Abstract
Anabolic androgenic steroids (AAS) are among the drugs most used by athletes for improving physical performance, as well as for aesthetic purposes. A number of papers have showed the side effects of AAS in different organs and tissues. For example, AAS are known to suppress gonadotropin‐releasing hormone, luteinizing hormone, and follicle‐stimulating hormone. This study investigates the effects of nandrolone on testosterone biosynthesis in Leydig cells using various methods, including mass spectrometry, western blotting, confocal microscopy and quantitative real‐time PCR. The results obtained show that testosterone levels increase at a 3.9 μM concentration of nandrolone and return to the basal level a 15.6 μM dose of nandrolone. Nandrolone‐induced testosterone increment was associated with upregulation of the steroidogenic acute regulatory protein (StAR) and downregulation of 17a‐hydroxylase/17, 20 lyase (CYP17A1). Instead, a 15.6 µM dose of nandrolone induced a down‐regulation of CYP17A1. Further in vivo studies based on these data are needed to better understand the relationship between disturbed testosterone homeostasis and reproductive system impairment in male subjects. J. Cell. Physiol. 231: 1385–1391, 2016. © 2015 The Authors. Journal of Cellular Physiology Published by Wiley Periodicals, Inc.
Anabolic androgenic steroids (AAS) are probably the most commonly used drugs among athletes for improving physical performance (Gheshlaghi et al., 2015; Piacentino et al., 2015). The use of AAS was forbidden by the International Olympic Committee in 1976, and the World Anti‐Doping Agency has included these compounds in the list of prohibited substances (Hartgens and Kuipers, 2004). Despite these bans, AAS consumption is constantly increasing particularly among the younger population at fitness centers (Hartgens and Kuipers, 2004), as well as among teenagers, also for aesthetic purposes (McPhail et al., 2014). Hence, the abuse of AAS is nowadays considered a major and widespread public health issue (Basaria, 2010).
Athletes usually take AAS in the form of intramuscular injections and it has been estimated that AAS abusers consume 600–5000 mg of AAS per week; this is 10–100 times more than the normal testosterone production level by testes (Fronczak et al., 2012). Such dosage may cause serious health consequences especially on male fertility, such as the suppression of the spermatogenesis, testicular atrophy, gynecomastia, and virilization (Basaria, 2010; Chimento et al., 2012; Fronczak et al., 2012). The use of synthetic testosterone and cognate substances leads to a reduction in natural testosterone production, mostly due to a disturbance of the feedback in the hypothalamic–pituitary‐gonadal axis, with consequent decrease in its levels in circulation (Kostic et al., 2011; Rahnema et al., 2014). AAS disturb the endogenous production of gonadotrophins and androgens, which can in turn affect the ultrastructure of the testes (Naraghi et al., 2010; Rahnema et al., 2014). This effect may persist for a long time after drug withdrawal (Keeney et al., 1990). Moreover, there are clear evidence of other serious side effects of AAS administration, that include increased chance of heart attacks, mood changes, liver cancer, and premature arrest of bone growth in younger males (Bhasin et al., 2006; Basaria, 2010; Bhasin, 2010; Turillazzi et al., 2011; Pope et al., 2014).
Nandrolone is considered to be one of the most commonly abused AAS worldwide (Shahidi, 2001; Hartgens and Kuipers, 2004). It has a structure very similar to testosterone and it is included in the class II of AAS which includes 19‐nor‐testosterone‐derivates. Its properties include boosting tissue‐building, maintenance of strength and muscle mass, libido, and bone health. Endogenous nandrolone is produced as a byproduct during biochemical transformational reactions, and its main metabolite (norandrosterone) can be detected in human urine samples in a concentration range of 0.01–0.14 mg/l (Reznik et al., 2001; Bjelic et al., 2014). Despite the beneficial effects of nandrolone and its use in the treatment of many clinical conditions (Wood, 2004), nandrolone administration to male rats induced spermatogenic cell apoptosis (Shokri et al., 2010). Nevertheless, the mechanisms underlying nandrolone‐induced testicular toxicity are not yet fully understood.
