Abstract
Background and Aims:
In laparoscopic surgeries, intraperitoneal instillation of local anaesthetics and opioids is gaining popularity, for better pain relief. This study compared the quality and duration of post-operative analgesia using intraperitoneal tramadol plus bupivacaine (TB) or magnesium plus bupivacaine (MB).
Methods:
In this study, 186 patients undergoing laparoscopic cholecystectomy were randomly divided into two groups: group TB received intraperitoneal tramadol with bupivacaine and group MB received intraperitoneal magnesium sulphate (MgSO4) with bupivacaine. The visual analogue scale (VAS) to assess pain, haemodynamic variables and side effects were noted and compared at different time points. The primary outcome was to compare the analgesic efficacy and duration of pain relief. The secondary outcomes included comparison of haemodynamic parameters and side effects among the two groups. The data analysis was carried out with unpaired Student's t-test and Chi-square test using software SPSS 20.0 version.
Results:
The mean of VAS pain score after 1, 2, 4, 6 and 24 h of surgery was more in TB group compared to MB group, and the difference was statistically significant (P < 0.05). The total rescue analgesia consumption in 24 h after surgery was 2.4 g (mean) of paracetamol in TB group and 1.4 g (mean) of paracetamol in MB group which was statistically significant (P < 0.05). There were no statistically significant differences in the secondary outcomes.
Conclusion:
Intraperitoneal instillation of bupivacaine-MgSO4 renders patients relatively pain-free in first 24 h after surgery, with longer duration of pain-free period and less consumption of rescue analgesic as compared to bupivacaine-tramadol combination.
Key words: Laparoscopic cholecystectomy, magnesium sulphate, post-operative analgesia
INTRODUCTION
There is a significant difference in the quality of pain related to laparoscopic surgery as compared to laparotomy. The pain after laparotomy is mostly parietal whereas it is more of visceral pain following laparoscopic cholecystectomy.[1] Characteristically, the pain following laparoscopic cholecystectomy is highly variable in intensity and duration and is largely unpredictable.[2]
The antinociceptive effect of magnesium sulphate (MgSO4) is quite useful in chronic pain and it also governs in part, the duration and intensity of post-operative pain.[3] Tramadol could also have a local effect when given intraperitoneally after laparoscopic surgery.[1]
The effectiveness of local anaesthetics, instilled intraperitoneally, solely or mixed with other drugs, has been shown in a number of studies on laparoscopic cholecystectomy, but there is no agreement regarding the dose, concentration, site and manner of administration.[4]
In this study, an effort, therefore, has been made to compare primarily the antinociceptive effects of MgSO4 with bupivacaine to tramadol with bupivacaine.
METHODS
This prospective, randomised, double-blind study was commenced following the ethics committee approval of the hospital. Written informed consent was obtained from all the patients before starting the study. A total of 186 patients belonging to the American Society of Anesthesiologists physical status 1 or 2, of either gender and aged between 18 and 65 years planned for laparoscopic cholecystectomy were selected for the study and randomly allocated into two groups of 93 each to be administered one of the following intraperitoneal instillations: tramadol plus bupivacaine (TB) group received 30 ml of 0.25% bupivacaine along with 100 mg tramadol and magnesium plus bupivacaine (MB) group received 30 ml of 0.25% bupivacaine along with 50 mg/kg of MgSO4 (dose of bupivacaine not exceeding 2.0 mg/kg BW) at the conclusion of surgery. Patients with allergy to study drugs (MgSO4, bupivacaine and tramadol), known hypomagnesaemia or hypermagnesaemia, chronic alcoholism, heart block, renal failure and those with peritoneal drain after surgery were not included in the study.
Randomisation was done by the lottery method to select the first group out of the two groups, after that the cases were allocated by systematic way (systematic randomised allocation method). Another anaesthesiologist not involved in the conduct of anaesthesia instilled the study drugs into the peritoneal cavity and the data collection and analysis was done by a separate anaesthesiologist.
