Abstract
Background
Receptor occupancy (RO) assays provide a means to measure the direct interaction of therapeutics with their cell surface targets. Free receptor assays quantify cell‐surface receptors not bound by a therapeutic while total receptor assays quantify the amount of target on the cell surface.
Methods
We developed both a flow cytometry‐based free RO assay to detect free surface CXCR4, and a total surface CXCR4 assay. In an effort to evaluate potential displacement interference, we performed in vitro experiments to compare on‐cell affinity with the IC50 values from in vitro and in vivo from the free CXCR4 assay. We determined free and total surface CXCR4 on circulating blood cells in cynomolgus monkeys dosed with MEDI3185, a fully human monoclonal antibody to CXCR4.
Results
We devised an approach to evaluate displacement interference during assay development and showed that our free assay demonstrated little to no displacement interference. After dosing cynomolgus monkeys with MEDI3185, we observed dose‐dependence in the magnitude and duration of receptor occupancy and found CXCR4 to increase on lymphocytes, monocytes, and granulocytes. In a multiple dose study, we observed time points where surface CXCR4 appeared fully occupied but MEDI3185 was not detectable in serum. These paradoxical results represented a type of assay interference, and by comparing pharmacokinetic, ADA and total CXCR4 results, the most likely reason for the free CXCR4 results was the emergence of neutralizing anti‐drug antibodies (ADA). The total CXCR4 assay was unaffected by ADA and provided a reliable marker of target modulation in both in vivo studies. © 2015 The Authors Cytometry Part B: Clinical Cytometry Published byWiley Periodicals, Inc.
Keywords: receptor occupancy, pharmacodynamic biomarker, biopharmaceutical, flow cytometry, antidrug antibodies, antitherapeutic antibodies, MEDI3185
Throughout the preclinical and clinical development of biotherapeutics, the establishment of quantitative PK/PD relationships helps to both select candidates based on pharmacokinetic (PK) and pharmacodynamic (PD) profiles and to design clinical studies with optimal dosing 1. A useful PD biomarker should be quantitative and represent aspects of the mechanism of action. Furthermore, accurate quantitative pharmacodynamic biomarkers are often necessary to evaluate the true in vivo potency of a therapeutic. Inaccurate measurements can lead to either over‐ or underestimation of potency, and in cases where pharmacodynamic biomarkers are used for human dose selection, inaccurate data can lead to over‐ or underdosing of patients. This may result respectively in either unnecessary high exposure possibly causing toxic effects or sub‐efficacious dosing.
Many biotherapeutics function as antagonists that bind to and inhibit cell surface molecules. In such a case, and, in cases where the biological effect of agonists is not easily measurable, target occupancy can provide quantitative evidence of target binding. Target occupancy is often determined using peripheral blood specimens as many targets are present on the surface of circulating blood cells and blood collections are relatively noninvasive. Studies that evaluate receptor occupancy in whole blood have been reported for CD862, β7 3, 4, CD14 5, IgF‐1 receptor 6, and IL21‐receptor 7. Some of these studies were performed with peripheral blood mononuclear cells (PBMCs), and it is likely that drug bound to the blood cells partially dissociates during PBMC preparation, leading to underestimation of drug binding. However, even in whole blood, receptor occupancy can be underestimated since the detection reagent is able to displace bound biotherapeutic during the staining procedure. This type of assay interference would be exacerbated by long incubation times and high concentration of detection reagent 8 analogous to bioanalytical assays that detect free soluble targets or biotherapeutics 9. Hence assay design is critical to obtain accurate receptor occupancy data that is unaffected by sample preparation or the assay procedure itself. Another type of possible assay interference is caused by antibodies against the therapeutic that can emerge after a single or several doses. This type of interference most commonly occurs when the detection reagent is the labeled biotherapeutic and emerging anti‐drug antibodies (ADA) 10 specifically bind the detection reagent resulting in either inhibition or crosslinking of the detection reagent 8. Validation guidelines for flow cytometric assays are beginning to be proposed 11, 12; however, the specific aspects of RO assays are not yet considered in these guidelines. Recently, considerations for RO assay development, sample testing, and data interpretation were published 13.
In this manuscript, we describe the development of a receptor occupancy assay and an approach to monitor displacement interference. CXCR4 is broadly expressed in cynomolgus blood and surface expression increases after dosing with MEDI3185, while free CXCR4 is fully occupied at doses of 0.1 mg/kg MEDI3185 and higher. MEDI3185 14 is a fully human, monoclonal IgG1 antibody to CXCR4 that bears three amino acid mutations in the FC region, designated as a “triple mutant” (TM) molecule, resulting in the ablation of antibody‐dependent cell‐mediated cytotoxicity and complement‐dependent cytotoxicity 15. Furthermore, we show how to identify and interpret assay interference that emerged during a preclinical study.
METHODS
CXCR4 Expression in Cynomolgus Monkey Blood
To determine the expression level of CXCR4 on lymphocytes, granulocytes, monocytes and T‐cell subsets, cynomolgus monkey blood was incubated with CD3‐PerCP (clone SP34‐2, BD552851), CD4‐FITC (clone L200, BD550628), CD8‐APC‐Cy7 (clone RPA‐T8, BD560662), CD95‐BV421 (clone DX2, BD562616), CD28‐BV510 (clone CD28.2, BD563075) per the manufacturers recommendations and 2.5 µg/ml MEDI3185‐AlexaFluor647 for 30 minutes at room temperature. Two milliliters of BD Pharm Lyse was added to lyse red blood cells, followed by washing with BD Cell Wash (BD Biosciences, San Jose, CA) and resuspended in 0.5 ml of BD Cell Wash. Samples were analyzed immediately on a BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA)
Measurement of Receptor Occupancy
Hundred microliters of cynomolgus monkey whole blood was incubated with 2.5 μg/ml AlexaFluor647‐labeled MEDI3185 and 1.5 μg/ml Pacific Blue‐labeled anti‐CD3 antibody (clone SP34‐2, BD Biosciences). Total CXCR4 was detected with 5 μg/ml Phycoerythrin‐labeled anti‐CD184 (CXCR4) (clone 1D9, BD Biosciences). The mixture was incubated for 1 hour at 2°C–8°C. In parallel, all blood samples were incubated with an isotype control antibody for MEDI3185‐AlexaFluor647 (IgG1TM‐AlexaFluor647) or an isotype control for clone 1D9‐PE (Phycoerythrin‐labeled‐rat IgG2a κ). 2 mL of BD Pharm Lyse was added to lyse red blood cells, followed by washing with BD Cell Wash and resuspension in 0.5 ml of BD Cell Wash. Fixation was performed with 2% paraformaldehyde and stored for up to 4 hours prior to analysis on a BD FACSCanto II flow cytometer. Lymphocytes, monocytes, and granulocytes were gated based on forward scatter (FSC) and side scatter (SSC) characteristics. In animal studies, the geometric mean of the CXCR4 signal was used to quantify staining signals and isotype control geometric mean values were subtracted from the specific staining geometric mean at each time point to derive the specific geometric mean signal. The assay was validated for precision and samples were stable for 48 hours (± 30%) regarding CXCR4 measurement on CD3+ cells. The baseline was calculated from the mean of multiple measurements before the first dose.
