Abstract
Background
Receptor occupancy, or saturation, assays are often utilized in preclinical and clinical development programs to evaluate the binding of a biologic to a cellular target. These assays provide critical information regarding the dose of drug required to “saturate” the target as well as important pharmacodymamic (PD) data. A flow cytometric method was developed to measure the degree of Semaphorin 4D (SEMA4D; CD100) saturation by VX15/2303, an investigational monoclonal antibody specific for SEMA4D.
Methods
The assay detects VX15/2503, a human IgG4 specific for SEMA4D, with an IgG4‐specific monoclonal antibody.
Results
Data generated allowed assessment of two related SEMA4D‐specific pharmacodynamic (PD) markers: (1) The measurement of cellular SEMA4D (cSEMA4D) saturation by VX15/2503, and (2) the cell membrane expression levels of cSEMA4D.
Conclusions
This assay specifically and reproducibly measured cSEMA4D saturation and expression levels. Evaluation of the SEMA4D‐specific PD markers were critical in determining the clinical saturation threshold of cSEMA4D by VX15/2503. © 2015 he Authors Cytometry Part B: Clinical Cytometry Published by Wiley Periodicals, Inc.
Keywords: Semaphorin4D, receptor‐occupancy, saturation, VX15/2503, clinical trials
Semaphorins consist of a family of soluble and transmembrane proteins, which function in axonal‐guidance, immune cell regulation, vascular growth, and tumor progression 1, 2, 3. They guide migration 4, 5, 6 and cytoskeletal changes in endothelial, immune, and tumor cells within the tumor microenvironment (TME). Semaphorin 4D (SEMA4D; CD100) contains a large N‐terminal propeller ‘‘sema’’ domain followed by an Ig‐like domain, a lysine‐rich domain, a transmembrane domain, and a cytoplasmic tail with consensus tyrosine and serine phosphorylation sites. SEMA4D functions both as a receptor, which signals through its cytoplasmic domain, and as a ligand 7. When activated, the cellular form of the molecule (cSEMA4D) is released from the cell surface via proteolytic cleavage to generate a physiologically active, soluble form of the protein (sSEMA4D) 8, 9.
A strong rationale exists for targeting SEMA4D in the treatment of solid tumors. SEMA4D is over‐expressed in head and neck, prostate, colon, breast, and lung cancers; moreover, elevated expression correlates with invasive disease and poor prognosis in human disease 10, 11, 12. Expression of SEMA4D at the invasive tumor edge creates a barrier to immune infiltration and antibody‐mediated SEMA4D blockade effectively facilitates access and amplification of anti‐tumorigenic immune activity within the tumor. In murine models, anti‐SEMA4D‐mediated tumor rejection, and increased efficacy of checkpoint blockade inhibitors, corresponding with increased immune activity in the TME 13.
SEMA4D is also involved in CNS physiology and homeostasis. SEMA4D knockout mice were resistant to the development of experimental allergic encephalomyelitis (EAE), a murine model for multiple sclerosis (MS), due to a reduced generation of immune response to the EAE‐inducing antigen 14. Anti‐SEMA4D has been shown to attenuate EAE in multiple rodent models 15, 16. In addition to the involvement with the immune response to CNS antigens, SEMA4D may play an important role in several other potentially pathogenic CNS processes, including migration of oligodendrocyte precursor cells, oligodendrocyte survival 1, remyelination 16 and the integrity of the neurovascular unit comprised of endothelial cells, astrocytes and pericytes 17.
VX15/2503 is a humanized IgG4 monoclonal antibody that binds specifically to SEMA4D and blocks the binding of SEMA4D to its receptors, plexinB1, plexinB2, and CD72. This biologic represents a novel therapeutic strategy for cancer, as well as for MS, and other neuroinflammatory/neurodegenerative diseases (for review see 11, 18, 19). Although the mechanism of action differs in each of these therapeutic indications, measurements of the degree of cSEMA4D saturation produce important pharmacodynamic data for each indication. An assay to measure the saturation of cSEMA4D by VX15/2503 in clinical whole blood samples was developed successfully and was effectively utilized in Phase 1 clinical trials for two therapeutic indications, oncology (Patnaik, A, et al. manuscript accepted, Clinical Cancer Research; Clintrials.gov identifier NCT01313065) and multiple sclerosis (Clintrials.gov identifier NCT01764737; study completed).
MATERIALS AND METHODS
Reagents, Instrumentation, Software
Mouse anti‐chicken IgG‐Biotin (clone G‐1) and mouse anti‐human IgG4‐Biotin (clone HP6025) were purchased from Southern Biotech (Birmingham, AL). Staining buffer, anti‐CD45 V500 (clone H13), anti‐CD3 AF488 (clone SP34‐2), Strepavidin APC (GMP Grade), FACS Lysing Solution, Cytofix Fixation Solution were purchased from (Becton Dickinson, San Jose, CA). Immunol‐Trol and Immunol‐Trol Low were purchased from Beckman Coulter, Brea, CA. Human IgG4 isotype control 2269‐Biotin (clone 2269), anti‐SEMA4D‐Biotin (clone 2282) and the study drug, VX15/2503, were provided by Vaccinex (Rochester, NY).