All patients were subjected to a thorough pre-anaesthetic evaluation, in which procedure was explained to the patient, and all patients were educated about the visual analogue scale (VAS) pain score of 0–10. All study drugs were prepared by an anaesthesiologist not involved in the study and data collection. A common conduct of anaesthesia was followed in all patients which included alprazolam 0.25 mg orally at night before surgery and ranitidine 150 mg orally at night and on the morning of surgery. Standard monitoring included pulse oximetry, non-invasive blood pressure, end-tidal CO2 and three-lead electrocardiogram. Induction of general anaesthesia was accomplished with intravenous (IV) lignocaine 1.0 mg/kg, fentanyl 2.0 μg/kg and propofol 2.0 mg/kg. IV vecuronium 0.1 mg/kg was used to facilitate endotracheal intubation. Maintenance of anaesthesia was with isoflurane along with 50% oxygen in air and positive pressure ventilation. Instillation of the study drugs into the peritoneal cavity through laparoscopic ports guided by the surgical camera was done at the conclusion of surgery. The residual effects of vecuronium were reversed with neostigmine and glycopyrrolate, and with adequate respiratory efforts, patients were extubated and transferred to the post-operative recovery room. When they were fully awake and responding to vocal commands, VAS pain score was recorded at 1, 2, 4, 6, 8, 12 and 24 h after surgery. The time duration of the first demand for analgesia and total paracetamol consumption in 24 h was recorded. IV paracetamol 1 g was given as rescue analgesic on demand or/and with VAS >3 up to a maximum dose of 4 g in 24 h.
Heart rate (HR), systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded 5 min before induction (i.e., baseline parameters) and thereafter every 5 min for the first 20 min after the administration of study drugs. SBP 20% below the baseline or <90 mmHg was treated with IV boluses of 250 ml lactated Ringer's solution or/and with ephedrine 6 mg, if required. The following side effects of the studied drugs were also observed during post-operative period at intervals of 30 min, 40 min, 1 h, 2 h, 4 h and 6 h after shifting to recovery room: nausea and vomiting (NV), loss of tendon reflexes and hypotension (defined as more than 20% reduction of SBP and/or DBP from baseline).
Sample size calculation was performed from the previous study results assuming that 20% difference in mean post-operative pain score would be detectable between the two groups using sample size of 93 patients per group for analysis of variance with a power of 80% and alpha level of 0.05. Statistical analysis was performed with SPSS for Windows: IBM Corp. Version 20.0. Armonk, NY, USA. Significance test was done with independent samples t-test. Results were displayed as mean ± standard deviation, and for all statistical tests, P < 0.05 was considered statistically significant.
RESULTS
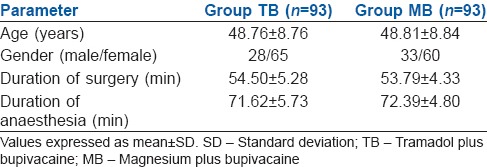
The two groups were comparable in terms of age, gender, weight and height. The percentage of females was found to be higher than males in each group showing no statistically significant difference (P > 0.05). No statistically significant difference was observed between the groups with respect to duration of anaesthesia and surgery as well (P > 0.05). We also analysed the total IV fluid required for patients in both groups during anaesthesia and found that the difference was not statistically significant (P > 0.05) [Table 1].
Table 1.
Demographic profile, duration of surgery and anaesthesia in two groups
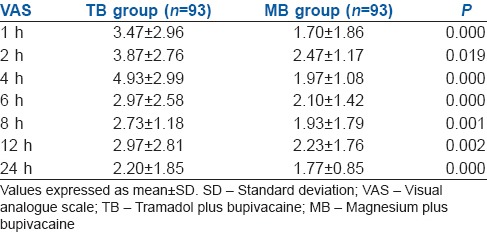
We analysed the VAS score seven times in 24 h, of which the cumulative mean of VAS pain score after 1, 2, 4, 6 and 24 h of surgery was more in TB group compared to MB group and the difference was statistically significant (P < 0.05) [Table 2].
Table 2.
Visual analogue scale pain score at different time intervals in the two groups (mean±standard deviation)
The mean time interval of the first rescue analgesia (paracetamol) demand was also longer in MB group compared to TB group which was also statistically significant (P < 0.05). TB group had mild to moderate pain and most of the patients in MB group had mild pain in first 24 h of surgery [Figure 1].
Figure 1.