Testosterone, like other androgens, is produced within the testis by specialized steroidogenic cells known as Leydig cells (Kostic et al., 2011). The production of this hormone is regulated by a complex neuroendocrine mechanism which involves the pulsatile release of luteinizing hormone (LH) and subsequent cAMP activation of a steroidogenic cascade. Numerous steroidogenic stimuli, as well as intratesticular factors, play a role in the intricate regulatory network of testosterone (Wu et al., 2007; De Maddalena et al., 2012; Janjic et al., 2012). Cholesterol is the common substrate for all types of steroid hormone biosynthesis (including the testosterone one) which begins in the cytosol and is completed in the mitochondria. Steroidogenic acute regulatory protein (StAR) transfers cholesterol to the inner membrane of mitochondria and, through the mobilization and delivery from the outer to the inner mitochondrial membrane, cholesterol is converted to pregnenolone by the cytochrome P450, family 11, subfamily A, polypeptide 1 (CYP11A1) (Stocco et al., 2005). Pregnenolone is further metabolized to progesterone by mitochondrial or microsomal hydroxy‐delta‐5‐steroid dehydrogenase, 3 beta‐, and steroid delta‐isomerase 1 (HSD3B1). In Leydig cells, maturation of progesterone to androstenedione is catalyzed by the 17a‐hydroxylase/17, 20 lyase (CYP17A1); further, the conversion of androstenedione to testosterone is dependent on the activity of 17b‐hydroxysteroid dehydrogenase, a steroid dehydrogenase specific for androgen production (Kostic et al., 2011; Macaluso et al., 2013).
The aim of this study was to investigate the effects of nandrolone on testosterone production by Leydig cells, by evaluating levels, and expression of the main enzymes involved in testosterone biosynthesis.
Materials and Methods
Cell cultures
R2C cells (cat. No. 89031606, ECACC, Health Protection Agency Culture Collections, Salisbury, United Kingdom), a rat Leydig cell line used for testing the steroidogenic function, were cultured in M‐199 medium (Invitrogen Corp., Carlsbad, CA) supplemented with 15% horse serum (HS; Invitrogen Corp.), 2.5% fetal bovine serum (FBS; Invitrogen Corp.), and an antibiotic and antimycotic solution (Invitrogen Corp.) containing 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin B. Cells were incubated at 37°C in a humidified atmosphere with 5% CO2, and maintained in culture as previously described (Macaluso et al., 2012; Barone et al., 2013). When cells reached approximately 80% confluence, they were treated for 48 h with different concentrations of nandrolone prepared by first dissolving Nandrolone Vetranal analytical standard (Sigma–Aldrich, St. Louis, MO) in absolute ethanol to obtain a working solution, and then in the culture medium without horse serum to reach the desired concentrations (0–15.6 μM).
Cell viability test
3‐[4,5‐Dimethylthiaoly]‐2,5‐diphenyltetrazolium bromide (MTT) assay was conducted to detect cell proliferation. A total of 5 × 103 cells per well were seeded in 96‐well plates, and after the incubation period of 24 and 48 h with serial dilutions of nandrolone working solution (0–500 μM), fresh MTT solution (100 μl), dissolved in PBS (0.5 mg/ml final concentration), was added to the cells. After 3 h of incubation at 37°C, the culture media were discarded and replaced with 100 µl of Dimethyl Sulfoxide. Absorbance was measured at 570 nm in a spectrophotometer. The colorimetric assay was performed in triplicate. Absorbance of untreated cells (basal) was considered 100%.
Liquid chromatography‐mass spectrometry
Testosterone measurements were performed by “Locorotondo Labs S.r.l.,” Palermo, Italy. Testosterone in the cell culture medium was quantified using a validated method for serum/plasma analysis of testosterone by liquid chromatography‐mass spectrometry (LC‐MS/MS). The method, developed by Chromsystems®, was fully validated on a AB Sciex API 5500 QTrap™ in terms of recovery (between 95% and 104%), linearity (0.01–6.00 μg/L), CV percentage for intra‐assay (2.6%), and CV percentage for inter‐assay (5.2%) in serum. The apparatus employed consists of an HPLC Eksigent ultra LC 110‐XL System, coupled with a AB Sciex API 5500 QTrap™ mass spectrometer equipped with a TurboIonSpray® Ion Source, working in positive ion mode. The scan Mode used was Multiple Ion Monitoring (MRM) following the characteristic transition 289.1→96.9 (quantifier ion) and 289.1→109.0 (qualifier ion) for Testosterone and 292.2→96.9 (quantifier ion) and 292.2→109.0 (qualifier ion) for the Internal Standard (Testosterone d3). MS parameters, optimized in order to achieve an improved sensitivity, were the following: Curtain gas (CUR): 20 a.u.; Ion Spray Voltage (IS): 5500 V; Ion Source Gas 1 (GS1): 40 a.u.; Ion Source Gas 2 (GS2): 40 a.u.; Temperature (TEM): 650°C; Declustering Potential (DP): 90 for Testosterone and Testosterone d3; Entrance Potential (EP): 8 for Testosterone and 11 for Testosterone d9; Collision Energy (CE): 25 and 29 for the product ion 96.0 and 109.0 respectively, both for Testosterone and for Testosterone d3; Collision Exit Potential (CXP): 10 for Testosterone and Testosterone d3. Chromatographic elution was performed on a reversed phase column. Prior to be injected into the HPLC system, the samples were subjected to a complex process of extraction, purification, and concentration. This sample pre‐treatment included the use of a reversed phase SPE plate (Solid Phase Extraction) that enabled an adequate degree of sensitivity even when analysing a complex matrix. Testosterone levels were also assayed in the culture medium alone as blank, and the value obtained was under the detection limit. Total testosterone analysis in cell cultures was performed in quadruplicate in four different independent experiments.
