Abstract
Aims
To determine the magnitude of the peripheral glucose gradient in patients with Type 1 diabetes in a real world setting and to explore its relationship with insulin dose and macronutrient intake.
Methods
All patients used mealtime analogue insulin. The glucose gradient was assessed using antecubital fossa venous and finger‐stick capillary samples, collected concurrently at room temperature. Baseline sampling occurred before the administration of an insulin dose and breakfast of the patient's choosing. Breakfast was consumed an average of 15 min after baseline. The macronutrient content of breakfast was documented. Sampling was repeated 1 and 2 h after baseline.
Results
The mean (95% CI) plasma capillary–venous glucose gradient values for 43 patients were: pre‐breakfast, 0.21 (0.08–0.34) mmol/l; 1 h after baseline, 0.87 (0.66–1.07) mmol/l; and 2 h after baseline, 0.52 (0.33–0.71) mmol/l. Glucose gradient and dietary carbohydrate intake (g/kg body weight) were positively correlated at both 1 h (P < 0.01) and 2 h after baseline (P < 0.01). No relationship was observed between this gradient and mealtime insulin dose, or the glucose concentration at either time point.
Conclusions
In patients with Type 1 diabetes, a clinically significant glucose gradient is present after the ingestion of a carbohydrate‐rich meal. As postprandial capillary and venous plasma glucose concentrations are not equivalent, defining the site of sample collection is important.
What's new?
This report details the magnitude of the postprandial capillary–venous glucose gradient and its clinical associations, in patients with Type 1 diabetes.
At 1 h from baseline, the capillary–venous glucose gradient was 0.87 mmol/l.
A positive association was seen between the postprandial glucose gradient and carbohydrate intake.
No association was seen with either preprandial insulin dose or baseline glucose concentration.
Study findings suggest that when a postprandial glucose concentration is within the biochemical hypoglycaemia range, the site of glucose sampling should be clarified to allow appropriate clinical interpretation of the result.
What's new?
This report details the magnitude of the postprandial capillary–venous glucose gradient and its clinical associations, in patients with Type 1 diabetes.
At 1 h from baseline, the capillary–venous glucose gradient was 0.87 mmol/l.
A positive association was seen between the postprandial glucose gradient and carbohydrate intake.
No association was seen with either preprandial insulin dose or baseline glucose concentration.
Study findings suggest that when a postprandial glucose concentration is within the biochemical hypoglycaemia range, the site of glucose sampling should be clarified to allow appropriate clinical interpretation of the result.
Introduction
Tissue uptake of glucose produces an arterial–capillary–venous gradient in glucose concentration, first described around 160 years ago 1. Multiple publications have confirmed that, after ingestion of an oral nutrient load, a glucose gradient occurs of ~1–2 mmol/l in magnitude 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13. In healthy volunteers, the gradient in peripheral tissue is minimal in the fasting state and maximal at ~30–60 min after oral carbohydrate intake 2. The gradient is attenuated in the presence of diabetes 4, 6, 12. Shortly after insulin was first manufactured, multiple small studies examined the effect of insulin injections on the gradient, but it was another 40 years before the pathophysiological distinction between Type 1 and Type 2 diabetes became well defined 14 and there is a gap in our knowledge about the effects of an enteral nutritional load on the peripheral capillary–venous glucose gradient in Type 1 diabetes.
In the present study we examined the peripheral glucose gradient in Type 1 diabetes in a clinical setting that reflects everyday life. There were several prespecified aims. The primary aim was to measure the capillary–venous glucose gradient, using a capillary sample taken from the finger and a venous sample taken from the antecubital fossa, before breakfast, then at 60 and 120 min after baseline glucose assessment. Additional prespecified aims included exploring the relationship between the postprandial glucose gradient and the following: breakfast insulin dose (U/kg); carbohydrate and total energy intake; baseline glucose; change in glucose; and percentage body fat.
