Abstract
The effects of hemodynamic and interstitial mechanical forces on endothelial biology in vivo have been appreciated for over half a century, regulating vessel network development, homeostatic function, and progression of vascular disease. Investigations using cultures of endothelial cells on two-dimensional (2D) substrates have elucidated important mechanisms by which microenvironmental stresses are sensed and transduced into chemical signaling responses. However recent studies in vivo and in three-dimensional (3D) in vitro models of vascular beds have enabled the investigation of forces and cellular behaviors previously not possible in traditional 2D culture systems. These studies support a developing paradigm that the 3D chemo-mechanical architecture of the vascular niche impacts how endothelial cells both sense and respond to microenvironmental forces. We present evolving concepts in endothelial force sensing and mechanical signaling and highlight recent insights gained from in vivo and 3D in vitro vascular models.
INTRODUCTION
Dynamic cellular response to mechanical forces is fundamental to vascular biology, regulating the development of the vascular plexus [1], vessel morphogenesis and sprouting [2,3], vessel barrier function [4], inflammatory signaling [5], gene transcription, and arteriosclerosis [6,7]. These mechanical forces are comprised of both extrinsic stresses, from blood flow-driven shear stress and circumferential stretch, extracellular matrix (ECM) ligation, and interstitial pressure, and intrinsic stresses from applied cellular tractions through cell-cell and cell-ECM adhesions. Precise sensing and integration of these stresses maintain vascular homeostasis and, when dysregulated, drive pathological progression [8,9].
Initial investigations of cells cultured on flat (2D) surfaces have identified key cellular structures and molecular machinery that sense and transduce forces of fluid shear and matrix stretch in endothelial cells and these observations continue to provide the scientific foundation for current studies. However, 2D endothelial cultures are inherently limited to recapitulating only a subset of relevant mechanical forces, such as interstitial flow, and cannot appropriately model some of the endothelial behaviors observed in vivo, such as angiogenic sprouting. Recent advances across scientific disciplines including tissue engineering, materials sciences, molecular sensors, mechanics, and computational methods have permitted the inclusion and tunability of distinct 3D hemodynamic and interstitial forces in both in vivo and 3D in vitro vascular models. Observations of dynamic endothelial behaviors in response to mechanical force stimuli in such systems have revealed that the distinct 3D chemo-mechanical architecture of the vascular microenvironment critically influences the endothelial response to force.
In this commentary, we first briefly provide a historical context of studies in endothelial mechanotransduction. We then will focus on recently identified molecular mechanisms of endothelial mechanotransduction, with highlights of conceptual advances derived from 3D in vivo and in vitro vascular models. We will explore the forces influencing vascular biology in microvasculature models, but allude to other vessel classes when appropriate.
EARLY INVESTIGATIONS OF ENDOTHELIAL MECHANOTRANSDUCTION
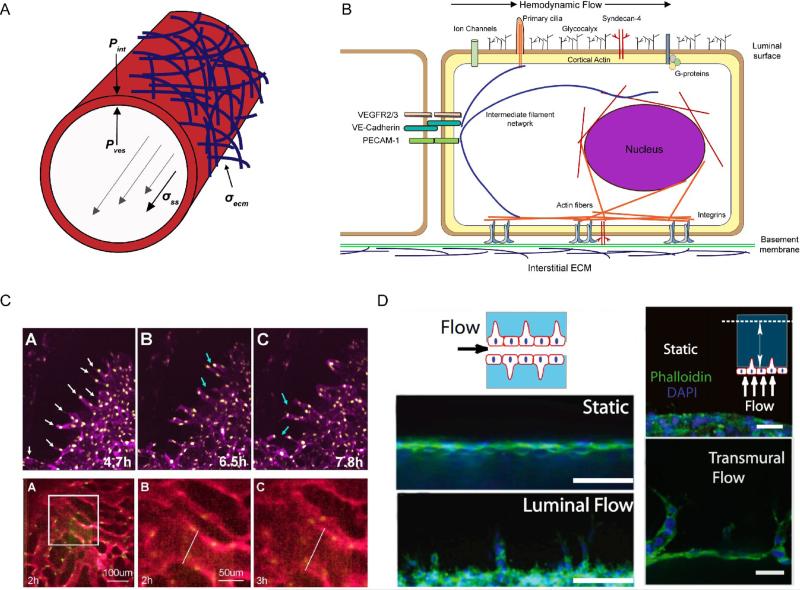
The field of endothelial mechanotransduction arose from the early observations in the arterial circulation that areas of disturbed blood flow were a critical determinant for where the early pathologic changes of atherosclerosis were initiated. Shortly thereafter, the ability of the endothelium to actively sense and respond to fluid shear stress was demonstrated by varying the viscosity of perfused medium in isolated arterial preparations [10]. Initial investigations into endothelial mechanotransduction thus focused on the mechanical stresses resulting from hemodynamic flow, which manifests as shear stress (σss), the frictional drag force per unit area from blood flow parallel to the vessel wall, and luminal blood pressure (Pves) which acts normal to the vessel wall to induce circumferential stretching (Figure 1A).