Western blotting analyses
Western blotting was performed as previously described (Barone et al., 2013). Equal amounts of proteins (40 μg) were separated on SDS–PAGE and transferred onto a nitrocellulose membrane (BioRad, Segrate, Italy). After blocking with 5% albumin bovine serum (Sigma–Aldrich), membranes were probed with primary antibodies [rabbit anti‐CYP17A1 (M‐80) clone polyclonal antibody, goat anti‐HSD3B1 (P‐18) clone polyclonal antibody, rabbit anti‐StAR (FL‐285) clone polyclonal antibody, rabbit anti‐CYP11A1 (H‐165) clone polyclonal antibody, and mouse anti‐β Actin C‐4 clone monoclonal antibody, Santa Cruz Biotechnology] diluted at 1:1000 overnight at 4°C. Protein bands were visualized using the enhanced chemiluminescence (ECL) detection system (GE Healthcare Life Sciences, Milan, Italy), the protein levels were normalized for β actin and the data were evaluated and quantified using ImageJ Free software (NIH, Bethesda, MD). Each experiment was performed in triplicate.
Confocal analyses
Immunofluorescence was performed as described previously (Gorska et al., 2013). Cells were fixed in ice‐cold methanol, incubated with unmasking solution (10 mM trisodium citrate, 0.05% Tween‐20) for 10 min, and treated with blocking solution (3% BSA in PBS) for 30 min at 24°C. Then, a primary antibody against CYP17A1 and StAR (Santa Cruz Biotechnology) was added and incubated in a humidified chamber overnight at 4°C, diluted 1:100. Cells were incubated for 1 h at RT with a conjugated secondary antibody (anti‐rabbit IgG–FITC antibody produced in goat, Sigma–Aldrich, 1:200 dilution); followed by 15 min incubation with HOECHT33342 (Sigma–Aldrich) diluted 1:1000. Finally, slides were mounted with cover slips, and images were taken immediately with a Leica Confocal Microscope TCS SP8 (Leica Microsystems, Heidelberg, Germany). The confocal microscopy analysis was carried out using the Leica application suite advanced fluorescences software. The staining intensity was measured as the mean pixel intensity (PI) normalized to the CSA (cross‐sectional area) of all cells in five fields per slide. Each experiment was quadrupled.
Quantitative real‐time PCR (qRT‐PCR)
The qRT‐PCR technique was performed as previously described (Barone et al., 2013; Di Felice et al., 2013). Total cellular RNA was isolated from cell cultures using TRIzol® REAGENT (Sigma–Aldrich), according to the manufacturer's instructions. RNA was retro‐transcribed using the ImProm‐II Reverse Transcriptase Kit (Promega Corporation, Madison, WI) to obtain cDNA, which was amplified using the StepOnePlus™ Real‐Time PCR System (Life Technologies, Monza, Italy). qRT‐PCR analysis was performed using GoTaq qPCR Master Mix (A6001, Promega). The mRNA levels were normalized to the levels obtained for hypoxanthine phosphoribosyltransferase 1 (HPRT1), for beta‐glucuronidase (GUSB) and for Glyceraldehyde 3‐phosphate dehydrogenase (GADPH). Changes in the transcript level were calculated using the 2‐ΔΔCT method (Livak and Schmittgen, 2001). The cDNA was amplified using the primers shown in Table I. cDNA was amplified using the Rotor‐gene™ 6000 Real‐Time PCR Machine (Qiagen GmbH, Germany). Each experiment was performed in triplicate.
Table I.