The following equation was used for normalization:
Animal Studies
Studies using Cynomolgus monkeys (Macaca fascicularis) were conducted at Covance Laboratories GmbH, Germany and SNBL USA Ltd according to protocols reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the testing facility and in compliance with the applicable national laws and regulations concerning the humane care and use of laboratory animals and the AstraZeneca Animal Welfare and Bioethics policies. Naïve cynomolgus monkeys were obtained from certified suppliers, group housed, allowed access to water ad libitum and fed a pelleted diet for monkeys supplemented with fresh fruit, vegetables, bread, etc.
Single Dose Study in Monkeys
The pharmacokinetics (PK) and pharmacodynamics (PD) of MEDI3185 after single administration were studied in cynomolgus monkeys as part of a non‐GLP study. Twelve male cynomolgus monkeys were randomized into 4 groups and received single intravenous (bolus) doses of MEDI3185 at dose levels of 0 (vehicle control; 25 mM histidine, 7% sucrose, 0.02% polysorbate 80, pH 5.9), 0.01, 0.1, and 1 mg/kg at a dose volume of 0.5 ml/kg.
Blood samples for preparation of serum and measurement of MEDI3185 were collected from all animals into plain tubes. Blood samples for measurement of receptor occupancy and total CXCR4 were collected from all animals into tubes containing K2EDTA, stored/shipped at 2°C–8°C and analyzed within 2 days of collection.
Repeat Dose Study in Monkeys
The toxicokinetics, immunogenicity, and receptor occupancy of MEDI3185 were studied in cynomolgus monkeys as part of a GLP toxicity study. Eighteen male and eighteen female cynomolgus monkeys were randomized into four groups and received weekly intravenous doses of MEDI3185 at dose levels of 0 (vehicle control; 25 mM histidine, 240 mM trehalose, 0.04% polysorbate 80, pH 6.2), 15, 50 and 150 mg/kg for 13 weeks (14 doses in total). The doses were administered by intravenous infusion over 30 minutes at a dose volume of 10 ml/kg except for one animal in the 15 mg/kg dose group which exhibited an infusion reaction after the 12th weekly dose. In this animal, the last two doses were infused over 60 minutes without incident. Each group consisted of five males and five females except for Group 3 (50 mg/kg dose), which consisted of three males and three females. The main study animals (three males and three females per group) were sacrificed at the end of the 13 week treatment period and additional animals (two males and two females per group) which had been dosed with 0, 15 and 150 mg/kg for 13 weeks were retained for a 12 week treatment‐free period to assess reversibility of any MEDI3185 mediated effects. Hence, scheduled necropsies were conducted at the end of the 13 week treatment period (three days after the final dose) or at the end of the 12 week treatment free period. Blood samples for preparation of serum for measurement of MEDI3185 and measurement of anti‐drug antibodies (ADA) were collected from all animals into plain tubes. Blood samples for measurement of receptor occupancy and total CXCR4 were collected from all animals into tubes containing EDTA. Samples were analyzed on the day of sampling.
Measurement of MEDI3185 in Cynomolgus Monkey Serum
Serum for measurement of MEDI3185 was stored at ≤ −70°C prior to analysis. MEDI3185 in serum obtained from the repeat dose cynomolgus monkey study was measured with a validated sandwich electrochemiluminescent (ECL) immunoassay using an MSD detection platform (MesoScale Discovery, Gaithersburg, MD). Prediluted standards, controls, and test samples were incubated with biotinylated anti‐MEDI3185 antibody, which had been immobilized on streptavidin‐coated microtiter plates. Following a wash step, MEDI3185 was detected with a sulfo‐tag labeled anti‐MEDI3185 antibody. After addition of read buffer, electrochemiluminescence was detected with an MSD Sector imager.
Determination of Antidrug Antibodies
Serum for measurement of ADA was stored at ≤ −70°C prior to analysis. Anti‐MEDI3185 antibodies in serum obtained from the repeat dose cynomolgus monkey study were measured with a validated bridging electrochemiluminescent (ECL) immunoassay using an MSD assay platform. Prediluted positive and negative controls and test samples were acid dissociated with acetic acid, neutralized with Tris buffer (pH 9.5), and coincubated with biotinylated MEDI3185 and sulfo‐tag labeled MEDI3185. The mixture was transferred to blocked streptavidin‐coated microtiter plates. Following a wash step and addition of read buffer, antibodies to MEDI3185 were detected with an MSD Sector imager.
Binding of MEDI3185 For Determination of On‐Cell Affinity
Cynomolgus monkey HSC‐F cells were harvested, washed in PBS, and approximately 500 cells/µl were incubated for 5 hours at 2 C–8 C with a titration of MEDI3185 ranging from 200 nM–0.381 pM. Cells were washed with PBS and stained with 99 nM Cy5 goat anti‐human IgG (Jackson ImmunoResearch Laboratories) for 20 minutes at 2 C–8 C. Cells were washed with PBS at 2 C–8 C and analyzed with a FACS Canto II HTS flow cytometer (BD Biosciences).
