Abstract
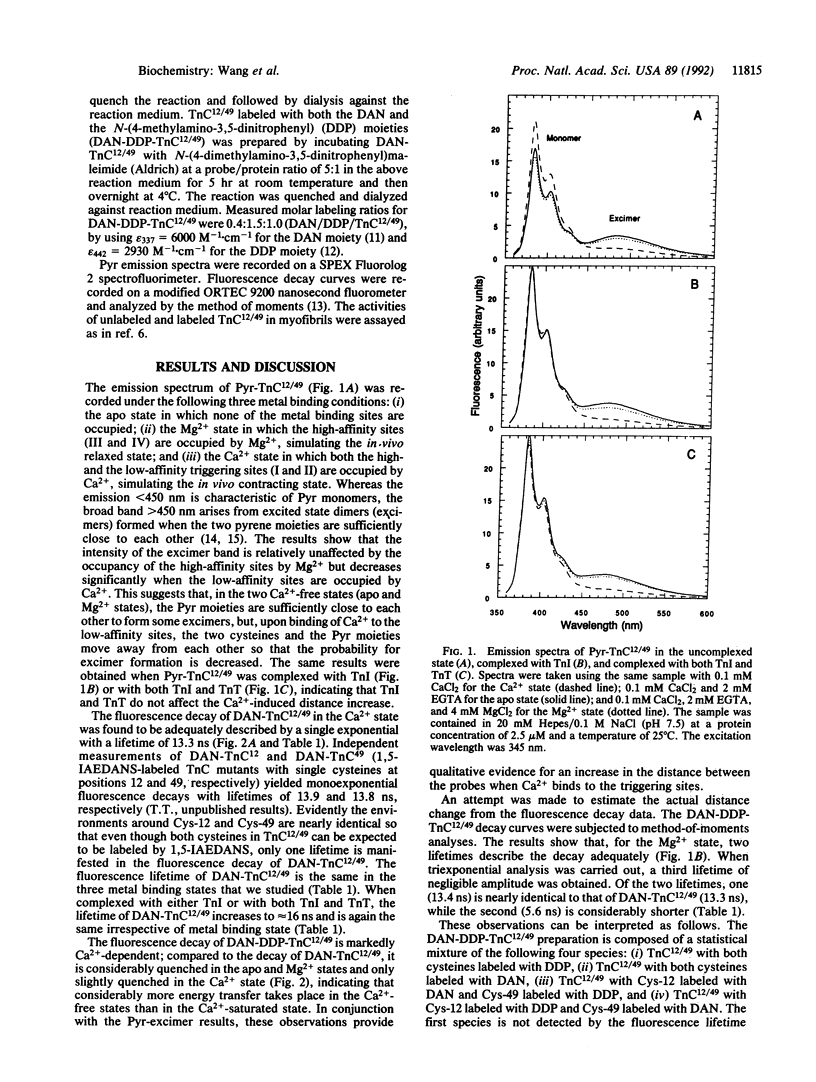
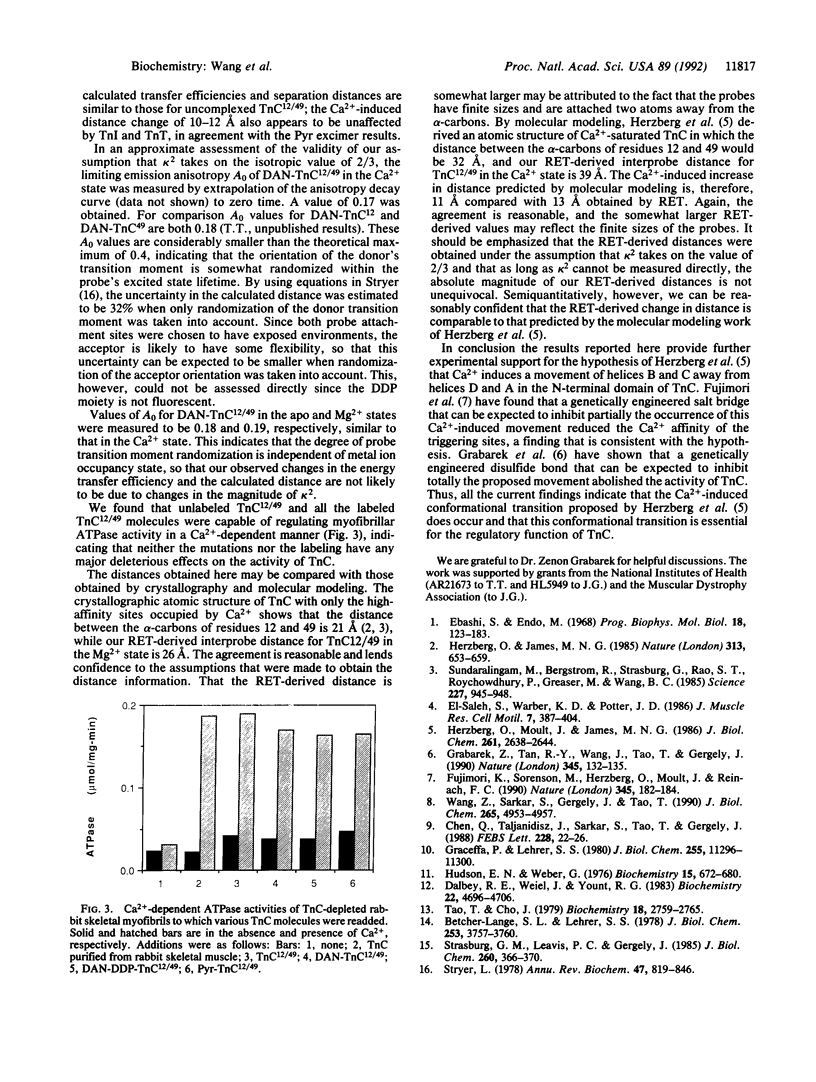
Troponin C is the Ca(2+)-binding subunit of troponin in vertebrate striated muscle. Binding of Ca2+ to troponin C is thought to induce a conformational change that triggers subsequent events in the initiation of muscle contraction. A molecular modeling study has proposed that, when Ca2+ binds to the N-terminal triggering sites, helices B and C separate from the helices D and A, thereby exposing a crucial interaction site for troponin I, the inhibitory subunit of troponin [Herzberg, O., Moult, J., and James, M. N. G. (1986) J. Biol. Chem. 261, 2638-2644]. In the present study the question of whether this separation actually occurs is addressed directly. A mutant rabbit skeletal troponin C containing a pair of cysteines at position 12 in helix A and position 49 in the polypeptide segment linking helices B and C was created by site-directed mutagenesis. Pyrene excimer fluorescence and resonance energy transfer studies on the labeled mutant troponin C reveal a Ca(2+)-induced increase in distance between the two cysteines. Under certain assumptions, the distance increase could be estimated from the extent of energy transfer to be approximately 13 A, in good agreement with the distance increase predicted by molecular modeling. Our results provide further experimental support for the model proposed by Herzberg et al. (above).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betcher-Lange S. L., Lehrer S. S. Pyrene excimer fluorescence in rabbit skeletal alphaalphatropomyosin labeled with N-(1-pyrene)maleimide. A probe of sulfhydryl proximity and local chain separation. J Biol Chem. 1978 Jun 10;253(11):3757–3760. [PubMed] [Google Scholar]
- Chen Q., Taljanidisz J., Sarkar S., Tao T., Gergely J. Cloning, sequencing and expression of a full-length rabbit fast skeletal troponin-C cDNA. FEBS Lett. 1988 Feb 8;228(1):22–26. doi: 10.1016/0014-5793(88)80576-3. [DOI] [PubMed] [Google Scholar]
- Dalbey R. E., Weiel J., Yount R. G. Förster energy transfer measurements of thiol 1 to thiol 2 distances in myosin subfragment 1. Biochemistry. 1983 Sep 27;22(20):4696–4706. doi: 10.1021/bi00289a014. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Eftink M. R., Ghiron C. A. Exposure of tryptophanyl residues in proteins. Quantitative determination by fluorescence quenching studies. Biochemistry. 1976 Feb 10;15(3):672–680. doi: 10.1021/bi00648a035. [DOI] [PubMed] [Google Scholar]