Figure 1.
A) Force diagram of a microvessel under flow. Hemodynamic flow (gray arrows) exerts frictional shear stress σss parallel to the vessel wall, and pressure Pves normal to the vessel wall. At the basal interface, cell-ECM stresses σecm are driven by integrin ligation to basement membrane and interstitial ECMs. Interstitial fluid accumulation increases interstitial pressures Pint that act on the outer vessel membrane. Transmural pressure PTM is defined by the difference vessel and interstitial pressures. B) Representative diagram of the intracellular localization of mechanosensors and transducers during endothelial exposure to flow, noting how individual elements are integrated in a force-sensitive continuum. Adapted from [6]. C) (top) Timelapse images of sprouting angiogenesis (white arrows) and anastomosis events (blue arrows) in E8.5 yolk sacs from [64]. (bottom) Timelapse images of flow-driven vessel arterial fusion events in yolk sacs from [47]. D) Using 3D in vitro vessel models, a shear stress threshold was identified for both luminal (left) and transmural (right) flows that drives vascular sprouting. Scale bars 50 and 100 microns. [3].
To study endothelial behaviors in response to shear stress, 2D parallel-plate flow chambers and cone-and-plate chambers were utilized to expose monolayers of endothelial cells to defined flow profiles. These seminal studies revealed that applied shear stress initiated mechanical changes within the cell, inducing cellular and cytoskeletal alignment in the direction of flow and the strengthening of cell-cell adherens junction complexes [11,12]. Fluid shear stress was further demonstrated to directly regulate endothelial cell proliferation, gene expression, lipid composition and metabolism, and inflammation [7,8,13]. Pulsatile or pathological changes in blood pressure can create an acute or chronic mechanical stimulus in the form of circumferential stretch. Early investigations into endothelial stretch sensing were conducted by culturing cell monolayers on deformable 2D silicone rubber membranes that were subjected to defined stretch. Endothelial cells were observed to remodel and orient their actin stress fibers perpendicular to the axis of stretch to bear less tension and thus minimize stretch-induced increases in intracellular mechanical energy [14]. These early shear and stretch studies demonstrated that hemodynamic mechanical forces directly modulate the structure and function of endothelial cells, providing the validation to explore further the forces and molecular mechanisms that influence endothelial mechanotransduction.
MOLECULAR FORCE SENSING AND MECHANOTRANSDUCTION
Despite a developed understanding of the importance of mechanical force on endothelial biology, surprisingly little is known about how endothelial cells sense force and transduce it into chemical signaling. This is due to the conundrum that, unlike chemical receptor-ligand signaling, mechano-receptors and transducers have no inherent “ligand” and researchers are thus forced to broadly probe cellular states before and after mechanical stimulus [15]. Many candidate mechanosensors have been proposed to function in endothelial force sensing, including the glycocalyx [16,17], plasma membrane fluidity [18], ion channels [19,20], primary cilia [21], nuclei [22], integrin-based focal adhesions [23-25], cell-cell adherens junctions, intermediate filament and actin networks [26,27], G proteins [28], and caveolae [29] (Figure 1B). In all likelihood each of these mechanically-sensitive structures acts in concert, or contextually, with the others to define a multimeric, force-sensitive network. However, we lack a precise molecular understanding of how, and in what context, these various elements conduct their signaling response to mechanical force.