Data Analysis
Flow cytometry data from in vivo studies were analyzed using BD Cell Quest™ Pro Software version 4.0.2. The average geometric mean fluorescence intensities of the specific and isotype control antibodies on CD3+ lymphocytes were calculated from duplicate staining reactions using FlowJo 7.5.5. Data were analyzed with Microsoft Excel (Microsoft, Redmond, WA) and plotted with Sigma Plot 11.5 (Systat Software).
For determination of on‐cell affinity and IC50, the mean fluorescence of Cy5 goat anti‐human IgG and normalized geometric mean fluorescence of MEDI3185‐AlexaFluor647 as a function of antibody concentration was fit to the four‐parameter nonlinear multiple independent binding site (MIBS) model 16. GraphPad Prism 6.0 software was used for analysis of data from the free CXCR4 assay and SAAM II software (The Epsilon Group, Charlottesville, VA) was used for analysis of on‐cell affinity data. For IC50 determination of in vivo data, only predose and time points in the first week after dosing were considered. Vehicle control group and animals dosed with 1 mg/kg were excluded from analysis. In time points where MEDI3185 was not measurable, MEDI3185 concentration was set to 0.
In this equation, = fluorescence (mean or geometric mean), = total MEDI3185 molecular concentration, = proportionality constant, = cell concentration in molarity, = number of receptors per cell, = background signal, and = equilibrium dissociation constant. was fixed for the experiments in blood at 0.01 pM (7000 cells per µl) and fixed at 0.00083 pM (500 cells/µl) for the on‐cell affinity determination with HSC‐F cells. , , and were assigned initial values of 0, 10000, 0, and 0 respectively and fit to the four‐parameter model.
RESULTS
To identify a cellular population with CXCR4 expression, we stained CXCR4 in cynomolgus monkey blood with fluorescent‐labeled MEDI3185 (MEDI3185‐AlexaFluor647). Staining was detected on lymphocytes, monocytes and granulocytes. Within CD3+ lymphocytes (T‐cells), CD4+ cells displayed higher surface expression levels of CXCR4 than CD8+ cells. In both CD4+ and CD8+ T‐cell subsets, naïve T‐cells 17 had the highest surface expression of CXCR4 as detected with MEDI3185‐AlexaFluor647. T effector memory cells (TEM) and T central memory cells (TCM) expressed surface CXCR4 at a lower level (Fig. 1)
Figure 1.
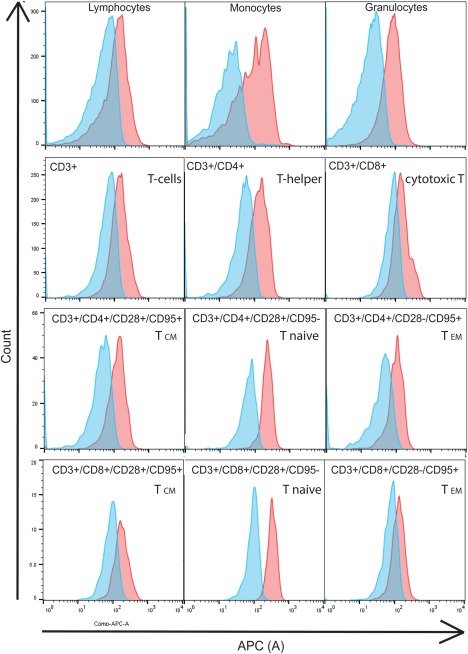
Surface expression of CXCR4 in cynomolgus blood cells. Whole Cynomolgus monkey blood was stained with, CD3‐PerCP, CD4‐FITC, CD8‐APC‐Cy7, CD95‐BV421, CD28‐BV510, and MEDI3185‐AlexaFluor647. After lysis of red blood cells, expression was determined by flow cytometry in the APC channel on lymphocytes, monocytes, and granulocytes (C), gated by forward and side scatter. Furthermore, CXCR4 staining on T‐cell subsets was determined on CD3+/CD4+ cells (T‐helper cells), CD3+/CD8+ cells (cytotoxic T‐cells), CD3+/CD4+/CD28+/CD95+ (CD4+ central memory T‐cell), CD3+/CD8+/CD28+/CD95+ (CD8+ central memory T‐cell), CD3+/CD4+/CD28+/CD95− (CD4+ naïve T‐cells), CD3+/CD8+/CD28+/CD95− (CD8+naïve T‐cells), CD3+/CD4+/CD28‐/CD95+ (CD4+ T‐effector memory cells), and CD3+/CD8+/CD28‐/CD95+ (CD8+ T‐effector memory cells). Staining with MEDI3185‐AlexaFluor647 is shown in red, isotype control staining in blue.
To measure receptor occupancy, we focused on lymphocytes, as granulocytes are a heterogeneous population of eosinophils, basophils, neutrophils, and mast cells and may exhibit different responses to MEDI3185, which could make interpretation of study data difficult. Within the lymphocytes, we selected CD3+ cells for further RO development.
To demonstrate specificity of staining and determine the optimal concentration of the detection reagent for the free CXCR4 assay, we titrated MEDI3185‐AlexaFluor647 and its isotype control IgG1TM‐AlexaFluor647. The signal increased with increasing concentration of MEDI3185‐AlexaFluor647 and saturated at 2.5 μg/ml (Fig. 2A). Isotype control signal at this concentration was low, as well as staining of blood that was preincubated with 20 μg/ml unlabeled MEDI3185. To detect total CXCR4 on the surface (bound and unbound to MEDI3185), we used phycoerythrin‐labeled clone 1D9. With increasing concentrations of 1D9‐PE, the signal increased and saturated at approximately 5 μg/ml (Fig. 2B). Isotype control staining at this concentration was low, as well as staining of blood that was preincubated with 20 μg/ml unlabeled 1D9. As expected, titration of unlabeled MEDI3185 led to complete inhibition of the signal from MEDI3185‐AlexaFluor647, confirming that only free CXCR4 is detected (Fig. 2C). MEDI3185 had a minor effect on the detection of total CXCR4 by Phycoerythrin‐labeled clone 1D9. At 0.5 µg/ml MEDI3185, the phycoerythrin geometric mean fluorescence increased by approximately 35% and returned to control levels at higher concentrations.