Samples were acquired on two FACSCanto™ II flow cytometers equipped with 405 nm, 488 nm, and 633 nm lasers from Becton Dickinson (San Jose, CA). Each instrument has the same configuration and version of the operating system FACSDiva™ software 6.1.2 from Becton Dickinson (San Jose, CA). The instruments were cross standardized in collaboration with BD Biosciences 20, 21. Briefly, several instruments were characterized using the BDTM Cytometer Setup and Tracking (CS&T) beads and BD FACSDiVaTM software. Next a virtual predicate instrument was created using the linearity and relative SD of electronic noise (rSDEN) baseline data from all instruments. The highest rSDEN values were selected for the virtual predicate instrument. Next Application Settings were created using 2.5 × rSDEN. After confirming that brightly stained cells would be within the linear range of the instrument, the brightest CS&T bead was used to create the corresponding target channels which were saved in worksheets as Application Settings. In order to cancel out day‐to‐day variations within individual instruments, the applications settings were updated daily based on the CS&T values. Data analysis was conducted with FACSDiva™ software 6.1.2. Quality control data were evaluated using Unity Real Time (URT) from Bio‐Rad Laboratories (Hercules, CA).
Samples: Peripheral whole blood sample was collected in Cyto‐Chex BCT tubes (Streck Labs, Omaha, NE). For validation, samples were obtained from healthy volunteers. Clinical specimens were obtained from patients enrolled in the first‐in‐human study involving VX15/2503 entitled, “Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of VX15/2503, a Humanized IgG4 anti‐SEMA4D Antibody, in a First‐In‐Human Phase 1 Study of Patients with Advanced Solid Tumors.” (Patnaik, A, et al. manuscript submitted, Clinical Cancer Research). Clinical specimens were also obtained from the Phase 1 MS study in humans involving VX15/2503 entitled, “A Phase 1, Multicenter, Randomized, Double‐Blind, Placebo‐Controlled, Ascending Single‐Dose Study of the Safety, Tolerability, and Pharmacokinetics of Intravenous VX15/2503 in Patients with Multiple Sclerosis.” Institutional Review Board approvals for the study protocols, amendments and informed consent documents were obtained prior to study initiation; study procedures were conducted in accordance with the Declaration of Helsinki.
cSEMA4D Saturation Assay: Two mL of whole blood was washed with PBS and resuspended in one mL of staining buffer. One hundred microliters of washed whole blood were dispensed into each of five polystyrene tubes and incubated with 100 µL of mAb VX15/2503 (20 µg/mL), or 100 μL staining buffer for 30 min at 4ºC. Treated cells were then washed with staining buffer and incubated 30 min at 4ºC with either 100 µL of mouse anti‐chicken IgG biotin (10 µg/mL), 100 μL of mouse anti‐human IgG4‐Biotin (10 µg/mL), 100 µL of mAb 2269 biotin (5 µg/mL), or 100 µL of mAb 2282 biotin (5 µg/mL). Samples were then washed with staining buffer and incubated 30 min at 4ºC with a cocktail containing 5 µL anti‐CD45 V500, 0.25 µL anti‐CD3 AF488 and 20 µL Strepavidin APC. Next, samples were incubated for 15 min at room temperature in 3 mL of FACS lysing solution, washed with 2 mL PBS and resuspended in 0.5 mL PBS and acquired on a BD FACSCanto II.
Method Validation: Samples for intra‐assay imprecision were created by spiking varying amounts of the compound VX15/2503 into peripheral whole blood collected from each of three apparently healthy volunteers. The resulting samples containing low, mid and high levels of VX15/2503 were then assayed in triplicate in a single analytical run. For each sample and each reportable result, the mean, SD and %CV were calculated. Inter‐assay imprecision was assessed by analyzing three replicates of Immuno‐Trol and Immuno‐Trol Low (described below) in four analytical runs performed by two technicians and distributed over each of two instruments. For each sample, the mean of replicates was calculated for each analytical run. The Grand Mean (mean of the daily means), SD (of the daily means) and %CV (of all four runs) were calculated.
Immuno‐Trol reagents are commercial whole blood products intended to deliver consistent cell populations that can be repeatedly measured to verify reagent performance as well as method performance for staining and analysis. For QC performance monitoring during sample analysis, the QC tubes contain all of the same reagents as the sample tubes, except that Immuno‐Trol is used as the sample instead of whole blood. We utilize Immuno‐Trol for both method validation and QC performance monitoring during sample analysis. Each product (Immuno‐Trol and Immuno‐Trol Low) is manufactured to deliver a specific concentration of cells to better ascertain performance across a range of numerical values. For example, a particular lot number of Immuno‐Trol reagent may be expected to generate a value of 46% CD4+ cells. A particular lot number of Immuno‐Trol Low may expect to generate a value of 21% CD4+ cells. As individual patient samples can contain varying SEMA4D levels, the use of a constant cell concentration can assist in ensuring the assay is performing as expected.
For the stability evaluation, peripheral whole blood was collected in Ethylenediaminetetraacetic acid (EDTA) and Acid Citrate Dextrose (ACD) Vacutainer® tubes (Becton‐Dickinson VACUTAINER Systems, Franklin Lakes, NJ) as well as Cyto‐Chex® Blood Collection Tube (BCT) (Streck, Inc., Omaha, NE). Samples were spiked ex vivo with 100 ng/mL of VX15/2503 and held at ambient temperature (18–22°C) or refrigerated (4°C) for 24 h, 48 h, 72 h, and 96 h. The percent change from baseline of the percent saturation of SEMA4D at each time point (baseline, 24, 48, 72, 96 h) at a concentration of 100 ng/mL VX15/2503 MAb was calculated using the following formula:
RESULTS
cSEMA4D Saturation Assay
The cSEMA4D Saturation Assay uses a peripheral whole blood matrix and a bound receptor design to calculate receptor saturation (Figs. 1A and 1B). The bound study drug, VX15/2503, a human IgG4 anti‐SEMA4D mAb, is detected with a fluorescently labeled anti‐IgG4 monoclonal antibody. Total available receptors were detected two ways; the first in the same manner as above except with specimens spiked ex vivo with super‐saturating levels of study drug. An advantage of this approach is that the bound drug and available receptors are detected with the same reagents, thus affinity differences and reagent competition do not confound the signal. In addition, a second measurement of total receptor is generated by using a proprietary, non‐competing anti‐SEMA4D mAb (clone 2282). The cSEMA4D saturation assay uses fewer fluorochromes and more assay tubes to further minimize the effects of unintended antibody and fluorochrome interactions. Assay specificity is increased by subtracting the geometric mean fluorescence intensity (GMFI) signal from control tubes with secondary reagents alone (tube 1) from tubes containing both primary and secondary reagents (tubes 2 and 3). T cells were selected as the cell of interest as they express the highest levels of cSEMA4D in human blood (unpublished results, Vaccinex).