- Fujimori K., Sorenson M., Herzberg O., Moult J., Reinach F. C. Probing the calcium-induced conformational transition of troponin C with site-directed mutants. Nature. 1990 May 10;345(6271):182–184. doi: 10.1038/345182a0. [DOI] [PubMed] [Google Scholar]
- Grabarek Z., Tan R. Y., Wang J., Tao T., Gergely J. Inhibition of mutant troponin C activity by an intra-domain disulphide bond. Nature. 1990 May 10;345(6271):132–135. doi: 10.1038/345132a0. [DOI] [PubMed] [Google Scholar]
- Graceffa P., Lehrer S. S. The excimer fluorescence of pyrene-labeled tropomyosin. A probe of conformational dynamics. J Biol Chem. 1980 Dec 10;255(23):11296–11300. [PubMed] [Google Scholar]
- Herzberg O., James M. N. Structure of the calcium regulatory muscle protein troponin-C at 2.8 A resolution. Nature. 1985 Feb 21;313(6004):653–659. doi: 10.1038/313653a0. [DOI] [PubMed] [Google Scholar]
- Herzberg O., Moult J., James M. N. A model for the Ca2+-induced conformational transition of troponin C. A trigger for muscle contraction. J Biol Chem. 1986 Feb 25;261(6):2638–2644. [PubMed] [Google Scholar]
- Strasburg G. M., Leavis P. C., Gergely J. Troponin-C-mediated calcium-sensitive changes in the conformation of troponin I detected by pyrene excimer fluorescence. J Biol Chem. 1985 Jan 10;260(1):366–370. [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Sundaralingam M., Bergstrom R., Strasburg G., Rao S. T., Roychowdhury P., Greaser M., Wang B. C. Molecular structure of troponin C from chicken skeletal muscle at 3-angstrom resolution. Science. 1985 Feb 22;227(4689):945–948. doi: 10.1126/science.3969570. [DOI] [PubMed] [Google Scholar]
- Tao T., Cho J. Fluorescence lifetime quenching studies on the accessibilities of actin sulfhydryl sites. Biochemistry. 1979 Jun 26;18(13):2759–2765. doi: 10.1021/bi00580a011. [DOI] [PubMed] [Google Scholar]
- Wang Z. Y., Sarkar S., Gergely J., Tao T. Ca2(+)-dependent interactions between the C-helix of troponin-C and troponin-I. Photocross-linking and fluorescence studies using a recombinant troponin-C. J Biol Chem. 1990 Mar 25;265(9):4953–4957. [PubMed] [Google Scholar]
- el-Saleh S. C., Warber K. D., Potter J. D. The role of tropomyosin-troponin in the regulation of skeletal muscle contraction. J Muscle Res Cell Motil. 1986 Oct;7(5):387–404. doi: 10.1007/BF01753582. [DOI] [PubMed] [Google Scholar]