Figure 2.
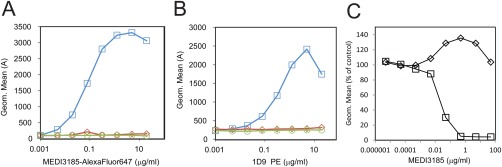
Assay development and reagent characterization. Staining of CXCR4 on CD3+ lymphocytes in cynomolgus blood. A: Titration of MEDI3185‐AlexaFluor647 (blue squares), isotype control‐AlexaFluor647 (green circles) and MEDI3185‐AlexaFluor647 blocked with 20 µg/ml MEDI3185 (red diamonds) in cynomolgus monkey blood. B: Titration of 1D9‐PE (blue circles), isotype control‐PE (green circles) and 1D9‐PE blocked with 20 μg/ml additional 1D9 (red diamonds) in Cynomolgus monkey blood. C: Staining of blood with MEDI3185‐AlexaFluor647 (squares) and 1D9‐PE (diamonds) in presence of increasing concentrations of unlabeled MEDI3185.
Free assays such as RO assays or plate based assays that measure a soluble free molecule, are subject to an intrinsic interference, since the detection or capture reagent competes with and displaces the bound biotherapeutic over time. Hence, during the incubation of blood samples with MEDI3185‐AlexaFluor647, labeled MEDI3185 over time will displace cell‐bound MEDI3185. This displacement effect with the labeled detection reagent can lead to a systematic overestimation of free CXCR4 and underestimation of MEDI3185 potency. We reasoned that displacement interference will lead to a shift of the inhibition curve. This shift would result in an apparent increase in the IC50 concentration. We therefore compared K D from on‐cell affinity determinations 16 with the IC50 from the free CXCR4 assay as the IC50 and K D values are expected to be similar. The on‐cell affinity of MEDI3185 was 221.0 pM (75.0–366.1) when determined in a cynomolgus monkey cell line (Fig. 3A). We titrated MEDI3185 in blood from 14 cynomolgus monkeys and subjected the sample to the free CXCR4 receptor occupancy measurement (Fig. 3B). The estimated IC50 values determined from the free CXCR4 assay were 241.1 pM (214.2–270.3), which is similar to the affinity obtained from the on‐cell affinity determination. As such, no obvious displacement interference was observed, and these results demonstrated that the free CXCR4 assay would accurately measure the actual receptor occupancy in a given blood sample. We also evaluated the baseline CXCR4 expression level in CD3+ cells among 14 animals. We observed moderate inter‐animal variability; the lowest specific geometric mean of MEDI3185‐AlexaFluor647 staining was 1074, the highest was 2986, the mean was 2016 and the median was 2041.
Figure 3.
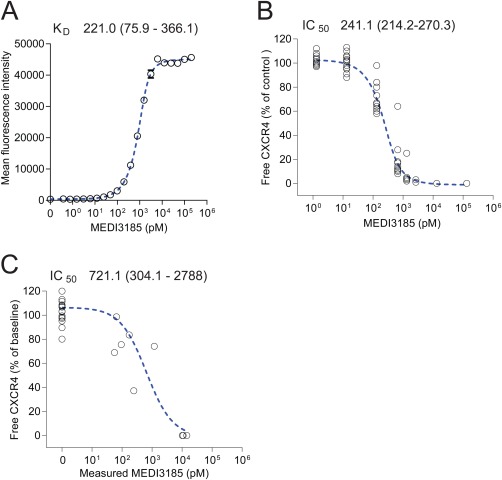
MEDI3185 on‐cell affinity determination and IC50 determination in RO assay. A: MEDI3185 on‐cell affinity determination by titration of MEDI3185 in a cynomolgus T‐cell line (HSC‐F), followed by detection with Cy5‐labeled polyclonal anti‐human IgG antibody. B: Titration of MEDI3185 in cynomolgus monkey blood from 14 animals and determination of receptor occupancy with the free CXCR4 assay. C: Plot of both measured MEDI3185 and corresponding measured free CXCR‐4 from a single dose cynomolgus monkey study up to 7 days after dosing (same study as shown in Figure 4A). IC50 and K D was determined with a four‐parameter model for all three experimental setups.
The free RO assay was employed for RO assessments after administration of a single dose of MEDI3185 at three dose levels. The lowest dose (0.01 mg/kg) decreased free CXCR4 to 62% of predose values at 1 hour after dosing and free CXCR4 fully recovered at 24 hours after dosing (Fig. 4A). Administration of 0.1 mg/kg resulted in full receptor occupancy (≤ 0.4% of baseline free CXCR4) at 1 h after dosing, and full recovery of free CXCR4 at 1 day after dosing. The highest dose (1 mg/kg) led to full receptor occupancy for 1 day followed by gradual recovery of free CXCR4 by day 7. The RO data set demonstrated dose‐dependent target engagement upon drug administration. We tested whether RO results from an in vivo study will be comparable with in vitro experiments regarding occupancy of CXCR4. We determined IC50 values with MEDI3185 study concentrations and corresponding RO. The approach seemed valid as we reasoned that there was no time delay between MEDI3185 concentration and RO effect on blood cells. The IC50 was 721.1 pM (304.1–2788), (Fig. 3C), which is higher than the IC50 determined in in vitro experiments.
Figure 4.
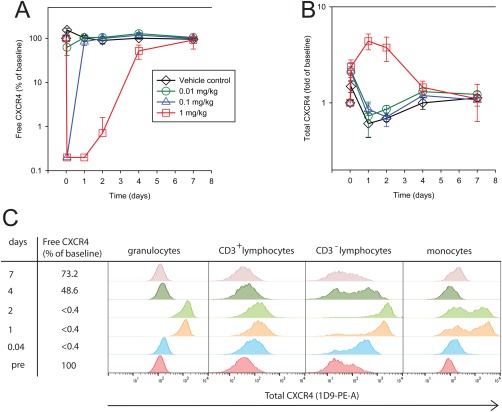
Free and total CXCR4 after administration of a single MEDIO3185 dose. Free surface CXCR4 (A) and total surface CXCR4 (B) on CD3+ lymphocytes after administration of a single dose of MEDI3185 to cynomolgus monkeys. Vehicle control (black diamond), 0.01 mg/kg (green circle), 0.1 mg/kg (blue triangle), and 1 mg/kg (red square). The group mean of three animals is plotted; error bars depict standard deviation. In one representative animal of the highest dose group, total CXCR4 (stained with 1D9‐PE) is shown in granulocuytes, CD3+ lymphocytes (T‐cells), CD3− lymphocytes, and monocytes.