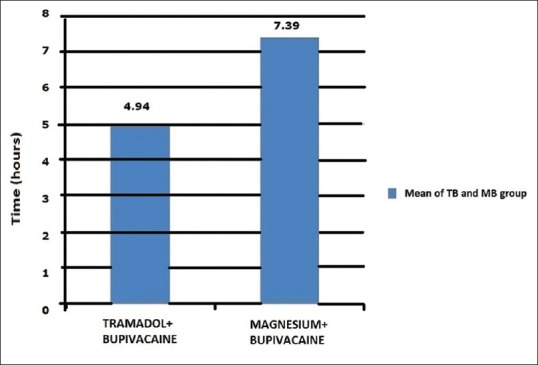
First rescue analgesia demand time (mean) in 24 h
Total rescue analgesia consumption in 24 h was analysed. TB group had 2.390 g (2.39 g, mean) and MB group had 1.430 g (1.43 g, mean) of paracetamol (rescue analgesia) consumption in 24 h which was statistically significant (P < 0.05) [Figure 2].
Figure 2.

Mean of total rescue analgesic consumption in 24 h
After intraperitoneal instillation of the study drugs, the HR was recorded at 5 min interval in TB group patients and compared with baseline HR and found that the difference was not statistically significant (P > 0.05). In MB group, HR was recorded at 5 min interval and compared with baseline HR and found that the difference was statistically significant (P < 0.05). The HR after MgSO4 plus bupivacaine instillation intraperitoneally was slightly less than baseline and stable thereafter [Figure 3].
Figure 3.
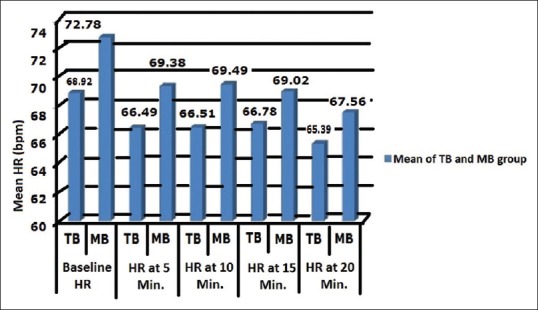
Comparison of mean heart rate in studied groups
After the study drugs instillation intraperitoneally, SBP was recorded at 5 min interval in both groups during our observation period and compared with baseline SBP and found that the difference was not statistically significant (P > 0.05).
After the study drugs instillation intraperitoneally, DBP was recorded at 5 min interval in both groups during our observation period and compared with baseline DBP and found that the difference was not statistically significant (P > 0.05).
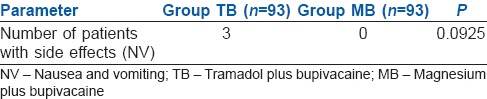
No statistically significant difference was found between the groups regarding side effects at 30 min after shifting to recovery room (P > 0.05). Side effects at 40 min, 1 h, 2 h, 4 h and 6 h after shifting of the patient to recovery room were also observed and found that no side effects occurred in patients of both groups except NV in three patients in ‘TB’ group which was statistically not significant [Table 3].
Table 3.
Intergroup comparison of side effects
DISCUSSION
Improved post-operative analgesia, using opioid-sparing regimens, may enable a high success rate of outpatient laparoscopic cholecystectomy as both pain and opioids may induce nausea.[5] In addition to the parenteral route of analgesic use, instillation of local anaesthetics and opioids intraperitoneally are attaining popularity for better pain relief.[1]
In the present study, we compared the analgesic effects of intraperitoneal instillation of MgSO4 with bupivacaine versus tramadol with bupivacaine in patients operated for laparoscopic cholecystectomy.