Identified endothelial mechanosensors, transducers, and associated signaling molecules are summarized in Table 1. Non-homogenous remodeling of cytoskeletal networks at lateral and basal structures in response to external stresses implicate cell-cell and cell-matrix adhesions as primary endothelial mechanotransducers [30]. At the apicolateral membrane, endothelial cells form mechanical connections to neighboring cells through a form of cell-cell adhesion termed adherens junctions (AJs). AJs resist dissociating forces, transmit forces to adjacent cells, and are remodeled in response to changes in internal and external tension. Within AJs is the shear stress-responsive complex of PECAM-1, VE-Cadherin, and VEGFR2/3, a complex that is both necessary and sufficient to impart flow-responsiveness [31], directs cellular alignments to flow, and is instrumental in promoting pro-atherosclerotic states. FRET-based molecular tension sensors elegantly demonstrated that shear stress triggers an increase in tension across junctional PECAM-1, but a decrease in tension across VE-Cadherin and cell-cell junctions [32]. Src-mediated phosphorylation of PECAM-1 was one of the first identified molecular modifications in endothelial cells in response to a variety of mechanical stimuli. PECAM-1 phosphorylation promotes Erk signaling, activation of VEGFR2, and production of nitric oxide in response to flow [15]. Despite changes in molecular tension, VE-Cadherin is not a direct mechanotransducer, rather a scaffolding molecule. Recent studies have articulated that the transmembrane domain of VE-cadherin facilitates the association of PECAM-1 and VEGFR2/3, and is required for the downstream activity of VEGFR2 in response to mechanical activation of PECAM-1 [31,33]. VEGFR2 undergoes ligand-independent phosphorylation in response to shear stress, leading to activation of MAPK, Akt, and PI3K pathways among others [34]. Cyclic strain has been demonstrated to trigger VEGFR2 dissociation from VE-cadherin at AJs and increase vascular permeability [35], implicating the complex in the transduction of mechanical stretch. VEGFR3 was recently identified as another member of this shear-responsive complex, signaling similarly to VEGFR2 and also dependent on VE-Cadherin transmembrane scaffolding [33].
Table 1.
Identified mechanical sensors and transducers in endothelial cells
| Structural mechanosensors | ||
|---|---|---|
| Cellular localization | Mechanical activation | |
| Stretch-induced ion channels [19,20] | Apical membrane | Fluid shear stress, circumferential strain |
| Membrane fluidity/lipid composition [18,61] | Apical membrane | Fluid shear stress, circumferential strain |
| Primary cilia [21] | Apical membrane | Fluid shear stress |
| Glycocalyx [16,17] | Apical membrane | Fluid shear stress |
| Caveole [29] | Internal plasma membrane | Fluid shear stress |
| Nucleus [22] | Cytoplasm | Fluid shear stress, circumferential strain, cell-ECM stress |
| Focal adhesions [25] | Basal ECM interface | Fluid shear stress, circumferential strain, cell-ECM stress |
| Heterotrimeric G-proteins [28] | Apical/basal membrane | Fluid shear stress, cell-ECM stress |
| Adherens junctions [31] | Apical/lateral membrane | Fluid shear stress, circumferential strain |
| Molecular mechanosensors, transducers, and associated signaling | ||
|---|---|---|
| Intracellular localization | Relevant function | |
| PECAM-1 | Adherens junctions, Apicolateral membrane | Phosphorylated in response to mechanical stimuli, transactivates VEGFR2/3 [15]. |
| VE-Cadherin | Adherens junctions | Transmembrane scaffolding of PECAM-1 and VEGFR2/3 [31]. |
| VEGFR2 | Adherens junctions, Apical membrane | Ligand-independent phosphorylation in response to shear stress, stretch, activates PI3K/Akt [33]. |
| VEGFR3 | Adherens junctions, Apical membrane | Ligand-independent phosphorylation in response to shear stress, activates PI3K/Akt [33]. |
| Cholesterol | Apical membrane | Composition in membrane alters bilayer viscosity, depletion abolishes shear responses [61]. |
| Piezo1 | Apical membrane | Shear regulation of Piezo1 ion channel currents during developmental vascular remodeling [19]. |
| Syndecan-4 | Apical and basal membranes | Shear-driven cell alignment independent of VEGFR2 pathway [60]. |
| α5, β1, αVβ3 integrins | Basal adhesion complexes | Activation by PI3K downstream of shear stress to controls cell alignment. Application and sensing of cell-ECM stresses [6,25]. |
| FAK, Src kinases | Focal adhesions, cortical membrane | Shear stress increases phosphorylation and associated signaling [62]. |
| Actin and intermediate filament cytoskeletons | Cortical plasma membrane, cytoplasmic, perinuclear | Fluid shear stress drives non-homogenous filament deformations. Inhibition blocks many responses to flow/ECM stress [26,30]. |
| Rap1b | Internal plasma membrane/cytoplasm | Activated by shear stress, promotes formation of PECAM-1, VE-Cadherin, VEGFR2 complex [63]. |
| Rho GTPases (RhoA, Cdc42, Rac1) | Internal plasma membrane/cytoplasm | Activity increased in response to shear-driven integrin activation to orchestrate cytoskeletal, junctional, and morphology dynamics [38,39]. |
At the basal interface, endothelial cells interact with basement membrane and interstitial ECM proteins through integrin-based focal adhesions. PI3K signaling downstream of the AJ mechanotransduction complex leads to the conformational activation of integrins and changes in cell-ECM stresses at the basal interface [23,25]. Integrin activation and ligation to ECM is required for endothelial adaptation to shear stress through the activation of the Rho family GTPases Rac1, RhoA, and Cdc42 [36,37], which coordinate the cytoskeletal rearrangements required for cellular alignment to flow, inflammatory signaling, and presentation of apical cell-adhesion receptors [38]. Rac1 activity also serves to promote VE-Cadherin stability by locally counteracting actomysoin force on VE-cadherin trans-dimers [39]. Recent evidence suggests that the AJ mechanotransduction complex may be able to spatially control the activation of Rac1 through the local recruitment of the GEF Trio to VE-cadherin [40].