We tested study samples using the total CXCR4 assay (Fig. 4B). Total CXCR4 on T‐cells (CD3+ lymphocytes) increased after the highest dose (1 mg/kg) to a maximum of 4.38 fold of baseline, whereas after dosing with 0.1 mg/kg and 0.01 mg/kg, total surface CXCR4 levels on CD3+ lymphocytes were indistinguishable from vehicle control. These data suggest that while total CXCR4 on CD3+ lymphocytes is a pharmacodynamic biomarker, it is less sensitive than free CXCR4 measurements as it does not detect effects at lower doses of MEDI3185. The observed increase of surface CXCR4 on T‐cells prompted us to investigate surface CXCR4 expression in granulocytes, monocytes, and CD3‐ lymphocytes (B‐cells and NK cells) after dosing with 1 mg/kg MEDI3185. We observed that surface CXCR4 was elevated on all studied cell populations (Fig. 4C). The increase in CXCR4 surface expression was highest at 1 and 2 days after dosing, when free CXCR4 levels, as measured in the free RO assay, remained fully suppressed. CXCR4 staining increased most dramatically on CD3‐ lymphocytes, followed by granulocytes and CD3+ lymphocytes. After dosing, monocytes split into high and low CXCR4 expressing populations.
We subsequently assessed free CXCR4 levels in a repeat dose cynomolgus monkey study, where animals were dosed weekly with MEDI3185 at 15 mg/kg, 50 mg/kg, or 150 mg/kg for 13 weeks. At all dose levels, the group median of free CXCR4 was less than 0.44% of baseline throughout the dosing period. Anti‐drug antibodies were detected in most dosed animals, and increased clearance of MEDI3185 was observed in 6 of 10 animals dosed at 15 mg/kg, while no impact of ADA on MEDI3185 clearance at 50 mg/kg or 150 mg/kg was observed (data not shown). In most animals dosed at 15 mg/kg, ADA were first detected at Day 21 after dosing, and the animals remained ADA positive at all consecutive time points (days 57, 85, 134, and 176). As expected, after the first dose, free CXCR4 levels decreased immediately to 0.38% of predose value (Fig. 5A, representative animals shown), consistent with the high concentration of MEDI3185 (479 μg/ml) in the blood (Fig. 5B). However, on Day 21 and 28 of the dosing period, MEDI3185 concentrations dropped to below 0.02 μg/ml in an ADA+ animal (Fig. 5B), a concentration where partial recovery of free CXCR4 would be expected. Surprisingly, free CXCR4 appeared fully suppressed, and results during the dosing period were indistinguishable from an ADA negative animal that maintained exposure throughout the dosing period. Furthermore, this paradoxical observation was more pronounced when MEDI3185 was cleared from circulation and undetectable (Fig. 5A,B, Day 133 and subsequent time points) and free CXCR4 appeared to remain fully suppressed in the ADA‐positive animal. The observation of full receptor occupancy in the absence of detectable drug likely represents an assay artifact (see discussion). In an animal that did not develop ADA, CXCR4 was fully occupied after the first dose and remained occupied throughout the dosing period (Fig. 5A), consistent with the concentration of MEDI3185 which remained high during the observed period (Fig. 5B).
Figure 5.
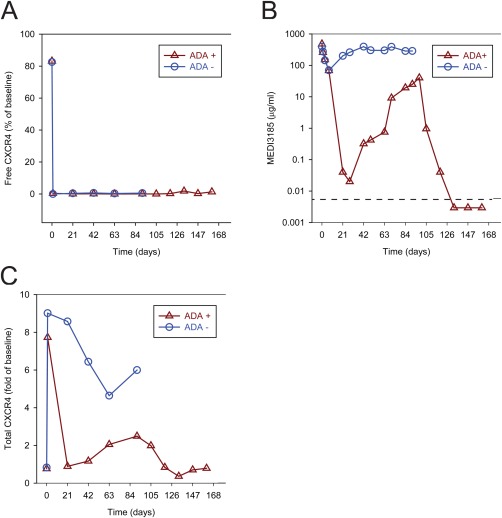
Concentration‐time profile of receptor occupancy in two animals in a multiple dose study with MEDI3185. Animals were dosed every 7 days; the last dosing occurred on day 91. Free surface CXCR4 on CD3+ lymphocytes was determined with MEDI3185‐AlexaFluor647 (A). MEDI3185 serum concentration (B) and total surface CXCR4 on CD3+ lymphocytes (C) is shown. Data from an animal that did not develop ADA (circles) and from an animal that was positive for ADA at day 21 and all following time points for ADA evaluation (triangles). Both animals were weekly intravenously dosed with 15 mg/kg MEDI3185.
Total CXCR4 on T‐cells (Fig. 5C) in an ADA‐negative animal dosed with 15 mg/kg, increased after the first dose 9.1 fold and then remained elevated above 4.6 fold throughout the dosing period (Fig. 5C). In an ADA‐positive animal, total CXCR4 increased initially after the first dose by 7.7 fold, followed by a sharp decline to 0.8 of baseline value at Day 21, consistent with a drop in MEDI3185 concentration at this time point. Then total CXCR4 increased to 2.5 fold of baseline at Day 91 and returned to baseline values at day 119. Strikingly, the concentration of MEDI3185 in this animal displayed similar kinetics (Fig. 5B), indicating that total CXCR4 is a valid pharmacodynamic biomarker in both ADA‐positive and ADA‐negative animals.
