normal saline was a safe alternative in right hepatectomy in healthy non-diabetic donors and does not increase morbidity, renal injury or recovery in hepatic function. However, it is associated with more adverse metabolic changes including acidosis, hyperchloraemia and hyperglycaemia. The study question evolved at a point when acetated solutions became available. This was conducted over a year to understand obvious benefits of acetated solutions over standard practice to improve the standard of care in hepatic donors. However, the inability to detect any difference of lactate levels during hepatic resection or postoperatively in our patients could be due to beta error from an underpowered study.
CONCLUSION
Administration of solutions of Ringer lactate before hepatic resection and normal saline subsequently during live donor hepatectomy did not affect the levels of lactate but decreased bicarbonate levels and increased base deficit in comparison to acetated solutions. However, randomised multicentric trials with larger numbers of patients may be needed to demonstrate the metabolic profiles for the future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Vohra S, Goyal N, Gupta S. Preoperative CT evaluation of potential donors in living donor liver transplantation. Indian J Radiol Imaging. 2014;24:350–9. doi: 10.4103/0971-3026.143897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soin AS, Kakodkar R. Living donor liver transplantation in India. Trop Gastroenterol. 2007;28:96–8. [PubMed] [Google Scholar]
- 3.Marko P, Gabrielli A, Caruso LJ. Too much lactate or too little liver? J Clin Anesth. 2004;16:389–95. doi: 10.1016/j.jclinane.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe I, Mayumi T, Arishima T, Takahashi H, Shikano T, Nakao A, et al. Hyperlactemia can predict the prognosis of liver resection. Shock. 2007;28:35–8. doi: 10.1097/shk.0b013e3180310ca9. [DOI] [PubMed] [Google Scholar]
- 5.Zander R. Fluid Management. Bibliomed-Medizinische Verlagsgesellschaft. 2008. [Last accessed on 2016 Jul 18]. pp. 18–30. Available from: https://www.physioklin.de/fileadmin/user_upload/./z/fluid_management_1109 .
- 6.Shaw AD, Bagshaw SM, Goldstein SL, Scherer LA, Duan M, Schermer CR, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012;255:821–9. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 7.Krajewski ML, Raghunathan K, Paluszkiewicz SM, Schermer CR, Shaw AD. Meta-analysis of high-versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg. 2015;102:24–36. doi: 10.1002/bjs.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meregalli A, Oliveira RP, Friedman G. Occult hypoperfusion is associated with increased mortality in hemodynamically stable, high-risk, surgical patients. Crit Care. 2004;8:R60–5. doi: 10.1186/cc2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care. 2013;3:12. doi: 10.1186/2110-5820-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Schryver N, Wittebole X, Hubert C, Gigot JF, Laterre PF, Castanares-Zapatero D. Early hyperlactatemia predicts pancreatic fistula after surgery. BMC Anesthesiol. 2015;15:109. doi: 10.1186/s12871-015-0093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingelfinger JR. Disorders of fluids and electrolytes. Lactic acidosis. N Engl J Med. 2014;371:2309–19. doi: 10.1056/NEJMra1309483. [DOI] [PubMed] [Google Scholar]
- 12.Chioléro R, Tappy L, Gillet M, Revelly JP, Roth H, Cayeux C, et al. Effect of major hepatectomy on glucose and lactate metabolism. Ann Surg. 1999;229:505–13. doi: 10.1097/00000658-199904000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansen TC, van Bommel J, Woodward R, Mulder PG, Bakker J. Association between blood lactate levels, sequential organ failure assessment sub scores, and 28-day mortality during early and late Intensive Care Unit stay: A retrospective observational study. Crit Care Med. 2009;37:2369–74. doi: 10.1097/CCM.0b013e3181a0f919. [DOI] [PubMed] [Google Scholar]
- 14.Guidet B, Soni N, Della Rocca G, Kozek S, Vallet B, Annane D, et al. A balanced view of balanced solutions. Crit Care. 2010;14:325. doi: 10.1186/cc9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reid F, Lobo DN, Williams RN, Rowlands BJ, Allison SP. (Ab) normal saline and physiological Hartmann's solution: A randomized double-blind crossover study. Clin Sci (Lond) 2003;104:17–24. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 16.O'Malley CM, Frumento RJ, Hardy MA, Benvenisty AI, Brentjens TE, Mercer JS, et al. A randomized, double-blind comparison of lactated Ringer's solution and 0.9% NaCl during renal transplantation. Anesth Analg. 2005;100:1518–24. doi: 10.1213/01.ANE.0000150939.28904.81. [DOI] [PubMed] [Google Scholar]
- 17.Montassier E, Batard E, Segard J, Hardouin JB, Martinage A, Le Conte P, et al. Base excess is an accurate predictor of elevated lactate in ED septic patients. Am J Emerg Med. 2012;30:184–7. doi: 10.1016/j.ajem.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 18.Wang WD, Liang LJ, Huang XQ, Yin XY. Low central venous pressure reduces blood loss in hepatectomy. World J Gastroenterol. 2006;12:935–9. doi: 10.3748/wjg.v12.i6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poon RT. Current techniques of liver transection. HPB (Oxford) 2007;9:166–73. doi: 10.1080/13651820701216182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar L, Kanneganti YS, Rajan S. Outcomes of implementation of enhanced goal directed therapy in high-risk patients undergoing abdominal surgery. Indian J Anaesth. 2015;59:228–33. doi: 10.4103/0019-5049.155000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas DJ, Alberti KG. Hyperglycaemic effects of Hartmann's solution during surgery in patients with maturity onset diabetes. Br J Anaesth. 1978;50:185–8. doi: 10.1093/bja/50.2.185. [DOI] [PubMed] [Google Scholar]
- 22.Richards RH, Vreman HJ, Zager P, Feldman C, Blaschke T, Weiner MW. Acetate metabolism in normal human subjects. Am J Kidney Dis. 1982;2:47–57. doi: 10.1016/s0272-6386(82)80043-7. [DOI] [PubMed] [Google Scholar]
- 23.Lee SW, Park SH, Kim KW, Choi EK, Shin YM, Kim PN, et al. Unenhanced CT for assessment of macrovesicular hepatic steatosis in living liver donors: Comparison of visual grading with liver attenuation index. Radiology. 2007;244:479–85. doi: 10.1148/radiol.2442061177. [DOI] [PubMed] [Google Scholar]
- 24.Shin WJ, Kim YK, Bang JY, Cho SK, Han SM, Hwang GS. Lactate and liver function tests after living donor right hepatectomy: A comparison of solutions with and without lactate. Acta Anaesthesiol Scand. 2011;55:558–64. doi: 10.1111/j.1399-6576.2011.02398.x. [DOI] [PubMed] [Google Scholar]