Figure 1.
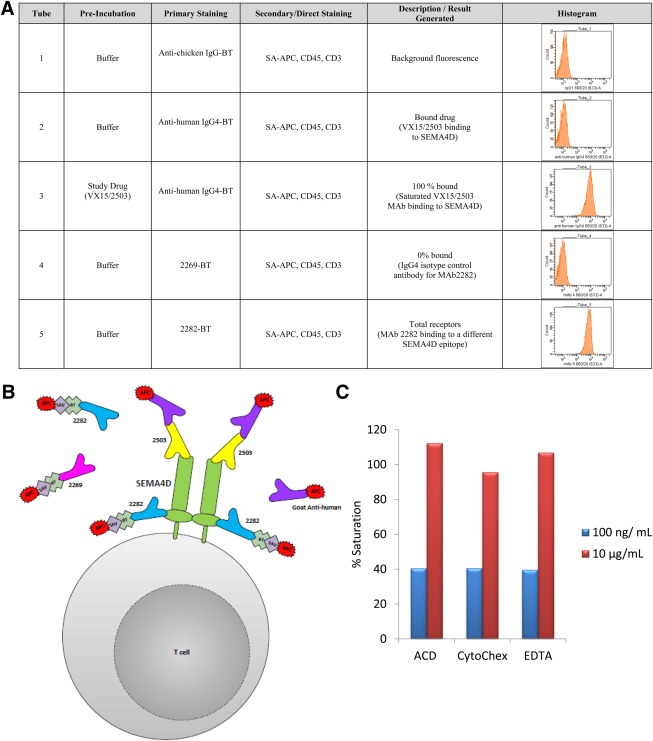
cSEMA4D Saturation Assay. The cSEMA4D Saturation Assay is a three‐color, five‐tube, wash/lyse/wash flow cytometric assay designed to evaluate the level of SEMA4D and SEMA4D saturation by VX15/2503 on peripheral T cells. A: Panel Configuration. B: Schematic Depiction. C: Percent Saturation in Different Anticoagulants. Peripheral whole blood was collected in EDTA, ACD, and CytoChex BCT blood collection tubes. Samples were spiked with samples spiked ex vivo with 10 µg/mL and 100 ng/mL of VX15/2503.
The three reportable results generated are: 1 the percent saturation of SEMA4D on peripheral T cells; 2 the fold over isotype (FOI) for VX15/2503; and 3 FOI for mAb 2282‐biotin. The geometric mean fluorescence intensity (GMFI) derived from the SA‐APC FL4 for each tube was used to calculate percent saturation and FOI using the following equations:
where:
A = FL4 GMFI generated from assay panel Tube 1 which represents the background signal.
B = FL4 GMFI generated from assay panel Tube 2 which represents the unknown sample signal.
C = FL4 GMFI generated from assay panel Tube 3 which represents the VX15/2503 mAb saturated (maximum) signal.
D = FL4 GMFI generated from assay panel Tube 4 which represents the mAb 2269 isotype.
E = FL4 GMFI generated from assay panel Tube 5 which represents the mAb 2282 saturated (maximum) signal.
The assay was capable of detecting differences in receptor saturation in whole blood “dosed” ex vivo with varying levels of VX15/2503. Assay performance was comparable when the whole blood was collected in a variety of anticoagulants. (Fig. 1C).
Analytical Method Validation. Samples spiked ex vivo with VX15/2503 were stable for up to 3 days in either EDTA held under refrigerated conditions or in ACD or CytoChex BCT held at ambient temperature (Fig. 2). CytoChex BCT was selected for further validation and clinical specimens, given that the variability up to day three was slightly less in CytoChex BCT (4.24%CV) compared to ACD (5.98%CV). The use of refrigerated samples in EDTA was not implemented due to logistical considerations and the expense of shipping refrigerated specimens. The mean intra‐assay imprecision for the samples spiked with low, mid, and high levels of VX15/2503 was 5.44%CV (range of 3.84 to 7.24%CV) (Table 1). The mean intra‐assay imprecision values for VX15/2503 FOI and for mAb 2282‐biotin FOI were 7.35%CV (range of 2.27 to 10.1%CV) and 4.85%CV (range of 2.71 to 7.30%CV), respectively. This % CV is acceptable, and please note that differences in absolute values of FOI may differ among donors due to different levels of surface expression of SEMA4D.
Figure 2.
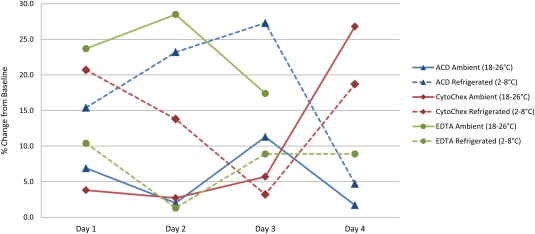
Stability evaluation.