DISCUSSION
We have developed an approach to evaluate free receptor assays (i.e. the free CXCR4 assay) for displacement interference in which we compare the on‐cell KD of the therapeutic with the IC50 determined from titration of the therapeutic. In our test for displacement interference, we found that the IC50 of the free CXCR4 from in vitro experiments (214.2‐270.3 pM) was very similar to the KD of MEDI3185 (75.9‐366.1 pM). However, the 95% confidence interval of the IC50 determined with data from the in vivo study was 304.1–2788 pM, which is somewhat higher than the in vitro IC50. Considering the small number of time points used to calculate the IC50 in the in vivo study, it is most likely that the in vivo IC50 is considerably less reliable than the in vitro affinity determination. It is also possible, that physiological changes (e.g. increase in CXCR4+ cells mobilized from the bone marrow or increase of CXCR4 on existing circulating cells) can account for a higher than expected IC50. Overall, considering the additional variability intrinsic to in vivo experiments, results from the in vitro and in vivo IC50 are still comparable, and the in vivo IC50 is concordant with the K D determined in vitro. To evaluate interference, it is also prudent to compare points along the curve as displacement interference might be more pronounced at almost saturating drug concentration. Optimally, in absence of any displacement interference, both curves should be mirror images of each other. In our case, we observed a congruence between the two curves, not only at EC50 but also at low and high MEDI3185 concentration.
The dose‐dependent magnitude and duration of suppression could be demonstrated with the free CXCR4 assay after a single dose of MEDI3185 in a cynomolgus monkey study (Fig. 4A) consistent with the in vitro potency of MEDI3185. However, in a repeat dose study, we observed paradoxical results in which full receptor occupancy was observed in the absence of detectable MEDI3185 levels (Fig. 5A,B). This effect is most likely an assay artifact, associated with the appearance of anti‐drug antibodies (ADA) that bind to MEDI3185 as well as neutralizing the detection reagent (MEDI3185‐AlexaFluor647). This appears as full receptor occupancy (no free CXCR4). This interference raises the question of the utility of an ADA‐sensitive receptor occupancy assay. As emerging ADA usually appear no earlier than two weeks, it is likely that free receptor measurements with labeled therapeutic should be free of ADA interference during this time period, and free CXCR4 results should be a reliable pharmacodynamic marker for up to 2 weeks post‐dosing. Also, when ADA are observed in only a few animals, these animals can be excluded from the PD analysis and a free receptor RO assay using labeled therapeutic as detection reagent can still be a valuable pharmacodynamic marker. This study illustrates the importance of evaluating RO in conjunction with PK, ADA and if possible a second pharmacodynamic marker to identify interference that occurs after dosing that cannot be assessed during assay development. However, in studies with longer duration, when ADA are present in a significant number of the animals, a receptor occupancy assay using the labeled drug as the detection reagent is unsuitable to monitor pharmacodynamic effect as ADA renders most animals (or individuals in a clinical study) unsuitable for evaluation. RO assays that utilize a competing detection antibody that is not identical to the study drug would completely prevent ADA interference that we observed. As discussed above, a competitive antibody needs to be more rigorously qualified to ensure full competition for effects that can arise due to different affinities of the competing and therapeutic antibodies. For free CXCR4 RO assessments, clone 12G5 18 may compete with MEDI3185 and could offer an ADA‐resistant alternative for monitoring free CXCR4 as it has been reported that both antibodies compete with the ligand SDF‐119 and bind to an epitope on the second extracellular loop 20.
We used clone 1D9, which binds to a different domain of CXCR4 compared to MEDI3185, to measure total CXCR4 levels. Interestingly, in in vitro binding experiments, 1D9 binding was increased at non‐saturating concentrations of MEDI3185. It is possible that this effect might arise because of the crosslinking of cells by MEDI3185, which is favored with low concentrations of MEDI3185 (one molecule of MEDI3185 binds two molecules of CXCR4 receptor with its two binding sites) whereas high MEDI3185 concentrations favor a 1:1 MEDI3185:CXCR4 stoichiometry. As such crosslinking would only occur at low concentrations where we observe an apparent increase in total CXCR4 signal. The cross‐linking of CXCR4 molecules by MEDI3185 and 1D9 may result in CXCR4 complexes that could yield a higher fluorescent signal. This minor interference was negligible as the increase of total CXCR4 was 8 fold after dosing with MEDI3185. Additionally, when total CXCR4 was upregulated in the in vivo studies, the concentration of MEDI3185 was 100 µg or higher. At this concentration, MEDI3185 did not interfere in the total CXCR4 assay.
Our use of both free and total RO assays revealed distinct advantages and disadvantages for each assay in the cynomolgus studies. The free surface CXCR4 assay is more sensitive than the total surface CXCR4 assay since the former revealed a pharmacodynamic effect at all dose levels, while the total CXCR4 assay only demonstrated an effect at 1 mg/kg (Figs. 4B, 5C). The apparent decreased sensitivity of the total CXCR4 assay most likely results from the time delay between MEDI3185 administration and increased CXCR4 expression levels. However, this was only observed for CD3+ lymphocytes. Assessment of total CXCR4 levels on CD3‐ lymphocytes (B‐cells and NK cells) revealed that a moderate increase is observable as early as 1 h post‐dose, and the maximal effect is reached at 1 day post dose. Hence, total CXCR4 measurements on CD3‐ lymphocytes (B‐cell or NK cells) could be a marker equally sensitive to free CXCR4 levels. The free CXCR4 assay measures receptor occupancy on circulating cells with a high degree of accuracy, and the marker represents a defining property of MEDI3185 – binding to CXCR4. Total CXCR4, on the other hand, is a downstream effect and has the advantage of representing a biological effect of CXCR4 binding by MEDI3185. However, depending on the intended use of MEDI3185, the desired mechanism of action and expected efficacy may be more correlated to either free or total CXCR4 expression levels. For example, MEDI3185 is likely to inhibit HIV‐infection as it competes with the clone 12G5, an inhibitor of HIV infection 18. In such case, free CXCR4 should be a very relevant readout as it measures the abundance of receptors available for infection.