Patients and methods
Patient inclusion criteria were adults (aged ≥18 years) with Type 1 diabetes who had previously received education in estimating their insulin:carbohydrate ratio and had a mealtime insulin:carbohydrate ratio that was stable on self‐report and were using fast‐acting analogue mealtime insulin, either with multiple daily insulin injections, or with a subcutaneous insulin infusion. Patients with poorly controlled diabetes (defined as HbA1c > 65 mmol/mol or >8.1%) and those who were pregnant were excluded. The diagnosis of Type 1 diabetes was made by the patients’ usual specialist diabetes physician and was confirmed by the diabetes specialist overseeing the present study.
On the morning of the research clinic visit, patients arrived having fasted, chose a continental breakfast then injected what they considered to be an appropriate amount of mealtime insulin immediately before the meal. Calculation of breakfast carbohydrate intake was undertaken by the study team, which included a dietician. Breakfast was commenced a median (range) of 15 (3–29) min after baseline sampling. Staggering the timing between baseline sampling and commencement of breakfast allowed subsequent exploration of the timing of the peak glucose gradient relative to the timing of nutrient consumption. Sampling was then repeated 60 and 120 min after baseline sampling. Blood was collected from the same arm, at all three sampling times. During the study, patients rested in a sitting position at room temperature; patients’ hands and forearms were not warmed.
Capillary and venous glucose was measured using two different brands of glucose meter [Accuchek® Performa (Roche Diagnostics, Mannheim Germany) and Nova StatStrip® (Nova Biomedical, Waltham, MA, USA)] and quadruplicate sampling. The use of both meters has been described previously in similar research settings 13, 15. In addition, at the 2‐h sampling point, both capillary and venous plasma samples were analysed in an accredited hospital laboratory. Details of the study methodology are outlined in the Supporting Information (Appendix S1).
Statistical analyses
The number of participants required to provide sufficient power to show a clinically relevant difference in glucose gradient at different time points as statistically significant using paired t‐tests (primary objective) was small when compared with the number of participants required to achieve the study's secondary objectives. The power calculation was therefore undertaken on the secondary objectives of exploring the association between the glucose gradient and prespecified clinical variables of interest. The study aimed to recruit a minimum of 40 patients, based on power calculations providing sufficient power (80%) to detect an r 2 of > 20% as statistically significant (two‐tailed α = 0.05).
The glucose gradient (capillary–venous glucose difference) was calculated from values obtained using the same measuring methodology; that is, either glucose meter plasma‐equivalent or laboratory plasma glucose measurements. Capillary and venous and also laboratory and meter‐derived values were compared using paired t‐tests. Associations between the glucose gradients and demographic, clinical and nutritional measures were tested using Spearman's rank correlation coefficients and independent t‐tests as appropriate. Bland–Altman statistics were used to compare glucose measurements obtained using different measurement techniques. medcalc software version 13.3.0.0 was used to calculate Bland–Altman statistics. spss software was used for all other calculations.
The study was registered with the Australian New Zealand Clinical Trials registry (ACTRN12613001162707) and approved by the Health and Disability Ethics Committee (New Zealand), reference 13/STH/148.
Results
Patients
Demographic and clinical characteristics of the 43 patients are shown in Table 1. Patients’ haematocrit values were within the range specified for the glucose meters used in the study. The median (range) temperature of the study room was 22 (19–26)°C.
Table 1.
Demographic and clinical characteristics of the 43 study participants
| No. of patients | |
|---|---|
| Gender, M:F | 23:20 |
| Ethnicity, New Zealand European: Other | 41:2 |
| Mode of insulin delivery, CSII:MDI | 13:30 |
| Median (range) | |
| Age, years | 47 (23–84) |
| Diabetes duration, years | 29 (1–68) |
| BMI, kg/m2 | 25.8 (20.1–41.7) |
| Body fat (%) | 36.4 (28.3–49.9) |
| HbA1c, mmol/mol | 56 (38–68) |
| HbA1c, % | 7.3 (5.6–8.4) |
| Breakfast carbohydrate intake, g | 48 (14–181) |
| Breakfast insulin dose, U | 7 (1.7–26.0) |
| Pre breakfast glucose, mmol/la | 9.7 (4.3–15.7) |
| 1‐h glucose, mmol/la | 12.1 (7.8–21.7) |
| 2‐h glucose, mmol/la | 10.8 (2.7–21.7) |
CSII, continuous subcutaneous insulin infusion; MDI, multiple daily insulin injections.