Whole blood was collected in EDTA, ACD, and CytoChex BCT blood collection tubes and spiked ex vivo with 100 ng/mL of VX15/2503. Samples were assayed on the day of collection and spiking (baseline) and at 24 h, 48 h, 72 h, and 96 h. Samples were maintained under two conditions: ambient temperature (18–22°C) or refrigerated (4°C). The percent change from baseline at each time point and condition was calculated with the following formula:
. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table 1.
SEMA4D Saturation Assay—Intra‐assay Imprecision.
| Reportable | Lowa | Midb | Highc | Mean | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Result | Mean | SD | %CV | Mean | SD | %CV | Mean | SD | %CV | %CV |
| Percent Saturation | 11.0 | 0.578 | 5.24 | 36.3 | 1.40 | 3.84 | 98.2 | 7.11 | 7.24 | 5.44 |
| VX15/2503 FOI | 203 | 19.7 | 9.68 | 246 | 5.60 | 2.27 | 34.2 | 3.45 | 10.1 | 7.35 |
| mAb 2282‐biotin FOI | 96.7 | 4.40 | 4.55 | 112 | 3.04 | 2.71 | 22.6 | 1.65 | 7.30 | 4.85 |
Whole blood collected from three apparently healthy volunteers was dosed ex vivo with varying concentrations of VX15/2503. Samples were then assayed in triplicate in a single analytical run.
Donor 3 spiked with approximately 10 ng/mL VX15/2503.
Donor 2 spiked with approximately 50 ng/mL VX15/2503.
Donor 1 spiked with approximately 10 μg/mL VX15/2503.
Inter‐assay imprecision was assessed using commercially available preserved whole blood material, Immuno‐Trol Cells and Immuno‐Trol Low Cells (Table 2). Given that control material was not pre‐spiked with VX15/2503, it was not possible to evaluate the percent saturation of SEMA4D on peripheral T cells; however, the FOI for mAb 2282‐biotin was evaluated. In addition, the APC GMFI from Tubes 2–4 was evaluated; the mean inter‐assay imprecision values were 18.0%CV (range of 13.6 to 22.3%CV) for mAb 2282‐biotin FOI, 10%CV (range of 9.84 to 10.2%CV) for the APC GMFI of Tube 2, 15.1%CV (range of 14.4 to 15.9%CV) for the APC GMFI of Tube 3, and 9.43%CV (range of 9.38 to 9.47% CV) for the APC GMFI of Tube 4. For the APC GMFI of Tube 5, the grand mean inter‐assay imprecision was 11.8%CV (range of 8.50 to 15.2%CV).
Table 2.
SEMA4D Saturation Assay — Inter‐assay Imprecision
| Population | %CV | ||
|---|---|---|---|
| Immuno‐Trol | Immuno‐Trol Low | Grand Mean %CV | |
| mAb 2282‐biotin FOI | 22.3 | 13.6 | 18.0 |
| GMFI Tube 2 | 9.84 | 10.2 | 10.0 |
| GMFI Tube 3 | 14.4 | 15.9 | 15.1 |
| GMFI Tube 4 | 9.47 | 9.38 | 9.43 |
| GMFI Tube 5 | 15.2 | 8.50 | 11.8 |
Inter‐Instrument variability was evaluated using the inter‐assay imprecision experiment (Table 3). For the APC GMFI of Tube 2, the inter‐instrument imprecision was 5.93%CV for QCL and 10.5%CV for QCN. Similarly, the inter‐instrument precision for the APC GMFI of Tube 3 was 0.388%CV for QCL and 3.67%CV for QCN. For the APC GMFI of Tube 4, the inter‐instrument precision was 4.04%CV for QCL and 11.1%CV for QCN. For the APC GMFI of Tube 5, the grand mean inter‐assay imprecision was 4.99%CV for QCL and 4.03%CV for QCN. These results indicated comparable assay performance between two instruments.
Table 3.
SEMA4D Saturation Assay — Inter‐instrument Imprecision
| Inst. 448 | Inst. 544 | Mean | SD | %CV | |
|---|---|---|---|---|---|
| Immuno‐Trol Low | |||||
| GMFI Tube 2 | 74.8 | 68.8 | 71.8 | 4.26 | 5.93 |
| GMFI Tube 3 | 4,092 | 4,115 | 4,103 | 15.9 | 0.388 |
| GMFI Tube 4 | 66.9 | 63.2 | 65.1 | 2.63 | 4.04 |
| GMFI Tube 5 | 4,913 | 5,273 | 5,093 | 254 | 4.99 |
| Immuno‐Trol | |||||
| GMFI Tube 2 | 74.5 | 64.2 | 69.4 | 7.32 | 10.5 |
| GMFI Tube 3 | 4,108 | 4,327 | 4,218 | 155 | 3.67 |
| GMFI Tube 4 | 68.6 | 58.6 | 63.6 | 7.06 | 11.1 |
| GMFI Tube 5 | 5,261 | 4,970 | 5,116 | 206 | 4.03 |
Performance Monitoring
In order to assess longitudinal assay performance, two levels of quality control samples (Immuno‐Trol & Immuno‐Trol Low) were included in each analytical run. Mean values, SD and %CV ranges were initially established during method validation from the inter‐assay imprecision data (repeat testing over a course of multiple days, instruments, and technicians). Mean values for each additional new lot of QC were determined and a fixed %CV applied. QC results were evaluated for acceptability and monitored for trending using BioRad Unity™ Real Time® 2.0 Statistical Package, Westgard Statistical Process Control (SPC) Rules, and Westgard Advisor™ 22.
As described in the discussion of the inter‐assay imprecision evaluation, the QC material was not pre‐spiked with VX15/2503, thus it was not possible to evaluate the percent saturation of SEMA4D on peripheral T cells during validation in monitoring assay performance in study. However, all other appropriate primary stains were added to the QC tubes and the APC GMFI for tubes 2, 3, 4, and 5 were monitored. The assay performance was within 1SD of the established mean throughout the life of the lot number of the QC (February – April 2014) (Fig. 3).