Forward and reverse primers used for qPCR
| Primer | Forward | Reverse |
|---|---|---|
| GUSB | 5′‐ACCACCCCTACCACCTATATC‐3′ | 5′‐ATCCAGTAGTTCACCAGCCC‐3′ |
| HPRT1 | 5′‐TGTCATGAAGGAGATGGGAG‐3′ | 5′‐ATCCAGCAGGTCAGCAAAG‐3′ |
| GAPDH | 5′‐GAAACCCATCACCATCTTCC‐3′ | 5′‐TCCACGACATACTCAGCAC‐3′ |
| CYP17A1 | 5′‐TGATCCAAAACTGACCGCC‐3′ | 5′‐TCCACCAGATTTCTGTCGCC‐3′ |
| STAR | 5′‐CCTTGGGCATACTCAACAAC‐3′ | 5′‐GCACCACCTTACTTAGCAC‐3′ |
Statistical analyses
A one‐way ANOVA followed by a Bonferroni post‐hoc test for multiple comparisons was used as an appropriate analysis for the data. All statistical analyses were performed using the GraphPad PrismTM 4.0 program (GraphPad Software Inc., San Diego, California). All data are presented as the mean ± SD, and the level of statistical significance was set at P < 0.05.
Results
Cell viability
The inhibitory effect of nandrolone on R2C cell growth was evaluated using MTT cell viability assays. The cells were treated with serial dilutions of nandrolone within the range of 0–500 μM, for 24 and 48 h. After 24 h of treatment, nandrolone did not induce significant cell death, while cell viability was affected by 48 h of nandrolone treatment at various concentrations. Figure 1 shows the mean value of cell viability after nandrolone treatment. To evaluate the effects of different nandrolone concentrations without significant cell death, 3.9 and 15.6 μM of nandrolone were used for 48 h for further experiments.
Figure 1.
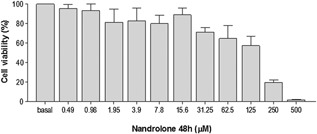
Effects of nandrolone on R2C cell viability. Effects of treatment of R2C cells with different nandrolone doses (0–500 μM) for 48 h evaluated by MTT cell viability tests. Vertical axis: percentage of cell viability. Horizontal axis: nandrolone concentration, in µM. Basal: untreated cells. Data are presented as the mean ± SD of triplicate experiments.
Testosterone production
To determine testosterone secretion, the medium was collected at the end of the incubation period and subsequently analyzed. The nandrolone working solution induced, after 48 h, an increase of the secreted testosterone in R2C cells treated with 3.9 µM nandrolone, compared to the basal sample (P < 0.05) (Fig. 2). Interestingly, a higher concentration (15.6 µM) did not induce a significant testosterone increment compared to basal values.
Figure 2.
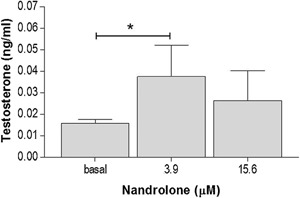
Testosterone production. Testosterone production in cells treated with 3.9 and 15.6 μM nandrolone concentrations for 48 h. Vertical axis: testosterone levels (ng/ml). Horizontal axis: nandrolone concentration, in µM. Basal: untreated cells. Data are presented as the mean ± SD of quadruplicate experiments. * = P < 0.001.
Levels of enzymes involved in testosterone synthesis
Lysates of R2C cells treated with different nandrolone concentrations were analyzed by western blotting analyses to verify the effects of nandrolone stimulation on the levels of proteins involved in testosterone synthesis. Our results showed that levels of StAR (Fig. 3) increased significantly in R2C cells treated with 3.9 µM nandrolone compared to both basal and 15.6 µM (P < 0.05). These results were confirmed by confocal analyses (Fig. 4A and C). Interestingly, the levels of CYP17A1 decreased upon treatment with 15.6 µM of nandrolone compared to both basal and 3.9 µM (P < 0.05) (Fig. 3). In addition, these results were in agreement with confocal analyses (Fig. 4B and D). Finally, western blotting analyses for CYP11A1 and HSD3B1 protein levels did not show any significant differences between untreated and treated cells (Fig. 3), and this datum was also in accordance with confocal analyses (not shown).
Figure 3.