Circumferential strains and changes in ECM stiffness modulate cell-ECM adhesion traction stresses (σecm) through conformational changes in integrin activation that manifest as alterations in endothelial phenotype, global cytoskeletal organization, and luminal shear responsiveness (Figure 1A) [6,41]. Endothelial cells plated on 2D polyacrylamide gels show that increased substrate stiffness alters the shear stress threshold required to induce morphological changes and alignment [41], ECM stiffening enhances VE-cadherin-mediated forces [42], and evidence suggests similar mechanisms exist in 3D vessels [43]. Rearrangements of actin stress fibers in response to cyclic stretch are also dependent on integrin activation and ECM ligation [8]. While the mechanisms of integrin force mechanotransduction have been reviewed in detail [44], in endothelial cells the downstream cytoskeletal changes in response to altered cell-ECM stresses are orchestrated primarily through the Rho GTPase RhoA, Src, and FAK kinases. Additionally, in vivo observations have shown that mural cells, pericytes and smooth muscle cells, can contribute to the basal contractile traction stresses translated to the endothelium.
The concept of decentralized endothelial mechanotransduction across a cellular continuum of force-sensitive molecules, adaptive structural units, and signal transmission elements is not novel [7,9] (Figure 1b), yet its relevance persists as our molecular understanding of endothelial mechanical signaling deepens. With the discoveries of new mechanosensors, transducers, and their associated signaling, and crosstalk mechanisms, it will be important to understand not only how these molecules function individually, but also how they mechanically integrate into this continuum. With advances in tunable biomaterials and 3D in vitro microfluidic platforms, it will be of interest to test the roles of identified endothelial force sensors under newly testable mechanical settings, such as 3D interstitial flow and pressure, which have been demonstrated to dictate 3D cancer cell migration and tumor phenotype [45,46].
FORCES AND 3D ENDOTHELIAL BEHAVIOR
While historic significance has been placed on correlations between anatomical vascular architectures, blood flow profiles, and sites of vascular pathogenesis, studies using in vivo vascular models have directly demonstrated the regulatory role of mechanical forces during vascular morphogenesis. Using a combination of genetic and mechanical manipulation, in vivo reduction of shear stress impaired developmental vascular remodeling at the onset of blood flow in the developing yolk sac [1]. In a recent follow-up study, Udan et al. elegantly demonstrated that vessel diameter changes during embryonic remodeling occurred via both vessel fusions events and directed endothelial migration. These dynamic endothelial behaviors were observed to be mechanically-dependent, dictated by local flow profiles and restricted to specific vessel classes [47]. Further, in vivo platforms permit the study of distinct force types, such as interstitial pressure (Figure 1A), on endothelial behavior. During tumor progression, local interstitial fluid accumulation due to lymphatic dysfunction, increased vascular permeability, and altered oncotic gradients elevate interstitial pressures. Resulting pressure gradients generate transmural flows which impair vascular transport and apply transmural shear stresses which influences endothelial function [48,49].
Intrinsically, force generation through RhoA-mediated contractility is critical for proper vessel architecture during in vivo angiogenesis [50]. Soluble morphogenic factors such as VEGF and sphingosine-1-phosphate, which stimulate angiogenic events in vivo, directly modulate applied cellular traction stresses through the RhoA-ROCK signaling axis [51]. Intriguingly, a novel antagonistic regulatory mechanism was recently identified between Notch and VEGFR signaling pathways during angiogenesis. Fluctuations in Notch/VEGFR signaling result in differential VE-cadherin dynamics, cell-cell adhesion phenotype, and endothelial cell migration during angiogenic sprouting [52]. These findings identify the regulation of cell-cell adhesions through intrinsic force-generation in response to chemical factors as critical for in vivo angiogenesis.