Figure 3.
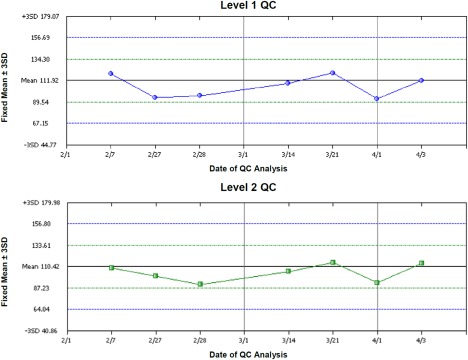
cSEMA4D saturation assay longitudinal performance.
QC performance (Level 1 Immunol Trol Low, Level 2 Immunol Trol Normal) of the APC GMFI of Tube 2 on each date of testing. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Clinical Utility
The cSEMA4D saturation assay provided saturation and ligand expression data which aided in evaluation of patients enrolled in the oncology study. Patients in the first two dose cohorts were initially treated on day −14, followed for two weeks, and then dosed again on Day 0 and weekly thereafter. Cellular SEMA4D expression levels and cSEMA4D saturation were evaluated (Fig. 4). Even at the lowest dose level (0.3 mg/kg) all patients showed complete saturation following the initial dose at day −14, and the cSEMA4D saturation level declined during the two week safety evaluation period prior to the second dose (Fig. 4A). This was expected based on the dose delivered and level of drug achieved in the serum. When the level of drug in the serum fell below approximately 0.1 to 0.3 µg/mL, complete T cell saturation was lost (Patnaik, A, et al. manuscript submitted, Clinical Cancer Research). Patients were dosed weekly thereafter and saturation results demonstrated that the assay was capable of detecting intermediate levels of T cell SEMA4D saturation as well as complete ligand desaturation. To note, in Figure 4A, patients #1 and 3 received their last dose on Day 29 and patient #2 also received doses on days 36 and 43. Analysis of the level of cSEMA4D on the surface of the T cells (Fig. 4B) demonstrated that the level of SEMA4D expressed on the cell surface decreased upon cellular saturation. Levels returned to near‐normal after cells became completely unsaturated, as can be seen by the rise in cSEMA4D expression prior to the second dose on Day 1. Clinical data was also obtained using serum samples collected from subjects enrolled in the placebo controlled, single‐ascending dose MS study (Fig. 5). Saturation (Fig. 5A and 5C) and cellular expression (Fig. 5B and 5D) data from the 1 mg/kg dose cohort is shown. As shown, saturation levels drop off sooner in patient #8 who is also the patient who had the highest level of anti‐drug antibodies. Overall, the clinical data demonstrate the fidelity, sensitivity, and significance of the clinical saturation assay.
Figure 4.
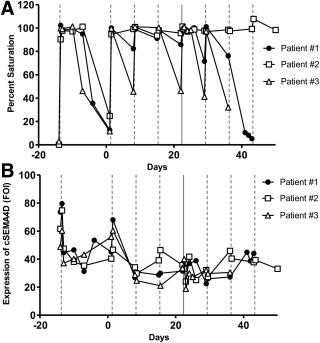
SEMA4D saturation in oncology clinical specimens. Patients from a select cohort in the Phase 1 Oncology trial were scheduled to be dosed with VX15/2503 at 0.3 mg/kg on Day −14, Day 0, and weekly thereafter (dotted lines). To note, patients #1 and 3 received their last dose on Day 29 and patient #2 also received doses on days 36 and 43. Blood was collected for cellular saturation analysis at several time points following the first dose and also PRE and EOI following each of the weekly doses. (A) Percent saturation values for three patients in the cohort (≤ 20% represent cells that are unsaturated and values ≥80% represent cells that are fully saturated). (B) Cellular levels of SEMA4D expressed as fold expression of MAb 2282 binding over isotype.
Figure 5.
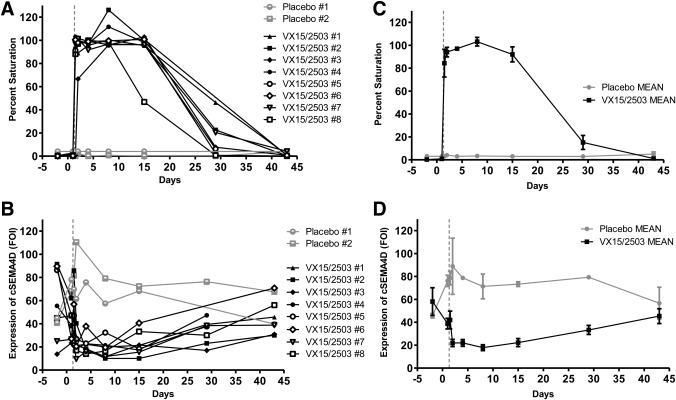
SEMA4D saturation in MS clinical specimens. Patients from a select cohort in the Phase 1 MS trial were dosed with VX15/2503 at 1 mg/kg (n = 8) or placebo (n = 2) on Day 0, and blood was collected for cellular saturation analysis at several time points through Day 45. Percent saturation values are shown for individual patients (A) and the mean of the VX15/2503 or placebo groups (B). Cellular levels of SEMA4D, expressed as fold expression of MAb 2282 binding over isotype, is also shown for individuals (C) and the mean of the VX15/2503 or placebo groups (D). Error bars in the mean graphs represent +/‐ SEM.