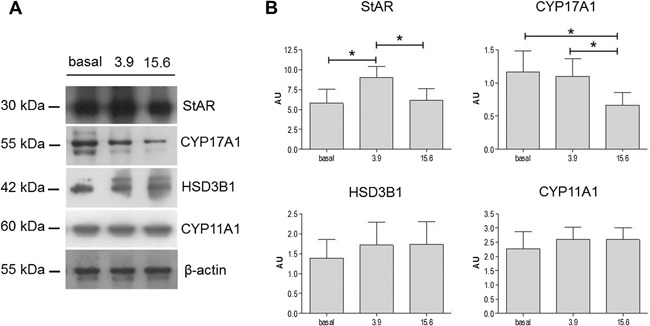
Effect of nandrolone stimulation on steroidogenic proteins. (A) Representative cropped blots for StAR (30 kDa), CYP11A1 (60 kDa), HSD3B1 (42 kDa), and CYP17A1 (55 kDa). The gels were run under the same experimental conditions and β‐actin was used as internal control. (B) Relative expression levels of StAR, CYP11A1, HSD3B1, and CYP17A1. Vertical axis: arbitrary units (AU). Horizontal axis: nandrolone concentration, in µM. Basal: untreated. Data are presented as the mean ± SD of triplicate experiments. * = P < 0.05.
Figure 4.
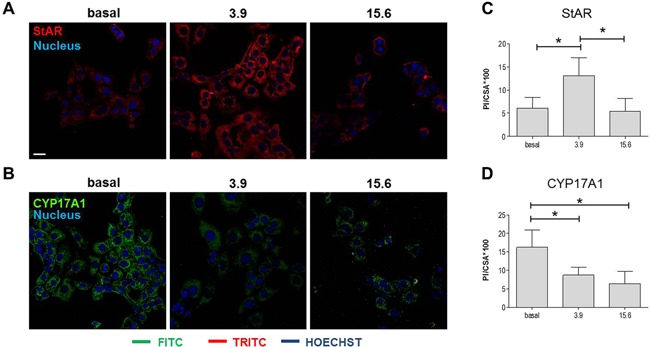
Effect of nandrolone supplementation on StAR and CYP17A1 levels: confocal analyses. (A and B) Representative microphotographs of immunofluorescence stain for StAR (A) and CYP17A1 (B) in cells treated with 3.9 and 15.6 μM of nandrolone. Basal: untreated cells. Bar = 25 μm. (C and D) Representative histograms of the immunofluorescence quantification of the staining intensity for StAR (C) and CYP17A1 (D). The stain intensity was expressed as the mean pixel intensity (PI) normalized to the cross‐sectional area (CSA) using the Leica application suite advanced fluorescence software. Vertical axis: PI/CSA *100. Horizontal axis: nandrolone concentration, in µM. Data are presented as the mean ± SD of quadruplicate experiments. * = P < 0.001.
Expression of StAR and CYP17A1 genes
In order to elucidate nandrolone‐induced modification of the levels of StAR and CYP17A1, qRT‐PCR analyses were performed for both molecules. The results showed that changes in both protein levels were related to variation in gene expression, as showed in Figure 5. In particular, expression of StAR mRNA increased significantly (Fig. 5A) while expression of CYP17A1 mRNA decreased significantly (Fig. 5B) in cells treated with both 3.9 and 15.6 µM concentrations of nandrolone, compared to basal levels (P < 0.05).
Figure 5.
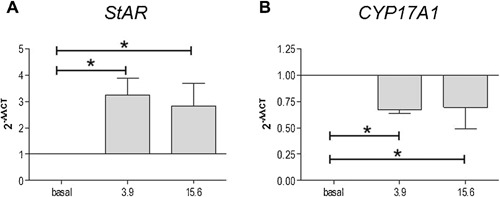
Effect of nandrolone on StAR and CYP17A1 gene expression. qRT‐PCR evaluation of StAR and CYP17A1 gene expression after nandrolone stimulation at 3.9 and 15.6 µM. The graphs show the normalization with the reference genes, according to the Livak Method (2‐ΔΔCT). Vertical axis: 2‐ΔΔCT. Horizontal axis: nandrolone concentration, in µM. Basal: untreated cells. Data are presented as the mean ± SD of triplicate experiments. * = P < 0.05.
Discussion
In many countries, the attention towards performance‐enhancing drug use has focused on elite athletes and the illicit competitive advantage gained from these drugs (Pope et al., 2014). However, performance‐enhancing drugs are also used by many non‐athletes not only for enhancing performance, but also their personal appearance. Moreover, there is widespread misperception on the safety of performance‐enhancing drug use or on the manageability of the adverse effects, when in fact these adverse effects still need to be better clarified (Pope et al., 2012). AAS is a category of performance‐enhancing drugs widely used to enhance muscular mass, which are also used for the treatment of many clinical conditions (Pope et al., 2014).