While these in vivo studies demonstrate elegantly how complex force fields appear to drive changes in vascular morphogenesis, our inability to model these processes in traditional 2D in vitro systems has limited detailed molecular mechanistic characterization of the mechanotransduction processes. Recently, engineering investigators have begun to develop 3D in vitro microfluidic devices in which one can seed endothelial cells to form perfusable vascular networks. The inherent similarity (vessel geometry, ECM composition and dimensionality, cellular architectures, flow profiles) between in vivo microvascular networks and these 3D in vitro vascular models provides an attractive approach to begin to investigate these questions. The first studies using these 3D in vitro microfluidic vessel models have focused on confirming many force-driven cell behavior observations made in in vivo and 2D culture: increased barrier function and junctional reorganization in response to elevated shear stress [53], changes in transmural pressure affect vessel permeability, sprouting, and monolayer integrity [54-56], and alterations in ECM stiffness dictate flow responsiveness and vessel barrier function [41,43]. Studies in 3D ex vivo and in vitro vascular beds have demonstrated that application of bulk tensile stress to alter 3D ECM mechanical properties can regulate neovessel sprouting, elongation during angiogenesis, and vascular network organization [57,58].
However the allure of in vitro microfluidic vessel platforms is identifying novel cellular behaviors and molecular functions that are uniquely observable in controlled 3D in vitro settings. Recently, our laboratory identified a previously uncharacterized endothelial response to shear stress using 3D in vitro microvascular vessel models. For both luminal and transmural flows there exists a shear stress threshold that, when surpassed, triggers local angiogenic sprouting. While matrix metalloproteinase 1 (MMP1) was identified as the dominant downstream effector regulating branching initiation [3], it remains unclear what shear-sensitive molecules initiate this response and whether similar processes govern luminal versus transmural shear sensing. Of interest will be the systematic combination of synthetic biomaterials that allow independently tunable ECM mechanical properties [59] and microfluidically controlled flow profiles in 3D in vitro vascular models to dissect the relative contributions of mechanical force stimuli and associated molecular mechnotransducers during dynamic endothelial force-responsive behaviors.
FUTURE OUTLOOK
Mechanical signaling in endothelial cells is at a compelling juncture. While numerous putative sensors and transducers of mechanical force have been identified, undoubtedly more remain undiscovered. Discoveries of candidate mechanosensors are confounded by an emerging theme that all proteins that are force-responsive also have secondary, non-mechanical functions. This is illustrated by the recently identified shear-responsiveness of the ion channel Piezo1 [19], the transmembrane proteoglycan Syndecan-4 [60], and the PECAM-1, VE-Cadherin, VEGFR2/3 complex [31,33]. While the emergence of CRISPR/Cas9 genome editing technology should facilitate high throughput screening for potential mechanical sensors and transducers, new discoveries will be driven by the combination of creative molecular approaches and engineered assays in response to this mechanical challenge. 3D microfluidic in vitro vascular models provide a platform to not only investigate novel influences of force on endothelial behavior, but also elucidate the contextual relevance and coordination of the growing list of mechanically-sensitive proteins. Advances in synthetic biomaterials compatible with microfluidic implementation will allow for the independent tuning of mechanical properties in the 3D perivascular environment. Combining these approaches with cellular and molecular engineering will begin to provide a framework for understanding how individual mechanical signaling elements contribute to a larger endothelial mechanosensitive continuum.
HIGHLIGHTS.
- Environmental and cell-generated forces profoundly influence endothelial behavior.
- 3D in vitro models allow the recapitulation of in vivo force-driven endothelial behaviors.
- Novel mechanosensory mechanisms provide insight into an endothelial force-sensitive continuum.