Similar to our observations, Uy et al. 21 observed an increase in CXCR4 expression in patients dosed with the CXCR4 inhibitor plerixafor, which is also known to mobilize cells into circulation 22. The apparent increase of surface CXCR4 could be due to an upregulation of CXCR4 or due to the mobilization of a cell population with high levels of surface CXCR4 into the peripheral circulation. Our data strongly indicate that CXCR4 is upregulated in granulocytes, CD3+ lymphocytes (T‐cells) and CD3‐ lymphocytes (B‐cells and NK cells) since we observed a uniform shift in CXCR4 expression. Like plerixafor, MEDI3185 very likely mobilizes cells into the circulation, but CXCR4 expression of mobilized cells and existing circulating cells after MEDI3185 dosing appeared to be not different in our study. Our interpretation is consistent with previous findings, as CXCR4 expression levels on lymphocytes and CD34+ cell in bone marrow, a major source of mobilized cells, is comparable with that of cells in peripheral blood 23. Subepithelial B‐cells store large amounts of CXCR4 intracellularly, and stimulation results in higher surface expression of CXCR4, indicating redistribution to the cell surface 24. CXCR4 expression on CD3‐ lymphocytes is not uniform (Fig. 4C), which is not surprising as this population is comprised of B‐cells and NK cells. NK cells are heterogeneous and populations characterized by CD16 and CD56 express different levels of surface CXCR4, independent of their localization in human peripheral blood or bone marrow 25. As such the increase of CXCR4 on peripheral NK cells is likely attributable to up regulation. However, monocytes split into two populations after dosing with MEDI3185, one with high and one with low expression of CXCR4. As monocytes are heterogeneous, it is possible that CXCR4 is only significantly upregulated on a monocyte subset or a monocyte population with higher CXCR4 expression is mobilized out of the tissue into the blood. The data for monocytes would support both mechanisms: upregulation of a subset of monocytes or mobilization of monocytes with higher CXCR4 expression into blood.
Previously published in vitro data also supports the notion that CXCR4 is upregulated on existing cells in the blood. Plerixafor induced up regulation of CXCR4 in Nalm‐6, a human B cell precursor leukemia, and more pronounced upregulation in HB‐119, a murine hybridoma B‐cell 19. Additionally, the CXCR4 ligand SDF‐1 down regulated CXCR4 on blood leukocytes in vitro through enhanced internalization 24 and, as such, displacement of SDF‐1 with MEDI3185 could result in upregulation of CXCR4.
Overall, we have demonstrated an assay development path for two RO assays to assess the PD effects of administration of MEDI3185 and means to assess displacement interference. Free CXCR4 on CD3+ demonstrated dose‐dependent degree and duration of CXCR4 suppression. At later timepoints in the preclinical study, by carefully assessing the RO results in the context of data from PK and ADA assays, we were able to identify substantial assay interference in the free CXCR4 assay that is most likely a result of emerging neutralizing antibodies to MEDI3185. Furthermore, we found that MEDI3185 upregulated CXCR4 on granulocytes, T‐cells, and CD3‐ lymphocytes (B‐cells and NK cells) in a pattern that is consistent with upregulation in circulating cells. If cells are mobilized, which is likely, the expression of freshly mobilized cells is not visibly different from already circulating cells.
ACKNOWLEDGEMENTS
The authors thank Brandon Lam and Hong Lu for providing ADA data and MEDI3185 concentrations in samples from the in vivo studies. They thank Werner Frings for execution of the GLP study and the receptor occupancy assay.
How to cite this article: Schwickart M, Chavez C, Henderson S, Vainshtein I, Standifer N, DelNagro C, Mehrzai F, Schneider A, Roskos L and Liang M. Evaluation of Assay Interference and Interpretation of CXCR4 Receptor Occupancy Results in a Preclinical Study with MEDI3185, a Fully Human Antibody to CXCR4. Cytometry Part B 2016; 90B: 209–219.
Conflict of Interest: Martin Schwickart, Carlos Chavez, Simon Henderson, Inna Vainshtein, Nathan Standifer, Christopher DelNagro, Freshta Mehrzai, Lorin Roskos, and Meina Liang are employees of MedImmune and own stock in Astra Zeneca.
LITERATURE CITED
- 1. Danhof M, Alvan G, Dahl SG, Kuhlmann J, Paintaud G. Mechanism‐based pharmacokinetic‐pharmacodynamic modeling—A new classification of biomarkers. Pharm Res 2005;22:1432–1437. [DOI] [PubMed] [Google Scholar]
- 2. Latek R, Fleener C, Lamian V, Kulbokas E, 3rd Davis PM, Suchard SJ, Curran M, Vincenti F, Townsend R. Assessment of belatacept‐mediated costimulation blockade through evaluation of CD80/86‐receptor saturation. Transplantation 2009;87:926–933. [DOI] [PubMed] [Google Scholar]
- 3. Stefanich EG, Danilenko DM, Wang H, O'Byrne S, Erickson R, Gelzleichter T, Hiraragi H, Chiu H, Ivelja S, Jeet S, Gadkari S, Hwang O, Fuh F, Looney C, Howell K, Albert V, Balazs M, Refino C, Fong S, Iyer S, Williams M. A humanized monoclonal antibody targeting the beta7 integrin selectively blocks intestinal homing of T lymphocytes. Br J Pharmacol 2011;162:1855–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rutgeerts PJ, Fedorak RN, Hommes DW, Sturm A, Baumgart DC, Bressler B, Schreiber S, Mansfield JC, Williams M, Tang M, Visich J, Wei X, Keir M, Luca D, Danilenko D, Egen J, O'Byrne S. A randomised phase I study of etrolizumab (rhuMAb beta7) in moderate to severe ulcerative colitis. Gut 2013;62:1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verbon A, Dekkers PE, ten Hove T, Hack CE, Pribble JP, Turner T, Souza S, Axtelle T, Hoek FJ, van Deventer SJ, van der Poll T. IC14, an anti‐CD14 antibody, inhibits endotoxin‐mediated symptoms and inflammatory responses in humans. J Immunol 2001;166:3599–3605. [DOI] [PubMed] [Google Scholar]