Plasma venous glucose, measured in the laboratory.
With regard to hypoglycaemia, patients reported no episodes of overnight or early‐morning hypoglycaemia immediately before the study. Three patients developed mild symptomatic hypoglycaemia at the 120‐min sampling point and were treated immediately after sampling was completed. Capillary and venous laboratory plasma glucose values (single capillary plasma sample/mean of two venous plasma values) for these three patients at 120 min were 3.2/2.75; 3.4/2.70 and 3.4/2.95 mmol/l. All three patients were hypoglycaemia‐aware on questionnaire review, which was completed before the 120‐min time point.
The comparability of meter‐ and laboratory‐derived glucose gradients are detailed in the Supporting Information (Appendix S2). In summary, only minor biases were seen between results from the two brands of glucose meter used in the study and between results obtained from glucose meter and plasma glucose, measured in an accredited laboratory.
Description of the capillary–venous glucose gradient
The mean glucose meter‐derived gradients were 0.21 (95% CI 0.08–0.34), 0.87 (95% CI 0.66–1.07) and 0.52 (95% CI 0.33–0.71) mmol/l at the 0‐, 60‐ and 120‐min time points, respectively. The corresponding 120‐min glucose gradient as measured in the laboratory was 0.40 mmol/l (95% CI 0.20–0.60). It was not significantly different (P > 0.05) from the meter‐derived value and these results are summarized in Fig. 1. The inter‐individual variability (dispersion) of the measured gradient at each time point was high, with sd values for the glucose meter‐derived gradients of 0.43, 0.66 and 0.62 mmol/l at 0, 60 and 120 min, respectively. Figure 2 highlights the lack of relationship between plasma glucose and the magnitude of the gradient and also shows the inter‐individual dispersion, at all time points. A weak correlation (r = 0.44, P = 0.003) was observed for the intra‐individual gradients measured at the 60‐ and 120‐min time points (Table 2).
Figure 1.
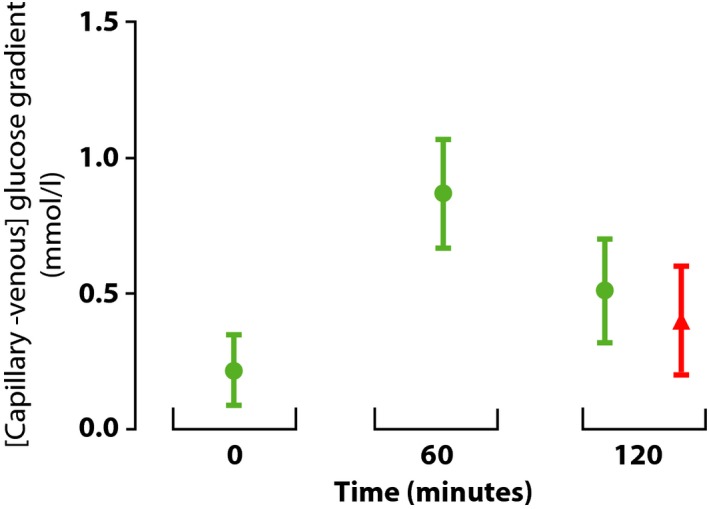
Magnitude of the [capillary – venous] glucose gradient at each sampling point, using either glucose meter derived measurements (green) or measurements derived from the laboratory measurement of plasma glucose (red). Bars represent 95% confidence intervals for the means indicating capillary values are significantly higher than venous values for all four sample collections. Breakfast was consumed an average of 15 min after the 0 min sampling point.
Figure 2.