ACKNOWLEDGEMENTS
The authors would like to thank Jeroen Eyckmans and William Polacheck for critical reading of the manuscript. This work was supported by T32 EB005583 (M.L.K.) and EB00262 (C.S.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chouinard-Pelletier G, Jahnsen ED, Jones EA. Increased shear stress inhibits angiogenesis in veins and not arteries during vascular development. Angiogenesis. 2013;16:71–83. doi: 10.1007/s10456-012-9300-2. [DOI] [PubMed] [Google Scholar]
- 3•.Galie PA, Nguyen DH, Choi CK, Cohen DM, Janmey PA, Chen CS. Fluid shear stress threshold regulates angiogenic sprouting. Proc Natl Acad Sci U S A. 2014;111:7968–7973. doi: 10.1073/pnas.1310842111. [Identifies a novel shear stress threshold from luminal and transmural flows that triggers endothelial sprouting in 3D in vitro engineered microfluidic vessels.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarbell JM. Mass transport in arteries and the localization of atherosclerosis. Annu Rev Biomed Eng. 2003;5:79–118. doi: 10.1146/annurev.bioeng.5.040202.121529. [DOI] [PubMed] [Google Scholar]
- 5.Helderman F, Segers D, de Crom R, Hierck BP, Poelmann RE, Evans PC, Krams R. Effect of shear stress on vascular inflammation and plaque development. Curr Opin Lipidol. 2007;18:527–533. doi: 10.1097/MOL.0b013e3282ef7716. [DOI] [PubMed] [Google Scholar]
- 6.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 9.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- 10.Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988;112:1018–1031. [PubMed] [Google Scholar]
- 11.Galbraith CG, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil Cytoskeleton. 1998;40:317–330. doi: 10.1002/(SICI)1097-0169(1998)40:4<317::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Dewey CF, Jr., Bussolari SR, Gimbrone MA,, Jr., Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981;103:177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 13.Johnson BD, Mather KJ, Wallace JP. Mechanotransduction of shear in the endothelium: basic studies and clinical implications. Vasc Med. 2011;16:365–377. doi: 10.1177/1358863X11422109. [DOI] [PubMed] [Google Scholar]
- 14.Kaunas R, Nguyen P, Usami S, Chien S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci U S A. 2005;102:15895–15900. doi: 10.1073/pnas.0506041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujiwara K. Platelet endothelial cell adhesion molecule-1 and mechanotransduction in vascular endothelial cells. J Intern Med. 2006;259:373–380. doi: 10.1111/j.1365-2796.2006.01623.x. [DOI] [PubMed] [Google Scholar]
- 16.Pahakis MY, Kosky JR, Dull RO, Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun. 2007;355:228–233. doi: 10.1016/j.bbrc.2007.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93:e136–142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto K, Ando J. Vascular endothelial cell membranes differentiate between stretch and shear stress through transitions in their lipid phases. Am J Physiol Heart Circ Physiol. 2015;309:H1178–1185. doi: 10.1152/ajpheart.00241.2015. [DOI] [PubMed] [Google Scholar]
- 19••.Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515:279–282. doi: 10.1038/nature13701. [Uses genetic manipulation, embryonic modeling, and in vitro assays to identify a critical mechanosensory role for the calcium ion channel Piezo1 during endothelial shear stress sensing.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naruse K, Yamada T, Sokabe M. Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am J Physiol. 1998;274:H1532–1538. doi: 10.1152/ajpheart.1998.274.5.H1532. [DOI] [PubMed] [Google Scholar]
- 21.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedorchak GR, Kaminski A, Lammerding J. Cellular mechanosensing: getting to the nucleus of it all. Prog Biophys Mol Biol. 2014;115:76–92. doi: 10.1016/j.pbiomolbio.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orr AW, Ginsberg MH, Shattil SJ, Deckmyn H, Schwartz MA. Matrix-specific suppression of integrin activation in shear stress signaling. Mol Biol Cell. 2006;17:4686–4697. doi: 10.1091/mbc.E06-04-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J Cell Biol. 2005;169:191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helmke BP, Goldman RD, Davies PF. Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Circ Res. 2000;86:745–752. doi: 10.1161/01.res.86.7.745. [DOI] [PubMed] [Google Scholar]
- 27.Osborn EA, Rabodzey A, Dewey CF, Jr., Hartwig JH. Endothelial actin cytoskeleton remodeling during mechanostimulation with fluid shear stress. Am J Physiol Cell Physiol. 2006;290:C444–452. doi: 10.1152/ajpcell.00218.2005. [DOI] [PubMed] [Google Scholar]
- 28.Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci U S A. 1998;95:2515–2519. doi: 10.1073/pnas.95.5.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizzo V, Morton C, DePaola N, Schnitzer JE, Davies PF. Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Am J Physiol Heart Circ Physiol. 2003;285:H1720–1729. doi: 10.1152/ajpheart.00344.2002. [DOI] [PubMed] [Google Scholar]