In this study, we investigated the effects of nandrolone on the main enzymes involved in testosterone biosynthesis in Leydig cells. Testosterone is mainly produced by the Leydig cells of the testes in males and by the theca cells of the ovaries in females, although smaller amounts are synthesized by the adrenal gland in both sexes (Kostic et al., 2011). Testosterone plays a significant role in the growth and development of the male reproductive organs and it is generally accepted that AAS exert their effects via androgen receptor (Hyyppä et al., 1997).
The present study demonstrates that nandrolone administration interferes with the biosynthesis of testosterone in a dose‐dependent fashion. The results showed a significant increase of testosterone levels in the culture medium of R2C cells treated with 3.9 µM of nandrolone, while the levels of this hormone did not change at higher doses (15.6 µM) of nandrolone, compared to basal condition. To the best of our knowledge, the present article is the first to describe the effects of nandrolone treatment on testosterone biosynthesis in Leydig cells, and our results reinforce the data available in the current literature on the deleterious effects of nandrolone on testis.
Recently, it was demonstrated that nandrolone decaonate, at the dose of 10 mg/kg/week for 8 weeks, induced a decrease of serum testosterone levels, a reduction of testicular weight, and an alteration of sperm characteristics in rats (Ahmed, 2015). Nandrolone administration in rats determines a number of morphological changes, such as reduction of the number and the size of Leydig cells, cytoplasmic vacuolization, and lipid droplet deposition. Moreover, from a biochemical point of view, it induces testicular damage by triggering oxidative stress, inflammatory cytokines, matrix metalloproteinases, cell‐adhesion molecules, apoptotic markers, and DNA damage (Nagata et al., 1999; Noorafshan et al., 2005; Naraghi et al., 2010; Ahmed, 2015; Bjelic et al., 2015).
In our experimental model, the most important data obtained show that testosterone increases when Leydig cells are stimulated with a lower concentration of nandrolone (3.9 μM), but this increment disappears when the cells are treated with higher concentrations (15.6 μM). These changes are accompanied by modification at either protein or gene level of some of the main factors involved in testosterone production, such as StAR and CYP17A1. The latter data suggests that nandrolone is able to interfere with testosterone biosynthesis. Interestingly, we reported some discrepancies between protein and gene levels in certain conditions. These differences could be due to a number of causes (including miRNA regulations, post‐translational modifications, protein degradation etc.) that are currently under investigation. However, the presence of these discrepancies could indicate that alternative (and probably unknown) routes for testosterone biosynthesis are stimulated by nandrolone, and should be further investigated in future studies.
In conclusion, our results support the hypothesis that nandrolone is able to modify testosterone production by interfering with StAR and CYP17A1 expression in Leydig cells. Further investigations are essential to better understand in vitro the molecular mechanisms responsible of these changes. Moreover, the data presented here also needs to be confirmed in in vivo models, in order to understand the relationship between AAS administration and testis impairment in human subjects.
Acknowledgments
This study was funded by “Ministero dell'Istruzione, dell'Università e della Ricerca” (FIRB Project code: RBFR12LD0W, titled “L'abuso/dipendenza da anabolizzanti e nuove sostanze psicoattive [Smart Drugs] quale piaga sociale di interesse sanitario e giuridico. Danni d'organo nella popolazione sportiva giovanile: evidenze epidemiologiche, biochimiche, patologiche, tossicologiche e meccanismi di controllo”). Part of this work was carried out using instruments provided by the Euro‐Mediterranean Institute of Science and Technology, and funded with the Italian National Operational Programme for Research and Competitiveness 2007–2013 (Project code: PONa3_00210, European Regional Development Fund). We are profoundly indebted to Amos Tambo (BSc) Hons and Mohsin Roshan (BSc) Hons for technical assistance and insight in the preparation of this article.
Cristoforo Pomara, Rosario Barone, and Antonella Marino Gammazza contributed equally to this work.
Conflicts of interest: The authors have no conflict of interest to declare.