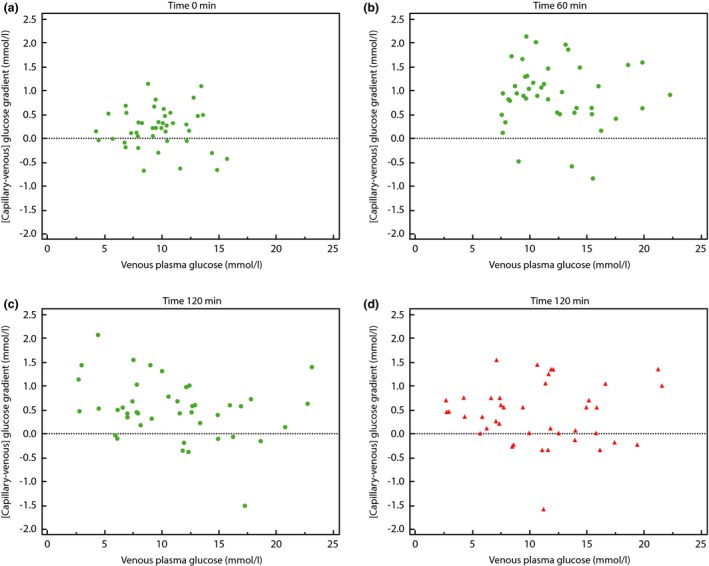
Points represent results from individual participants. The x axis shows antecubital venous plasma glucose and the y axis shows the [capillary – venous] glucose gradient. The horizontal dashed line is for orientation; values above this line represent those results which show a higher capillary than venous glucose value. The glucose gradient is derived either from glucose meter measurements (Figs. 2 a–c, green), or derived from the laboratory measurement of plasma glucose (red). (a) Baseline (time 0 min) sampling point. (b) Time 60 min sampling point. (c) Time 120 min sampling point. (d) Time 120 min sampling point (plasma capillary samples).
Table 2.
Relationship between 60‐ and 120‐min glucose gradients and breakfast nutrient intake and insulin dose
| 60 min | 120 min | |||||
|---|---|---|---|---|---|---|
| Meter‐derived glucose gradient | Meter‐derived glucose gradient | Laboratory‐derived glucose gradient | ||||
| Rho | P | Rho | P | Rho | P | |
| Total energy intake (kJ) | 0.31 | 0.04 | 0.28 | 0.07 | 0.37 | 0.02 |
| Total energy/kga | 0.37 | 0.02 | 0.32 | 0.04 | 0.31 | 0.05 |
| Carbohydrate intake (g) | 0.32 | 0.03 | 0.31 | 0.05 | 0.36 | 0.02 |
| Carbohydrate /kg | 0.45 | 0.002 | 0.43 | 0.005 | 0.35 | 0.03 |
| Insulin (U) | −0.07 | 0.68 | 0.10 | 0.51 | 0.18 | 0.25 |
| Insulin/kg | −0.03 | 0.86 | 0.16 | 0.31 | 0.14 | 0.39 |
| Total energy – carbohydrate | 0.14 | 0.38 | 0.06 | 0.68 | 0.20 | 0.20 |
| [Total energy– carbohydrate]/kg | 0.19 | 0.21 | 0.12 | 0.45 | 0.21 | 0.19 |
| Lag between time 0 sampling and breakfast start time (min) | −0.13 | 0.39 | −0.00 | 0.98 | −0.11 | 0.50 |
| Meter‐derived glucose gradient at 60 min | – | – | 0.44 | 0.003 | 0.16 | 0.33 |
| Meter‐derived glucose gradient at 120 min | – | – | – | – | 0.60 | <0.001 |
Rho = Spearman's rank correlation coefficient.
Total body weight in kg.
Predictors of capillary–venous glucose gradient
The relationships between the glucose gradient at 60 and 120 min and clinical variables is summarized in Table 2. A significant association was observed between the glucose gradient and carbohydrate intake at both the 60‐ and 120‐min time points. This relationship was present when considering absolute carbohydrate intake, but became stronger when carbohydrate intake was adjusted for body weight. In contrast, no association was seen between the glucose gradient and total energy intake minus energy derived from carbohydrates, irrespective of adjustment for body weight (Table 2). Supplementary analyses of other macronutrients showed a very weak positive relationship between the gradient and total protein, adjusted for body weight (g/kg): at time point zero, r 2 < 0.01, P = 0.60; at time 60 min, r 2 = 0.10, P = 0.04; at time 120 min, r 2 = 0.11, P = 0.03. No relationship was seen between the gradient and unadjusted protein intake, nor between fat intake, either unadjusted or adjusted for body weight.