DISCUSSION
SEMA4D is a multifunctional molecule with at least three known receptors, high affinity plexin‐B1, intermediate affinity plexin‐B2 and low affinity CD72 3. The blocking human IgG4 monoclonal antibody VX15/2503 is being developed in a number of different indications. A Phase 1 trial has been successfully completed in solid tumor patients (Patnaik A, et al. submitted Clin Can Res; Clintrials.gov identifier NCT01313065), and a second single‐ascending dose Phase 1 trial was completed in MS patients (Clintrials.gov identifier NCT01764737). A Phase 2 study in Huntington's Disease has been initiated based on preclinical data obtained using the YAC‐128 mouse model 17. Much is known about different potential mechanisms of action in these indications, but, in all cases, SEMA4D is blocked from binding to any of its receptors, inhibiting downstream physiological effects. The SEMA4D Saturation Assay described herein is a real‐time flow cytometric method to measure the extent of this important initial blocking step.
Like many biomarker assays, the cSEMA4D Saturation Assay was developed in parallel with VX15/2503 throughout the lifecycle of the compound from the drug discovery phase to the pre‐clinical toxicology phase to clinical testing 23. (Fig. 6) The fit‐for‐purpose, iterative approach was applied to the validation strategy in that, as the intended use of the data and the associated regulatory requirements changed, the assay was re‐optimized and re‐validated appropriately 24, 25, 26. The prototype assay was developed during the drug discovery phase and initially used to detect binding of anti‐SEMA4D candidates to cellular SEMA4D. During the evaluation of in‐vivo murine models for both oncology and neurology applications, the cSEMA4D Saturation Assay provided proof‐of‐concept data confirming that the compound was binding as expected to murine splenocytes. For the murine models a mouse anti‐mouse SEMA4D antibody (MAb 67–2) variant of the drug was used. VX15/2503 is a humanized version of 67–2 and both antibodies have very similar binding and functional characteristics (Fisher et al. manuscript accepted, mAbs). The assay was then further optimized and appropriately validated to support formal GLP toxicology studies in rats and cynomolgus monkeys (using VX15/2503). Given that the toxicology studies required serial sampling, the assay was adapted to a whole blood matrix rather than splenocytes. For the primate studies, assay tubes 4 and 5, which contain an additional isotype and mAb 2282, respectively, were incorporated in the panel. MAb 2282 is another human anti SEMA4D antibody that recognizes primate SEMA4D even in the presence of bound VX15/2503 and thus provides the measurement for total cell‐associated receptor. This reagent has the advantage of not being affected by anti‐SEMA4D drug levels and can serve as an independent measure of antigen levels. Next a clinical assay based on the primate assay was developed to support the first‐in‐man trials. This assay incorporated process improvements and more sensitive secondary reagents. Finally, the initial clinical assay was optimized and validated once more to make additional improvements to the process, and this final process is the one described here in the M&M. Changes were made which allowed for improved assay performance yet allowed for a comparison of data generated with each method. These improvements included changing to a GMP‐grade SA‐APC as lot‐to‐lot variability was observed in the non‐GMP ‐grade reagent from another vendor. In the first iteration of the clinical assay, whole blood was pre‐lysed in an ammonium chloride solution; there were concerns that this step was too operator dependent and thus might introduce variability in the final data set. In the next generation of the assay, whole blood was first washed to remove free drug and serum Ig prior to staining and finally a more robust lyse‐fixation step was introduced. QC tubes (Immuno‐trol) were added and CD45 staining was added to the final assay to further increase specificity as well as the time and debris gating strategy. The over‐all assay configuration and calculations were not modified so that data generated in both versions of the assay could be compared.
Figure 6.
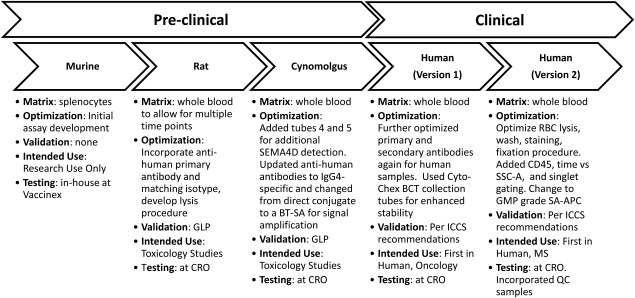
Progression of the cSEMA4D saturation assay from discovery through clinical. Highlighted list of assay modifications and improvements as the assay transitioned from preclinical to clinical development.
Flow cytometry, the premier technology for single cell analysis, is ideal for receptor occupancy measurements as it allows for multiparametric analysis. Flow cytometry based saturation assays using whole blood provide a quick turn‐around time for data (a few days), compared to traditional PK batch testing in frozen samples (up to a few months). Thus insights into PK trends can be gleaned prior to availability of the drug plasma PK data. Another advantage in using whole blood is the lack of manipulation as compared to frozen serum or plasma. In this case, SEMA4D is expressed in both cellular and soluble forms 8, 9 and in assessing the level of target saturation, it is possible we could have chosen either form to analyze. The use of fresh whole blood and flow cytometry avoids ELISA‐based issues with frozen samples, including dilutional or serum‐mediated disruption of MAb‐ligand equilibrium kinetics, which can result in false positives and negatives 27. Flow cytometry also allows one to gate on specific populations when analyzing saturation, and allowed this assay to focus on T cells, which is the immune cell type in peripheral blood that expresses the highest level of SEMA4D.