Instillation of local anaesthetics intraperitoneally around the operative site is used as an analgesic technique on the assumption that conduction from visceral sites is obstructed and may lessen the intensity of referred pain to the shoulder, which results from irritation of diaphragm innervations, i.e., C3, C4, C5 and diaphragmatic shifting due to gaseous distension, in the post-operative period.[6] Absorption from the large peritoneal surface into systemic circulation may also contribute to analgesia.[7] The timing of administration of the local anaesthetic during surgery is a matter of debate. Some trials advocated early instillation of intraperitoneal local anaesthetics as it provided better control of post-operative pain when compared with instillation at the end of the surgery,[7] but was contradicted by other trials.[8]
Magnesium reduces calcium influx to the cell, and also antagonises N-methyl-D-aspartate (NMDA) receptors, which are vital for neuronal signalling as well as pain processing in the central nervous system. Due to blockade of this receptor, MgSO4 decreases post-operative pain as both somatic and visceral pain fibres are blocked.[7] MgSO4 has been used in laparoscopic cholecystectomy as an IV bolus, continuous infusion,[9] epidural infusion and in the subarachnoid space.[10] In addition, intraperitoneal local anaesthetics alone or in addition to MgSO4 have been shown to improve post-operative pain after laparoscopic cholecystectomy.[2]
Our results show that the addition of MgSO4 to bupivacaine decreases post-operative pain and analgesic consumption in first 24 h after surgery along with longer pain-free period compared to patients who were given TB after laparoscopic cholecystectomy. The results of several studies are in accordance with ours. A study that compared intraperitoneal instillation of bupivacaine, solely and in addition to MgSO4 in a dose of 50 mg/kg, concluded that patients receiving intraperitoneal bupivacaine with MgSO4 at the end of the surgery had better pain relief for a period of 2–5 h compared with patients who were given only intraperitoneal bupivacaine.[11] Our study also shows 2–5 h of relatively pain-free period in patients with intraperitoneal instillation of bupivacaine and MgSO4. An earlier study, in which experiments were done on frog sciatic nerves, found a better conduction block with MgSO4 plus bupivacaine but not with TB. Inhibition of the compound nerve action potentials induced by bupivacaine was markedly enhanced by MgSO4, but was not changed by tramadol. The amplitude of compound nerve action potentials increased from 15.10% with bupivacaine alone to 35.43% with bupivacaine plus MgSO4.[12]
A previous study revealed that IV tramadol produces better post-operative analgesia in the early post-operative period after laparoscopic cholecystectomy compared with an identical intraperitoneal dose of tramadol in patients undergoing laparoscopic cholecystectomy. Parietal and visceral pain scores were lowest in the IV tramadol group during the first post-operative hour.[13] Another study, conducted in India, concluded that intraperitoneal instillation of bupivacaine in combination with dexmedetomidine is superior to bupivacaine alone and may be better than bupivacaine with tramadol.[14]
We also analysed that the addition of MgSO4 to bupivacaine decreased the HR which was slightly less than baseline and became stable thereafter compared to patients who were given TB after laparoscopic cholecystectomy. This is in accordance with the results of a previous study where MgSO4 was administered 50 mg/kg IV in patients undergoing laparoscopic cholecystectomy and a decrease in the HR was observed; however, a significant reduction in the mean arterial pressure was also found, which was not found in our study.[15]
In this study, post-operative NV was observed in three patients in the TB group but not in any patient in the MB group, the difference being insignificant statistically. The results of a previous study, which used pre-operative infusion of MgSO4 in a dose of 50 mg/kg in laparoscopic cholecystectomy, were similar with ours as the incidence of nausea was lower in the MgSO4 group than in the TB group.[16] MgSO4 blocks NMDA receptors found in emetic pathways and structures related with the final common pathway for vomiting. NMDA antagonists have the potential to be broad-spectrum anti-emetics.[17] However, no current data are available on the direct effects of MgSO4 on post-operative NV.
Several reports have revealed that the range of mean plasma concentration (0.92–1.14 μg/ml) after plain intraperitoneal bupivacaine administration (100–150 mg) is quite below the toxic concentration of 3 μg/ml.[18] We used lower doses of bupivacaine in our study than those believed to cause systemic toxicity and none of our patients exhibited features of local anaesthetic toxicity.