A secondary study hypothesis was that the magnitude of the glucose gradient would show a dose–response relationship with the prandial insulin injection, expressed either as total dose of insulin or dose per kg body weight. No association was evident.
On case note review, the attending physician considered six patients to have symptoms, signs and investigative results consistent with diabetic gastroparesis. There was, however, no difference in the glucose gradient of these six patients compared with the gradients of the 37 patients without gastroparesis. One patient had treated coeliac disease. Glucose results showed no substantive differences when compared with other patients.
Details of the ancillary clinical analyses are provided in the Supporting Information (Appendix S3). In summary, these ancillary analyses did not show any statistically or clinically significant associations with the capillary–venous glucose gradient. In particular, no relationship was seen between the delay in commencing breakfast and the magnitude of the gradient measured at 60 min, on either formal analysis or visual inspection of graphed results. This suggests that in the real world setting of a wide range of patient‐selected food choices, with minimal restrictions placed on speed of nutrient consumption, there was no single peak time at which the gradient occurred, when considering the 30–60‐min time period after starting breakfast. Other variables that showed no relationship with the magnitude of the gradient included gender, mode of insulin delivery (subcutaneous injection or insulin pump), venous plasma glucose concentration, change in venous plasma glucose concentration over the 2 h of study, BMI and percentage body fat.
Discussion
In this study we measured the magnitude of the postprandial capillary–venous glucose gradient between finger and antecubital fossa samples in patients with Type 1 diabetes. A gradient of 0.87 mmol/l was measured an average of 45 min after breakfast. This gradient was present even in patients with suboptimum ‘insulin on board’, as judged by the lack of any relationship between the level of hyperglycaemia and the gradient. The gradient showed a positive correlation with carbohydrate intake and a weak positive correlation with protein intake, but not with insulin dose, nor with any of the additional demographic or clinical variables studied.
A strength of the study lies in its real world setting, making its findings directly applicable to the everyday clinical care of patients with Type 1 diabetes. As an example, it has been suggested that patients assess their glucose meter's performance by comparing their routinely collected laboratory venous plasma glucose with a concomitant glucose meter finger‐stick value 16. When this comparison is undertaken shortly after a meal, the capillary finger‐stick value (which glucose meters read as plasma glucose), will be subjected to a systematic positive bias compared with the laboratory plasma glucose, measured from a sample taken from the antecubital fossa. This positive bias is additional to acceptable analytical variations in meter‐derived capillary glucose levels 17 and is therefore likely to affect the interpretation of comparison of the results obtained from these two distinct sampling sites. We therefore recommend that this comparison should be undertaken before, rather than shortly after meals.
The study results may also inform the reporting of biochemical hypoglycaemia, usually defined as a plasma glucose ≤3.9 mmol/l 18. The present study showed that, after a carbohydrate load, it is possible to observe discordance between the presence/absence of biochemical hypoglycaemia as assessed by concomitant finger‐stick capillary and antecubital fossa venous results. This may have clinical implications. One example is consideration of possible hypoglycaemic unawareness based on a postprandial laboratory plasma glucose just below the threshold for biochemical hypoglycaemia, as it cannot be assumed that a concomitant finger‐stick glucose result would be in the hypoglycaemic range.
The study also has several limitations. Firstly, it was not designed to explore mechanisms that might be responsible for the observed glucose gradient, and therefore does not offer any explanation for the genesis of the gradient. Secondly, the laboratory measurement of plasma glucose was used as an ancillary method of measurement only at final sample collection. Bland–Altman analysis did, however, support the robustness of the glucose meter recordings relative to the measurement of laboratory plasma glucose. A third limitation relates to the constraints associated with this study's real‐world design, which included a wide choice of breakfast foods, consumed over a variable period of time at the discretion of the patient. A more detailed assessment of the characteristics of the gradient would require a prescriptive, rather than a real‐world study design. For example, it might include a prespecified breakfast of known glycaemic load, consumed over a short time frame, with frequent glucose sampling which continued into the post‐absorptive period. This approach would allow assessment of the exact time to peak glucose gradient and of the magnitude of this peak (which is likely to be larger than the 1‐h gradient reported in the present study) and would also allow assessment of the length of time required for the gradient to return to baseline. Finally, there was a small inevitable time lag, averaging < 1 min, between venous sample collection and later collection of the capillary sample from the same upper limb. This is likely to have had a minor overall impact on the estimation of the glucose gradient.