Literature Cited
- Ahmed MA. 2015. Amelioration of nandrolone decanoate‐induced testicular and sperm toxicity in rats by taurine: Effects on steroidogenesis, redox and inflammatory cascades, and intrinsic apoptotic pathway. Toxicol Appl Pharmacol 282:285–296. [DOI] [PubMed] [Google Scholar]
- Barone R, Macaluso F, Catanese P, Marino Gammazza A, Rizzuto L, Marozzi P, Lo Giudice G, Stampone T, Cappello F, Morici G, Zummo G, Farina F, Di Felice V. 2013. Endurance exercise and conjugated linoleic acid (CLA) supplementation up‐regulate CYP17A1 and stimulate testosterone biosynthesis. PLoS ONE 8:e9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaria S. 2010. Androgen abuse in athletes: Detection and consequences. J Clin Endocrinol Metab 95:1533–1543. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Calof OM, Storer TW, Lee ML, Mazer NA, Jasuja R, Montori VM, Gao W, Dalton JT. 2006. Drug insight: Testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab 2:146–159. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S. 2010. The brave new world of function‐promoting anabolic therapies: Testosterone and frailty. J Clin Endocrinol Metab 95:509–511. [DOI] [PubMed] [Google Scholar]
- Bjelic MM, Stojkov NJ, Baburski AZ, Sokanovic SJ, Mihajlovic AI, Janjic MM, Kostic TS, Andric SA. 2014. Molecular adaptations of testosterone‐producing Leydig cells during systemic in vivo blockade of the androgen receptor. Mol Cell Endocrinol 396:10–25. [DOI] [PubMed] [Google Scholar]
- Bjelic MM, Stojkov NJ, Radovic SM, Baburski AZ, Janjic MM, Kostic TS, Andric SA. 2015. Prolonged in vivo administration of testosterone‐enanthate, the widely used and abused anabolic androgenic steroid, disturbs prolactin and cAMP signaling in Leydig cells of adult rats. J Steroid Biochem Mol Biol 149:58–69. [DOI] [PubMed] [Google Scholar]
- Chimento A, Sirianni R, Zolea F, De Luca A, Lanzino M, Catalano S, Andò S, Pezzi V. 2012. Nandrolone and stanozolol induce Leydig cell tumor proliferation through an estrogen‐dependent mechanism involving IGF‐I system. J Cell Physiol 227:2079–2088. [DOI] [PubMed] [Google Scholar]
- De Maddalena C, Vodo S, Petroni A, Aloisi AM. 2012. Impact of testosterone on body fat composition. J Cell Physiol 227:3744–3748. [DOI] [PubMed] [Google Scholar]
- Di Felice V, Serradifalco C, Rizzuto L, De Luca A, Rappa F, Barone R, Di Marco P, Cassata G, Puleio R, Verin L, Motta A, Migliaresi C, Guercio A, Zummo G. 2013. Silk fibroin scaffolds enhance cell commitment of adult rat cardiac progenitor cells. J Tissue Eng Regen Med 9:E51–E64. [DOI] [PubMed] [Google Scholar]
- Fronczak CM, Kim ED, Barqawi AB. 2012. The insults of illicit drug use on male fertility. J Androl 33:515–528. [DOI] [PubMed] [Google Scholar]
- Gheshlaghi F, Piri‐Ardakani MR, Masoumi GR, Behjati M, Paydar P. 2015. Cardiovascular manifestations of anabolic steroids in association with demographic variables in body building athletes. J Res Med Sci 20:165–168. [PMC free article] [PubMed] [Google Scholar]
- Gorska M, Marino Gammazza A, Zmijewski MA, Campanella C, Cappello F, Wasiewicz T, Kuban‐Jankowska A, Daca A, Sielicka A, Popowska U, Knap N, Antoniewicz J, Wakabayashi T, Wozniak M. 2013. Geldanamycin‐induced osteosarcoma cell death is associated with hyperacetylation and loss of mitochondrial pool of heat shock protein 60 (hsp60). PLoS ONE 8:e1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartgens F, Kuipers H. 2004. Effects of androgenic‐anabolic steroids in athletes. Sports Med 34:513–554. [DOI] [PubMed] [Google Scholar]
- Hyyppä S, Karvonen U, Räsänen LA, Persson SG, Pösö AR. 1997. Androgen receptors and skeletal muscle composition in trotters treated with nandrolone laureate. Zentralbl Veterinarmed A 44:481–491. [DOI] [PubMed] [Google Scholar]
- Janjic MM, Stojkov NJ, Andric SA, Kostic TS. 2012. Anabolic‐androgenic steroids induce apoptosis and NOS2 (nitric‐oxide synthase 2) in adult rat Leydig cells following in vivo exposure. Reprod Toxicol 34:686–693. [DOI] [PubMed] [Google Scholar]
- Keeney DS, Sprando RL, Robaire B, Zirkin BR, Ewing LL. 1990. Reversal of long‐term LH deprivation on testosterone secretion and Leydig cell volume, number and proliferation in adult rats. J Endocrinol 127:47–58. [DOI] [PubMed] [Google Scholar]