- 30.Helmke BP, Thakker DB, Goldman RD, Davies PF. Spatiotemporal analysis of flow-induced intermediate filament displacement in living endothelial cells. Biophys J. 2001;80:184–194. doi: 10.1016/S0006-3495(01)76006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 32•.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol. 2013;23:1024–1030. doi: 10.1016/j.cub.2013.04.049. [Elegantly utilizes molecular tension sensors to map junctional forces under shear stress. Identifies that intramolecular tension increases across PECAM-1, but decreases across VE-cadherin and cell-cell adhesions in response to flow.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Coon BG, Baeyens N, Han J, Budatha M, Ross TD, Fang JS, Yun S, Thomas JL, Schwartz MA. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J Cell Biol. 2015;208:975–986. doi: 10.1083/jcb.201408103. [Defines the role of VE-cadherin during shear sensing as a critical adapter which binds the transmembrane regions of VEGFR2 and the newly identified VEGFR3 to assemble the mechanosensory complex.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shay-Salit A, Shushy M, Wolfovitz E, Yahav H, Breviario F, Dejana E, Resnick N. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc Natl Acad Sci U S A. 2002;99:9462–9467. doi: 10.1073/pnas.142224299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian Y, Gawlak G, O'Donnell JJ, 3rd, Birukova AA, Birukov KG. Activation of VEGF receptor-2 mediates endothelial permeability caused by cyclic stretch. J Biol Chem. 2016 doi: 10.1074/jbc.M115.690487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kutys ML, Yamada KM. An extracellular-matrix-specific GEF-GAP interaction regulates Rho GTPase crosstalk for 3D collagen migration. Nat Cell Biol. 2014;16:909–917. doi: 10.1038/ncb3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz MA, Shattil SJ. Signaling networks linking integrins and rho family GTPases. Trends Biochem Sci. 2000;25:388–391. doi: 10.1016/s0968-0004(00)01605-4. [DOI] [PubMed] [Google Scholar]
- 38.Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, Schwartz MA. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 2002;21:6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Daneshjou N, Sieracki N, van Nieuw Amerongen GP, Schwartz MA, Komarova YA, Malik AB, Conway DE. Rac1 functions as a reversible tension modulator to stabilize VE-cadherin trans-interaction. J Cell Biol. 2015;208:23–32. doi: 10.1083/jcb.201409108. [Local manipulation of Rac1 activity using a photoactivable probe directly confirms a model where VE-cadherin adhesive stabilization is mediated by Rac1-induced reduction of mechanical tension at AJs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Timmerman I, Heemskerk N, Kroon J, Schaefer A, van Rijssel J, Hoogenboezem M, van Unen J, Goedhart J, Gadella TW, Jr., Yin T, et al. A local VE-cadherin and Trio-based signaling complex stabilizes endothelial junctions through Rac1. J Cell Sci. 2015;128:3041–3054. doi: 10.1242/jcs.168674. [Identifies a moelcular mechanism by which the localizaiton of the GEF Trio to AJs locally increases Rac1 activity to stabilize VE-cadherin transdimers and regulate vessel barrier funciton.] [DOI] [PubMed] [Google Scholar]
- 41•.Galie PA, van Oosten A, Chen CS, Janmey PA. Application of multiple levels of fluid shear stress to endothelial cells plated on polyacrylamide gels. Lab Chip. 2015;15:1205–1212. doi: 10.1039/c4lc01236d. [Using a systematic combination of microfrabrication and microfluidic approaches, illustrates that underlying substrate stiffness influences endothelial shear stress responsiveness.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnan R, Klumpers DD, Park CY, Rajendran K, Trepat X, van Bezu J, van Hinsbergh VW, Carman CV, Brain JD, Fredberg JJ, et al. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am J Physiol Cell Physiol. 2011;300:C146–154. doi: 10.1152/ajpcell.00195.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan KL, Khankhel AH, Thompson RL, Coisman BJ, Wong KH, Truslow JG, Tien J. Crosslinking of collagen scaffolds promotes blood and lymphatic vascular stability. J Biomed Mater Res A. 2014;102:3186–3195. doi: 10.1002/jbm.a.34990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross TD, Coon BG, Yun S, Baeyens N, Tanaka K, Ouyang M, Schwartz MA. Integrins in mechanotransduction. Curr Opin Cell Biol. 2013;25:613–618. doi: 10.1016/j.ceb.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polacheck WJ, German AE, Mammoto A, Ingber DE, Kamm RD. Mechanotransduction of fluid stresses governs 3D cell migration. Proc Natl Acad Sci U S A. 2014;111:2447–2452. doi: 10.1073/pnas.1316848111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munson JM, Bellamkonda RV, Swartz MA. Interstitial flow in a 3D microenvironment increases glioma invasion by a CXCR4-dependent mechanism. Cancer Res. 2013;73:1536–1546. doi: 10.1158/0008-5472.CAN-12-2838. [DOI] [PubMed] [Google Scholar]