- 6. Tolcher AW, Sarantopoulos J, Patnaik A, Papadopoulos K, Lin CC, Rodon J, Murphy B, Roth B, McCaffery I, Gorski KS, Kaiser B, Zhu M, Deng H, Friberg G, Puzanov I. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin‐like growth factor receptor 1. J Clin Oncol 2009;27:5800–5807. [DOI] [PubMed] [Google Scholar]
- 7. Hua F, Comer GM, Stockert L, Jin B, Nowak J, Pleasic‐Williams S, Wunderlich D, Cheng J, Beebe JS. Anti‐IL21 receptor monoclonal antibody (ATR‐107): Safety, pharmacokinetics, and pharmacodynamic evaluation in healthy volunteers: a phase I, first‐in‐human study. J Clin Pharmacol 2014;54:14–22. [DOI] [PubMed] [Google Scholar]
- 8. Roskos LK, Schneider A, Vainshtein I, Schwickart M, Lee R, Lu H, Faggioni R, Liang M. PK‐PD modeling of protein drugs: Implications in assay development. Bioanalysis 2011;3:659–675. [DOI] [PubMed] [Google Scholar]
- 9. Lee JW, Kelley M, King LE, Yang J, Salimi‐Moosavi H, Tang MT, Lu JF, Kamerud J, Ahene A, Myler H, Rogers C. Bioanalytical approaches to quantify “total” and “free” therapeutic antibodies and their targets: Technical challenges and PK/PD applications over the course of drug development. AAPS J 2011;13:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chirmule N, Jawa V, Meibohm B. Immunogenicity to therapeutic proteins: Impact on PK/PD and efficacy. AAPS J 2012;14:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Hara DM, Xu Y, Liang Z, Reddy MP, Wu DY, Litwin V. Recommendations for the validation of flow cytometric testing during drug development. II. Assays. J Immunol Methods 2011;363:120–134. [DOI] [PubMed] [Google Scholar]
- 12. Green CL, Brown L, Stewart JJ, Xu Y, Litwin V, Mc Closkey TW. Recommendations for the validation of flow cytometric testing during drug development. I. Instrumentation. J Immunol Methods 2011;363:104–119. [DOI] [PubMed] [Google Scholar]
- 13. Liang MD, Schwickart MSI, Schneider AKSI, Vainshtein ISS, Del Nagro CSI, Standifer NSI, Roskos LKVP. Receptor occupancy assessment by flow cytometry as a pharmacodynamic biomarker in biopharmaceutical development. Cytometry B Clin Cytom 2015, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adeela Kamal YW, Philipp S, Anne‐Marie M, Leslie W, Melissa P, Brenda M, Keven H, Vahe B, Norman G. MEDI3185, a potent anti‐CXCR4 antibody, inhibits tumor cell migration, signaling and tumor growth in preclinical models. AACR 104th Annual Meeting 2013; Apr 6–10, 2013; Washington, DC.
- 15. Oganesyan V, Gao C, Shirinian L, Wu H, Dall'Acqua WF. Structural characterization of a human Fc fragment engineered for lack of effector functions. Acta Crystallogr D Biol Crystallogr 2008;64:700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drake AW, Klakamp SL. A rigorous multiple independent binding site model for determining cell‐based equilibrium dissociation constants. J Immunol Methods 2007;318:147–152. [DOI] [PubMed] [Google Scholar]
- 17. Nadazdin O, Boskovic S, Murakami T, O'Connor DH, Wiseman RW, Karl JA, Tuscher JJ, Sachs DH, Madsen JC, Tocco G, Kawai T, Cosimi AB, Benichou G. Phenotype, distribution and alloreactive properties of memory T cells from cynomolgus monkeys. Am J Transplant 2010;10:1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Endres MJ, Clapham PR, Marsh M, Ahuja M, Turner JD, McKnight A, Thomas JF, Stoebenau‐Haggarty B, Choe S, Vance PJ, Wells TN, Power CA, Sutterwala SS, Doms RW, Landau NR, Hoxie JA. CD4‐independent infection by HIV‐2 is mediated by fusin/CXCR4. Cell 1996;87:745–756. [DOI] [PubMed] [Google Scholar]
- 19. Sison EA, Magoon D, Li L, Annesley CE, Rau RE, Small D, Brown P. Plerixafor as a chemosensitizing agent in pediatric acute lymphoblastic leukemia: Efficacy and potential mechanisms of resistance to CXCR4 inhibition. Oncotarget 2014;5:8947–8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brelot A, Heveker N, Pleskoff O, Sol N, Alizon M. Role of the first and third extracellular domains of CXCR‐4 in human immunodeficiency virus coreceptor activity. J Virol 1997;71:4744–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uy GL, Rettig MP, Motabi IH, McFarland K, Trinkaus KM, Hladnik LM, Kulkarni S, Abboud CN, Cashen AF, Stockerl‐Goldstein KE, Vij R, Westervelt P, DiPersio JF. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood 2012;119:3917–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dar A, Schajnovitz A, Lapid K, Kalinkovich A, Itkin T, Ludin A, Kao WM, Battista M, Tesio M, Kollet O, Cohen NN, Margalit R, Buss EC, Baleux F, Oishi S, Fujii N, Larochelle A, Dunbar CE, Broxmeyer HE, Frenette PS, Lapidot T. Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4‐dependent SDF‐1 release from bone marrow stromal cells. Leukemia 2011;25:1286–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dabusti M, Lanza F, Campioni D, Castagnari B, Tieghi A, Moretti S, Punturieri M, De Angeli C, Spanedda R, Ferrazzi E, Castoldi G. CXCR‐4 expression on bone marrow CD34+ cells prior to mobilization can predict mobilization adequacy in patients with hematologic malignancies. J Hematother Stem Cell Res 2003;12:425–434. [DOI] [PubMed] [Google Scholar]
- 24. Forster R, Kremmer E, Schubel A, Breitfeld D, Kleinschmidt A, Nerl C, Bernhardt G, Lipp M. Intracellular and surface expression of the HIV‐1 coreceptor CXCR4/fusin on various leukocyte subsets: Rapid internalization and recycling upon activation. J Immunol 1998;160:1522–1531. [PubMed] [Google Scholar]
- 25. Stabile H, Nisti P, Morrone S, Pagliara D, Bertaina A, Locatelli F, Santoni A, Gismondi A. Multifunctional human CD56 low CD16 low natural killer cells are the prominent subset in bone marrow of both healthy pediatric donors and leukemic patients. Haematologica 2015;100:489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]