The observation in the present study of a lack of relationship between insulin dose and the magnitude of the gradient suggests that the mechanism(s) responsible for the gradient may have a non‐insulin‐mediated glucose uptake component to them. In hyperglycaemia, non‐insulin‐mediated glucose uptake is responsible for most of the postprandial uptake of glucose 19, 20. As the majority of our study patients had glucose levels above the physiological range for all or part of the study, the lack of a relationship between the glucose gradient and dose of insulin is perhaps not surprising.
The presence of a capillary–venous glucose gradient is likely to be of importance when considering calibration of continuous glucose monitoring systems 21, as calibration may include postprandial capillary finger‐stick glucose values 22. Results from the present study would suggest that inclusion of multiple postprandial calibration points would produce continous glucose monitoring system outputs that are more aligned to capillary rather than to venous glucose values.
In conclusion, the present study shows the presence of a clinically significant postprandial capillary–venous glucose gradient in patients with Type 1 diabetes, studied in a real‐world setting. This gradient was not unexpected when considering information available from previous studies. It has important implications, particularly for the detection of biochemical hypoglycaemia and for the postprandial calibration and validation of glucose measurements that involve the use of both venous and capillary samples. In clinical settings, where postprandial sampling for plasma glucose measurement is undertaken and precise estimation of glucose is required, it is important to define the sampling site, for example, by stating either capillary finger‐stick plasma glucose or venous antecubital fossa plasma glucose, as appropriate.
Funding sources
I.L. was sponsored by the Diabetes Education and Research Trust, New Zealand. The funder had no input into any aspect of the study.
Competing interests
H.L. is on the speakers’ bureau for Sanofi (New Zealand).
Supporting information
Appendix S1. Study methodology.
Appendix S2. Results.
Appendix S3. Relationship between glucose gradient and demographic and clinical variables.
Acknowledgements
I.L. undertook work on this study as a University of Otago, Christchurch summer student. We are grateful for the input of the following staff from the Diabetes Centre, Canterbury District Health Board: Stacey Hodgman, (Research Nurse), Deborah Kendall (Research Co‐ordinator) and also Hilary Totty (Diabetes Dietitian), who provided oversight and interpretation of dietary data. Finally, we thank our patients, both for their willingness to participate in this study and also for offering useful suggestions about possible future study design.
Diabet. Med. 33, 998–1003 (2016)
An earlier version of this research was presented as a poster at the Australian Diabetes Society and the Australian Diabetes Educators Association Annual Scientific Meeting 2014 (abstract can be accessed at http://ads-adea-2014.p.asnevents.com.au/days/2014-08-28/abstract/18029).