The assay has performed well in two clinical trials. The information provided by the saturation assay has contributed to the VX15/2503: SEMA4D data package including target biology, PD, and PK data that helped select dosing schedules for future trials. For example, it was determined employing this assay that cellular SEMA4D expression levels decrease after dosing. This pattern of expression confirmed preclinical data showing that VX15/2503 binding to cellular SEMA4D induced internalization of the complex (Fisher et al. manuscript manuscript accepted, mAbs). Also, the saturation data was critical to correlate clinical PK and PD results, demonstrating that approximately 0.1 to 0.3 µg/mL VX15/2503 was the concentration of drug in serum below which complete T cell saturation was lost (Patnaik A, et al. manuscript accepted, Clinical Cancer Research). In pre‐clinical toxicology studies, the approximate concentration of drug in serum at which complete saturation was lost was 1 to 5 µg/mL 28. It is possible this difference from the clinical saturation threshold could be due to slightly different binding kinetics in humans vs. rats/monkeys, allometric scaling factors, the unavailability of appropriate samples needed at frequent time points to determine an accurate threshold, or slight differences in the clinical vs. non‐clinical PK assays. This assay has also helped to provide real‐time data regarding drug exposure; for example patient #3 in Figure 4 and patient #8 in Figure 5 demonstrated decreases in saturation earlier than other patients in their respective cohorts, and these were the same two patients that demonstrated the higher titer anti‐drug antibody responses (data not shown), indicating that the saturation assay is sensitive to immunogenic responses. In addition, the data from this assay has also supplied critical safety information. In the Phase 1 oncology trial, a patient in the 1 mg/kg cohort had an infusion reaction following their second thru fourth injections of VX15/2503. The patient was treated after each infusion to control the reaction but it was unknown whether the patient was indeed clearing the drug due to an anti‐drug response, in which case it would be logical to remove the patient from the study due to lack of exposure to drug. The saturation assay showed in real‐time that the patient was becoming unsaturated between doses, and following the last infusion, even immediately after the dose was administered. The patient was appropriately removed, in part because the saturation data indicated that the patient was not being effectively exposed to test article due to a likely immunogenic response. This was confirmed later with the batched PK and immunogenicity data.
This receptor occupancy assay has served to provide valuable information throughout the development of VX15/2503. It has demonstrated that flow‐ based assays can be validated and controlled in a manner suitable for clinical data collection and in some cases provide advantages over more traditional assay platforms. As immune‐based targets and monoclonal antibody therapeutics continue to expand, successful validation and control of these flow‐based assays will become increasingly important.
ACKNOWLEDGMENTS
The assistance of Ms. Sarah Livingston in figure preparation is gratefully acknowledged. The authors have no conflict of interest to declare.
How to cite this article: Fisher, TL , Seils, J , Reilly, C. , Litwin, V , Green, L , Salkowitz-Bokal, J , Walsh, R , Harville, S , Leonard, JE , Smith, E and Zauderer, M. Saturation Monitoring of VX15/2503, a Novel Semaphorin 4D‐Specific Antibody, in Clinical Trials. Cytometry Part B 2016; 90B: 199–208.
LITERATURE CITED
- 1. Giraudon P, Vincent P, Vuaillat C, Verlaeten O, Cartier L, Marie‐Cardine A, Mutin M, Bensussan A, Belin MF, Boumsell. L. Semaphorin CD100 from activated T lymphocytes induces process extension collapse in oligodendrocytes and death of immature neural cells. J Immunol 2004;172:1246–1255. [DOI] [PubMed] [Google Scholar]
- 2. Giraudon P, Vincent P, Vuaillat. C. Tcells in neuronal injury and repair: Semaphorins and related T‐cell signals. Neuromol Med 2005;7:207–216. [DOI] [PubMed] [Google Scholar]
- 3. Worzfeld T, Offermanns S. Semaphorins and plexins as therapeutic targets. Nat Rev Drug Discov 2014;13:603–621. [DOI] [PubMed] [Google Scholar]
- 4. Mendes‐da‐Cruz DA, Lepelletier Y, Brignier AC, Smaniotto S, Renand A, Milpied P, Dardenne M, Hermine O, Savino W. Neuropilins, semaphorins, and their role in thymocyte development. Ann N Y Acad Sci 2009;1153:20–28. [DOI] [PubMed] [Google Scholar]
- 5. Takamatsu H, Takegahara N, Nakagawa Y, Tomura M, Taniguchi M, Friedel RH, Rayburn H, Tessier‐Lavigne M, Yoshida Y, Okuno T, et al. Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat Immunol 2010;11:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delaire S, Billard C, Tordjman R, Chedotal A, Elhabazi A, Bensussan A, Boumsell. L. Biological activity of soluble CD100. II. Soluble CD100, similarly to H‐SemaIII, inhibits immune cell migration. J Immunol 2001;166:4348–4354. [DOI] [PubMed] [Google Scholar]