CONCLUSION
Intraperitoneal instillation of MgSO4 with bupivacaine after completion of laparoscopic surgery renders patients relatively pain-free in first 24 h after surgery, with longer duration of pain-free period and less consumption of rescue analgesics in post-operative period as compared to intraperitoneal instillation of tramadol with bupivacaine, although haemodynamic parameters and side effects were comparable in both groups.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Golubovic S, Golubovic V, Cindric-Stancin M, Tokmadzic VS. Intraperitoneal analgesia for laparoscopic cholecystectomy: Bupivacaine versus bupivacaine with tramadol. Coll Antropol. 2009;33:299–302. [PubMed] [Google Scholar]
- 2.Bisgaard T. Analgesic treatment after laparoscopic cholecystectomy: A critical assessment of the evidence. Anesthesiology. 2006;104:835–46. doi: 10.1097/00000542-200604000-00030. [DOI] [PubMed] [Google Scholar]
- 3.Shoeibi G, Sadegi M, Firozian A, Tabassomi F. The additional effect of magnesium sulphate to lidocaine in spinal anaesthesia for caesarean section. Int J Pharmacol. 2007;3:425–7. [Google Scholar]
- 4.Pasqualucci A, de Angelis V, Contardo R, Colò F, Terrosu G, Donini A, et al. Preemptive analgesia: Intraperitoneal local anesthetic in laparoscopic cholecystectomy. A randomized, double-blind, placebo-controlled study. Anesthesiology. 1996;85:11–20. doi: 10.1097/00000542-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Kehlet H, Rung GW, Callesen T. Postoperative opioid analgesia: Time for a reconsideration? J Clin Anesth. 1996;8:441–5. doi: 10.1016/0952-8180(96)00131-6. [DOI] [PubMed] [Google Scholar]
- 6.Gvozdenović L, Cvijanović R, Kolak R, Pjević M, Gavrilović S, Veljković R, et al. Anaesthesiology in current world of laparoscopic surgery. Med Danas. 2003;2:298–301. [Google Scholar]
- 7.Marks JL, Ata B, Tulandi T. Systematic review and metaanalysis of intraperitoneal instillation of local anesthetics for reduction of pain after gynecologic laparoscopy. J Minim Invasive Gynecol. 2012;19:545–53. doi: 10.1016/j.jmig.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Paulson J, Mellinger J, Baguley W. The use of intraperitoneal bupivacaine to decrease the length of stay in elective laparoscopic cholecystectomy patients. Am Surg. 2003;69:275–8. [PubMed] [Google Scholar]
- 9.Saadawy IM, Kaki AM, Abd El Latif AA, Abd-Elmaksoud AM, Tolba OM. Lidocaine vs. magnesium: Effect on analgesia after a laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2010;54:549–56. doi: 10.1111/j.1399-6576.2009.02165.x. [DOI] [PubMed] [Google Scholar]
- 10.Kesavan S. Appropriate timing of administration of magnesium sulphate during spinal anaesthesia to enhance postoperative analgesia. Br J Anaesth. 2010;104:89–93. doi: 10.1093/bja/aep334. [DOI] [PubMed] [Google Scholar]
- 11.Maharjan SK, Shrestha S. Intraperitoneal magnesium sulphate plus bupivacaine for pain relief after laparoscopic cholecystectomy. J Kathmandu Med Coll. 2012;1:21–5. [Google Scholar]
- 12.Büyükakilli B, Doruk N, Çömelekoğlu Ü, Çamdeviren H, Güneş S. Do adjuncts (tramadol and magnesium) potentiate impulse inhibition by a local anesthetic in isolated frog sciatic nerves? Turk J Med Sci. 2006;36:201–6. [Google Scholar]
- 13.Akinci SB, Ayhan B, Aycan IO, Tirnaksiz B, Basgul E, Abbasoglu O, et al. The postoperative analgesic efficacy of intraperitoneal tramadol compared to normal saline or intravenous tramadol in laparoscopic cholecystectomy. Eur J Anaesthesiol. 2008;25:375–81. doi: 10.1017/S0265021508003694. [DOI] [PubMed] [Google Scholar]
- 14.Shukla U, Prabhakar T, Malhotra K, Srivastava D, Malhotra K. Intraperitoneal bupivacaine alone or with dexmedetomidine or tramadol for postoperative analgesia following laparoscopic cholecystectomy: A comparative evaluation. Indian J Anaesth. 2015;59:234–9. doi: 10.4103/0019-5049.155001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jee D, Lee D, Yun S, Lee C. Magnesium sulphate attenuates arterial pressure increase during laparoscopic cholecystectomy. Br J Anaesth. 2009;103:484–9. doi: 10.1093/bja/aep196. [DOI] [PubMed] [Google Scholar]
- 16.Mentes O, Harlak A, Yigit T, Balkan A, Balkan M, Cosar A, et al. Effect of intraoperative magnesium sulphate infusion on pain relief after laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2008;52:1353–9. doi: 10.1111/j.1399-6576.2008.01816.x. [DOI] [PubMed] [Google Scholar]
- 17.Lucot JB. Effects of N-methyl-D-aspartate antagonists on different measures of motion sickness in cats. Brain Res Bull. 1998;47:407–11. doi: 10.1016/s0361-9230(98)00093-8. [DOI] [PubMed] [Google Scholar]
- 18.Gvozdenovic L, Pajtic V, Ivanov D, Cvijanovic R, Gavrilovic S, Gojkovic Z, et al. Acute postoperative pain relief, by intraperitoneal application of local anaesthetics, during laparoscopic cholecystectomy. J Health Sci. 2011;1:90–5. [Google Scholar]