References
- 1. Chauveau M. Nouveles recherches sur Ia question glycogenique. Compt Rend Soc Biol 1856; 42: 108. [Google Scholar]
- 2. Förster H, Haslbeck M, Mehnert H. Metabolic studies following the oral Ingestion of different doses of glucose. Diabetes 1972; 21: 1102–1108. [DOI] [PubMed] [Google Scholar]
- 3. Cori CF, Pucher GW, Bowen BD. Comparative study of the blood sugar concentration in the arterial and venous blood of diabetic patients during insulin action. Exp Biol Med 1923; 21: 122–123. [Google Scholar]
- 4. Lawrence RD. Effect of insulin on the sugar content of arterial and venous blood in diabetes. Br Med J 1924; 3299: 516–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pemberton HS, Cunningham L. The behaviour of the diabetic towards circulating glucose. Lancet 1925; 206: 1222–1224. [Google Scholar]
- 6. Rabinowitch IM. Simultaneous determination of arterial and venous blood‐sugars in diabetic individuals. Br J Exp Pathol 1927; 8: 76–84. [Google Scholar]
- 7. Friedenson M, Rosenbaum MK, Thalheimer EJ, Peters JP. Cutaneous and venous blood glucose curves: II In benign glycosuria and in diabetes. Arch Intern Med 1929; 43: 633–652. [Google Scholar]
- 8. Kuwa K, Nakayama T, Hoshino T, Tominaga M. Relationships of glucose concentrations in capillary whole blood, venous whole blood and venous plasma. Clin Chim Acta 2001; 307: 187–192. [DOI] [PubMed] [Google Scholar]
- 9. Colagiuri S, Sandbaek A, Carstensen B, Christensen J, Glumer C, Lauritzen T et al Comparability of venous and capillary glucose measurements in blood. Diabet Med 2003; 20: 953–956. [DOI] [PubMed] [Google Scholar]
- 10. Eriksson KF, Fex G, Trell E. Capillary‐venous differences in blood glucose values during the oral glucose tolerance test. Clin Chem 1983; 29: 993. [PubMed] [Google Scholar]
- 11. Larsson‐Cohn U. Differences between capillary and venous blood glucose during oral glucose tolerance tests. Scand J Clin Lab Invest 1976; 36: 805–808. [DOI] [PubMed] [Google Scholar]
- 12. Haeckel R, Brinck U, Colic D, Janka H‐U, Puntmann I, Schneider J, Viebrock C. Comparability of blood glucose concentrations measured in different sample systems for detecting glucose intolerance. Clin Chem 2002; 48: 936–939. [PubMed] [Google Scholar]
- 13. Swaminathan A, Lunt H, Chang WS, Logan FJ, Frampton CM, Florkowski CM. Impact of prandial status on the comparison of capillary glucose meter and venous plasma glucose measurements in healthy volunteers. Ann Clin Biochem 2013; 50: 6–12. [DOI] [PubMed] [Google Scholar]
- 14. Gale EAM. The Discovery of Type 1 diabetes. Diabetes 2001; 50: 217–226. [DOI] [PubMed] [Google Scholar]
- 15. Lindquist KA, Chow K, West A, Pyle L, Isbell TS, Cree‐Green M et al The StatStrip glucose monitor is suitable for use during hyperinsulinemic euglycemic clamps in a pediatric population. Diabetes Technol Ther 2014; 16: 298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration . Blood Glucose Monitoring Device. 2014. Available at http://www.fda.gov/medicaldevices/productsandmedicalprocedures/InVitroDiagnostics/GlucoseTestingDevices/default.htm Last accessed 8 June 2015.
- 17. International Organization for standardization ISO 15197 :2013. In vitro diagnostic test systems – Requirements for blood‐glucose monitoring systems for self‐testing in managing diabetes mellitus. Available at http://www.iso.org/iso/catalogue_detail?csnumber=26309 Last accessed 12 November 2015.
- 18. Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo‐Jack S, Fish L et al Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36: 1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Best JD, Kahn SE, Ader M, Wantanabe RM, Ni TC, Bergman RN. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care 1996; 19: 1018–1030. [DOI] [PubMed] [Google Scholar]
- 20. Shumak SL, Gulan M, Zinman B, Gottesman IS. Determination and kinetic analysis of non‐insulin mediated glucose uptake in type 1 (insulin‐dependent) diabetes mellitus. Diabetologia 1989; 32: 28–33. [DOI] [PubMed] [Google Scholar]
- 21. Rossetti P, Bondia J, Vehí J, Fanelli CG. Estimating plasma glucose from interstitial glucose: the issue of calibration algorithms in commercial continuous glucose monitoring devices. Sensors 2010; 10: 10936–10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diabetes Research In Children Network (Direcnet) Study Group , Buckingham BA, Kollman C, Beck R, Kalajian A, Fiallo‐Scharer R et al Evaluation of factors affecting CGMS calibration. Diabetes Technol Ther 2006; 8: 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Study methodology.
Appendix S2. Results.
Appendix S3. Relationship between glucose gradient and demographic and clinical variables.