- 7. Hall KT, Boumsell L, Schultze JL, Boussiotis VA, Dorfm M, Cardoso AA, Bensussan A, Nadler LM, Freeman. GJ. Human CD100, a novel leukocyte semaphorin that promotes B‐cell aggregation and differentiation. Proc Natl Acad Sci USA 1996;93:11780–11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kikutani H, Kumanogoh. A. Semaphorins in interactions between T cells and antigen‐presenting cells. Nat Rev Immunol 2003;3:159–167. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki K, Kumanogoh A, Kikutani. H. Semaphorins and their receptors in immune cell interactions. Nat Immunol 2008;9:17–23. [DOI] [PubMed] [Google Scholar]
- 10. Campos M, Campos SGDE, Ribeiro GG, Eguchi FC, Silva SR, Oliveira CZDE, Costa AMDA, Curcelli EC, Nunes MC, Penna V, et al. Ki‐67 and CD100 immunohistochemical expression is associated with local recurrence and poor prognosis in soft tissue sarcomas, respectively. Oncol Lett 2013;5:1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ch'ng ES, Kumanogoh A. Roles of Sema4D and Plexin‐B1 in tumor progression. Mol Cancer 2010;9:251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kato S, Kubota K, Shimamura T, Shinohara Y, Kobayashi N, Watanabe S, Yoneda M, Inamori M, Nakamura F, Ishiguro H, et al. Semaphorin 4D, a lymphocyte semaphorin, enhances tumor cell motility through binding its receptor, plexinB1, in pancreatic cancer. Cancer Sci. 2011;102:2029–2037. [DOI] [PubMed] [Google Scholar]
- 13. Evans EE, Jonason AS, Jr ., Bussler H, Torno S, Veeraraghavan J, Reilly C, Doherty MA, Seils J, Winter LA, Mallow C, et al. Antibody blockade of semaphorin 4D promotes immune infiltration into tumor and enhances response to other immunomodulatory therapies. Cancer Immunol Res 2015;3:689–701. [DOI] [PubMed] [Google Scholar]
- 14. Kumanogoh A, Suzuki K, Ch'ng E, Watanabe C, Marukawa S, Takegahara N, Ishida I, Sato T, Habu S, Yoshida K, et al. Requirement for the lymphocyte semaphorin, CD100, in the induction of antigen‐specific T cells and the maturation of dendritic cells. J Immunol 2002;169:1175–1181. [DOI] [PubMed] [Google Scholar]
- 15. Okuno T, Nakatsuji Y, Moriya M, Takamatsu H, Nojima S, Takegahara N, Toyofuku T, Nakagawa Y, Kang S, Friedel RH, et al. Roles of Sema4D‐Plexin‐B1 Interactions in the Central Nervous System for Pathogenesis of Experimental Autoimmune Encephalomyelitis. J Immunol 2010;184:1499–1506. [DOI] [PubMed] [Google Scholar]
- 16. Smith ES, Jonason AJ, Reilly C, Veeraraghavan J, Fisher T, Doherty M, Klimatcheva E, Mallow C, Cornelius C, Leonard JE, et al. SEMA4D compromises blood‐brain barrier, activates microglia, and inhibits remyelination in neurodegenerative disease. Neurobiol Dis 2014;73:254–268. [DOI] [PubMed] [Google Scholar]
- 17. Southwell AL, Franciosi S, Villanueva EB, Xie Y, Winter LA, Veeraraghavan J, Jonason A, Felczak B, Zhang W, Kovalik V, et al. Anti‐semaphorin 4D immunotherapy ameliorates neuropathology and some cognitive impairment in the YAC128 mouse model of Huntington disease. Neurobiol Dis 2015;76:46–56. [DOI] [PubMed] [Google Scholar]
- 18. Nkyimbeng‐Takwi E, Chapoval. SP. Biology and function of neuroimmune semaphorins 4A and 4D. Immunol Res 2011;50:10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Liu B, Ma Y, Jin. B. Sema 4D/CD100‐plexin B is a multifunctional counter‐receptor. Cell Mol Immunol 2012;10:97–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoffman RA, Wang L, Bigos M, Nolan. JP. NIST/ISAC standardization study: variability in assignment of intensity values to fluorescence standard beads and in cross calibration of standard beads to hard dyed beads. Cytometry A 2012;81:785–796. [DOI] [PubMed] [Google Scholar]
- 21. Meinelt E, Reunanen M, Edinger M, Jaimes M, Stall A, Sasaki D, Trotter J. Standardizing application setup across multiple flow cytometers using BD FACVSDiva version 6 software. BD Biosci Techn Bull 2012:1–16. [Google Scholar]
- 22. Westgard JO, Quam EF, Barry PL, Ehrmeyer SS. Basic Method Validation: Training in Analytical Quality Management for Healthcare Laboratories, 2nd ed. Westgard Quality Corporation, Madison, WI, 2003.
- 23. Litwin V, Andahazy J. Monitoring the Cellular components of the Immune System during Clinical Trials ‐ A translational medicine approach In: Litwin V, Marder P, editors. Flow Cytometry in Drug Discovery and Development. New Jersey: Wiley‐Blackwell, John Wiley & Sons, Inc;. 2010. pp 189–204. [Google Scholar]
- 24. Lee JW, Weiner RS, Sailstad JM, Bowsher RR, Knuth DW, O'Brien PJ, Fourcroy JL, Dixit R, Pandite L, Pietrusko RG, et al. Method validation and measurement of biomarkers in nonclinical and clinical samples in drug development: a conference report. Pharm Res 2005;22:499–511. [DOI] [PubMed] [Google Scholar]
- 25. Lee JW, Devanarayan V, Barrett YC, Weiner R, Allinson J, Fountain S, Keller S, Weinryb I, Green M, Duan L, Rogers JA, Millham R, et al. Fit‐for‐purpose method development and validation for successful biomarker measurement. Pharm Res 2006;23:312–328. [DOI] [PubMed] [Google Scholar]
- 26. O'Hara DM, Xu Y, Liang Z, Reddy MP, Wu DY, Litwin V. Recommendations for the validation of flow cytometric testing during drug development: II assays. J Immunol Methods 2011;363:120–134. [DOI] [PubMed] [Google Scholar]
- 27. Nilsson LB. The bioanalytical challenge of determining unbound concentration and protein binding for drugs. Bioanalysis 2013;5:3033–3050. [DOI] [PubMed] [Google Scholar]
- 28. Leonard JE, Fisher TL, Winter LA, Cornelius CA, Reilly C, Smith ES, Zauderer M. Nonclinical safety evaluation of VX15/2503, a humanized IgG4 anti‐SEMA4D antibody. Mol Cancer Ther 2015;14:964–972. [DOI] [PubMed] [Google Scholar]