- 47.Udan RS, Vadakkan TJ, Dickinson ME. Dynamic responses of endothelial cells to changes in blood flow during vascular remodeling of the mouse yolk sac. Development. 2013;140:4041–4050. doi: 10.1242/dev.096255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boucher Y, Baxter LT, Jain RK. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res. 1990;50:4478–4484. [PubMed] [Google Scholar]
- 49.Jain RK. Vascular and interstitial barriers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev. 1990;9:253–266. doi: 10.1007/BF00046364. [DOI] [PubMed] [Google Scholar]
- 50.Hoang MV, Whelan MC, Senger DR. Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proc Natl Acad Sci U S A. 2004;101:1874–1879. doi: 10.1073/pnas.0308525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang MT, Reich DH, Chen CS. Measurement and analysis of traction force dynamics in response to vasoactive agonists. Integr Biol (Camb) 2011;3:663–674. doi: 10.1039/c0ib00156b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52••.Bentley K, Franco CA, Philippides A, Blanco R, Dierkes M, Gebala V, Stanchi F, Jones M, Aspalter IM, Cagna G, et al. The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat Cell Biol. 2014;16:309–321. doi: 10.1038/ncb2926. [Combines computational and experimental approaches to identify a regulatory feedback mechanism between Notch and VEGFR signaling that controls angiogenic sprouting through modulation of adherens junction dynamics.] [DOI] [PubMed] [Google Scholar]
- 53.Price GM, Wong KH, Truslow JG, Leung AD, Acharya C, Tien J. Effect of mechanical factors on the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials. 2010;31:6182–6189. doi: 10.1016/j.biomaterials.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeMaio L, Tarbell JM, Scaduto RC, Jr., Gardner TW, Antonetti DA. A transmural pressure gradient induces mechanical and biological adaptive responses in endothelial cells. Am J Physiol Heart Circ Physiol. 2004;286:H731–741. doi: 10.1152/ajpheart.00427.2003. [DOI] [PubMed] [Google Scholar]
- 55.Wong KH, Truslow JG, Khankhel AH, Chan KL, Tien J. Artificial lymphatic drainage systems for vascularized microfluidic scaffolds. J Biomed Mater Res A. 2013;101:2181–2190. doi: 10.1002/jbm.a.34524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song JW, Munn LL. Fluid forces control endothelial sprouting. Proc Natl Acad Sci U S A. 2011;108:15342–15347. doi: 10.1073/pnas.1105316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krishnan L, Underwood CJ, Maas S, Ellis BJ, Kode TC, Hoying JB, Weiss JA. Effect of mechanical boundary conditions on orientation of angiogenic microvessels. Cardiovasc Res. 2008;78:324–332. doi: 10.1093/cvr/cvn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Rosenfeld D, Landau S, Shandalov Y, Raindel N, Freiman A, Shor E, Blinder Y, Vandenburgh HH, Mooney DJ, Levenberg S. Morphogenesis of 3D vascular networks is regulated by tensile forces. Proc Natl Acad Sci U S A. 2016;113:3215–3220. doi: 10.1073/pnas.1522273113. [Utilizes 3D microengineered in vitro vascular tissues to demonstrate that uniaxial cell-induced and externally applied tensile forces regulate angiogenesis and vessel structure organization.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baker BM, Trappmann B, Wang WY, Sakar MS, Kim IL, Shenoy VB, Burdick JA, Chen CS. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat Mater. 2015;14:1262–1268. doi: 10.1038/nmat4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baeyens N, Mulligan-Kehoe MJ, Corti F, Simon DD, Ross TD, Rhodes JM, Wang TZ, Mejean CO, Simons M, Humphrey J, et al. Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc Natl Acad Sci U S A. 2014;111:17308–17313. doi: 10.1073/pnas.1413725111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park H, Go YM, St John PL, Maland MC, Lisanti MP, Abrahamson DR, Jo H. Plasma membrane cholesterol is a key molecule in shear stress-dependent activation of extracellular signal-regulated kinase. J Biol Chem. 1998;273:32304–32311. doi: 10.1074/jbc.273.48.32304. [DOI] [PubMed] [Google Scholar]
- 62.Berk BC, Corson MA, Peterson TE, Tseng H. Protein kinases as mediators of fluid shear stress stimulated signal transduction in endothelial cells: a hypothesis for calcium-dependent and calcium-independent events activated by flow. J Biomech. 1995;28:1439–1450. doi: 10.1016/0021-9290(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 63.Lakshmikanthan S, Zheng X, Nishijima Y, Sobczak M, Szabo A, Vasquez-Vivar J, Zhang DX, Chrzanowska-Wodnicka M. Rap1 promotes endothelial mechanosensing complex formation, NO release and normal endothelial function. EMBO Rep. 2015;16:628–637. doi: 10.15252/embr.201439846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Udan RS, Piazza VG, Hsu CW, Hadjantonakis AK, Dickinson ME. Quantitative imaging of cell dynamics in mouse embryos using light-sheet microscopy. Development. 2014;141:4406–4414. doi: 10.1242/dev.111021. [DOI] [PMC free article] [PubMed] [Google Scholar]