- Kostic TS, Stojkov NJ, Bjelic MM, Mihajlovic AI, Janjic MM, Andric SA. 2011. Pharmacological doses of testosterone upregulated androgen receptor and 3‐Beta‐hydroxysteroid dehydrogenase/delta‐5‐delta‐4 isomerase and impaired Leydig cells steroidogenesis in adult rats. Toxicol Sci 121:397–407. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Macaluso F, Morici G, Catanese P, Ardizzone NM, Marino Gammazza A, Bonsignore G, Lo Giudice G, Stampone T, Barone R, Farina F, Di Felice V. 2012. Effect of conjugated linoleic acid on testosterone levels in vitro and in vivo after an acute bout of resistance exercise. J Strength Cond Res 26:1667–1674. [DOI] [PubMed] [Google Scholar]
- Macaluso F, Barone R, Catanese P, Carini F, Rizzuto L, Farina F, Di Felice V. 2013. Do fat supplements increase physical performance? Nutrients 5:509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail SM, Schippers M, Marshall AL. 2014. Age, physical inactivity, obesity, health conditions, and health‐related quality of life among patients receiving conservative management for musculoskeletal disorders. Clin Interv Aging 10:1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S, Kurosawa M, Mima K, Nambo Y, Fujii Y, Watanabe G, Taya K. 1999. Effects of anabolic steroid (19‐nortestosterone) on the secretion of testicular hormones in the stallion. J Reprod Fertil 115:373–379. [DOI] [PubMed] [Google Scholar]
- Naraghi MA, Abolhasani F, Kashani I, Anarkooli IJ, Hemadi M, Azami A, Barbarestani M, Aitken RJ, Shokri S. 2010. The effects of swimming exercise and supraphysiological doses of nandrolone decanoate on the testis in adult male rats: A transmission electron microscope study. Folia Morphol (Warsz) 69:138–146. [PubMed] [Google Scholar]
- Noorafshan A, Karbalay‐Doust S, Ardekani FM. 2005. High doses of nandrolone decanoate reduce volume of testis and length of seminiferous tubules in rats. APMIS 113:122–125. [DOI] [PubMed] [Google Scholar]
- Piacentino D, Kotzalidis GD, Del Casale A, Aromatario MR, Pomara C, Girardi P, Sani G. 2015. Anabolic‐androgenic steroid use and psychopathology in athletes. A Systematic Review. Curr Neuropharmacol 13:101–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr. , Kanayama G, Hudson JI. 2012. Risk factors for illicit anabolic‐androgenic steroid use in male weightlifters: A cross‐sectional cohort study. Biol Psychiatry 71:254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr. , Wood RI, Rogol A, Nyberg F, Bowers L, Bhasin S. 2014. Adverse health consequences of performance‐enhancing drugs: An endocrine society scientific statement. Endocr Rev 35:341–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahnema CD, Lipshultz LI, Crosnoe LE, Kovac JR, Kim ED. 2014. Anabolic steroid‐induced hypogonadism: Diagnosis and treatment. Fertil Steril 101:1271–1279. [DOI] [PubMed] [Google Scholar]
- Reznik Y, Dehennin L, Coffin C, Mahoudeau J, Leymarie P. 2001. Urinary nandrolone metabolites of endogenous origin in man: A confirmation by output regulation under human chorionic gonadotropin stimulation. J Clin Endocrinol Metab 86:146–150. [DOI] [PubMed] [Google Scholar]
- Shahidi NT. 2001. A review of the chemistry, biological action, and clinical applications of anabolic‐androgenic steroids. Clin Ther 23:1355–1390. [DOI] [PubMed] [Google Scholar]
- Shokri S, Aitken RJ, Abdolvahhabi M, Abolhasani F, Ghasemi FM, Kashani I, Ejtemaeimehr S, Ahmadian S, Minaei B, Naraghi MA, Barbarestani M. 2010. Exercise and supraphysiological dose of nandrolone decanoate increase apoptosis in spermatogenic cells. Basic Clin Pharmacol Toxicol 106:324–330. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Wang X, Jo Y, Manna PR. 2005. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: More complicated than we thought. Mol Endocrinol 19:2647–2659. [DOI] [PubMed] [Google Scholar]
- Turillazzi E, Perilli G, Di Paolo M, Neri M, Riezzo I, Fineschi V. 2011. Side effects of AAS abuse: An overview. Mini Rev Med Chem 11:374–389. [DOI] [PubMed] [Google Scholar]
- Wood RI. 2004. Reinforcing aspects of androgens. Physiol Behav 83:279–289. Review. [DOI] [PubMed] [Google Scholar]
- Wu X, Wan S, Lee MM. 2007. Key factors in the regulation of fetal and postnatal Leydig cell development. J Cell Physiol 213:429–433. [DOI] [PubMed] [Google Scholar]


