Abstract
BACKGROUND & AIMS
We sought to determine the efficacy of psyllium fiber treatment on abdominal pain and stool patterns in children with irritable bowel syndrome (IBS). We evaluated effects on breath hydrogen and methane production, gut permeability, and microbiome composition. We also investigated whether psychological characteristics of children or parents affected the response to treatment.
METHODS
We performed a randomized, double-blind trial of 103 children (mean age, 13 ± 3 y) with IBS seen at primary or tertiary care settings. After 2 weeks on their habitual diet, children began an 8-day diet excluding carbohydrates thought to cause symptoms of IBS. Children with ≥75% improvement in abdominal pain were excluded (n = 17). Children were assigned randomly to groups given psyllium (n = 37) or placebo (maltodextrin, n = 47) for 6 weeks. Two-week pain and stool diaries were compared at baseline and during the final 2 weeks of treatment. We assessed breath hydrogen and methane production, intestinal permeability, and the composition of the microbiome before and after administration of psyllium or placebo. Psychological characteristics of children were measured at baseline.
RESULTS
Children in the psyllium group had a greater reduction in the mean number of pain episodes than children in the placebo group (mean reduction of 8.2 ± 1.2 after receiving psyllium vs mean reduction of 4.1 ± 1.3 after receiving placebo; P = .03); the level of pain intensity did not differ between the groups. Psychological characteristics were not associated with response. At the end of the study period, the percentage of stools that were normal (Bristol scale scores, 3–5), breath hydrogen or methane production, intestinal permeability, and microbiome composition were similar between groups.
CONCLUSIONS
Psyllium fiber reduced the number of abdominal pain episodes in children with IBS, independent of psychological factors. Psyllium did not alter breath hydrogen or methane production, gut permeability, or microbiome composition. ClinicalTrials.gov no: NCT00526903.
Keywords: Irritable Bowel Syndrome, Fiber, Microbiome, Abdominal Pain, Psyllium
Childhood irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder affecting up to 20% of school-aged children and characterized by abdominal discomfort associated with defecation, or changes in stool frequency or stool form.1
It is well recognized that Westernized countries have deficient fiber intake.2 Both soluble and insoluble fibers improve laxation. Fiber with high water-holding capacity, such as psyllium, can soften hard stool or, alternatively, firm diarrheal stool. Supplemental fiber has been used to treat IBS. However, the efficacy of fiber in IBS is unclear.3,4 Although not conclusive, psyllium, a soluble fiber, may improve abdominal pain and/or stooling symptoms in adults with IBS.3,4
In children, there are few data from randomized trials regarding fiber in IBS. Thus, we performed a randomized, double-blind trial evaluating the efficacy of psyllium fiber in children with IBS. We also assessed whether fiber treatment affected stooling, breath hydrogen/methane production, and gut permeability, all thought to play roles in IBS symptoms. Because child and parent psychological factors may impact pain report, we determined whether they affected the response to fiber treatment. In a subset of children, we examined the effect of fiber treatment on stool microbiome composition.
Patients and Methods
Subjects
Children 7 to 18 years of age meeting pediatric Rome III IBS criteria were identified as described previously.5 Screening methods and inclusion/exclusion criteria are shown in Supplementary Table 1.
This investigator-initiated study was approved by the Baylor College of Medicine Institutional Review Board (protocol H-17388). Consent was obtained from parents and assent from children. The study was registered as clinicaltrials.gov identifier NCT00526903. Subjects were recruited between January 2009 and March 2014. Recruitment was paused for 6 months in 2009 because of an interruption in funding. All authors had access to the study data and reviewed and approved the final manuscript.
Study Design
Research coordinators conducted a home visit in which children completed the psychological questionnaires and received pain/stool diary and elimination diet instructions (Supplementary Figure 1). During the 2-week baseline on their habitual diet, the pain/stool diary was completed and stool was collected for microbiome analysis. Children then began an 8-day exclusion diet eliminating carbohydrates described as potentially causing symptoms in IBS and kept an additional 8-day pain/stool diary.6 After completion of the diet, a second home visit was made. Children who had 75% or less improvement in abdominal pain frequency and severity were eligible and underwent breath and permeability testing on their habitual diet and were randomized to either fiber or placebo for 6 weeks. During the final 2 weeks they kept a pain/stool diary and on the final treatment weekend again underwent breath and permeability testing and stool was collected.
The primary outcomes were changes in the number and severity of abdominal pain episodes and the percentage of stools that were normal (see later) during the last 2 weeks of treatment compared with the 2-week baseline period. Secondary outcomes included changes in breath hydrogen/methane production and gut permeability. We evaluated whether psychological factors affected the response to fiber treatment. In a subset of children, we examined the effect of fiber treatment on stool microbiome composition.
Randomization
Randomization was performed in blocks of 6 to either fiber or placebo (1:1) using a random number generator. Subjects were stratified by age (ages, 7–11 and 12–18 y), sex, and baseline mean pain frequency (≤21 vs >21 episodes during the baseline period). Investigators, research coordinators, and subjects were blinded to treatment assignment for the duration of the study.
Treatment
The fiber used was psyllium (Konsyl Pharmaceuticals, Easton, MD); children 7 to 11 years of age received 6 g, children 12 to 18 years of age received 12 g. The total daily amount of fiber was within the range endorsed by a conference on dietary fiber.7 The placebo fiber was maltodextrin (Maltrin; Grain Processing Corporation, Muscatine, IA), which was similar in appearance to the psyllium. Treatment was taken as a single daily dose provided as powder in identical packets.
Procedures and Measures
Pain/stool diary
Subjects entered responses daily using a designated recorded telephone line linked to the study database. Abdominal pain ratings were made 3 times per day using a validated scale.8 Pain frequency was defined as the number of ratings with pain of 1 or greater over the 2-week diary. Mean pain severity was the mean value of ratings when pain was present.
All stools during the diary intervals were characterized by the children using the Bristol Stool Form Scale, which also was used to determine IBS subtype.9,10 The proportion of stools that were constipated (rated 1–2), normal (rated 3–5), or diarrheal (rated 6–7) was calculated.10
Breath hydrogen/methane testing
Breath samples were collected at baseline and at 15-minute intervals for 3 hours after ingestion of 10 g of lactulose (EasySampler System; QuinTron Instrument Co, Milwaukee, WI) and analyzed for hydrogen and methane (MicroLyzer Model SC, QuinTron Instrument Co). Values were calculated as the area under the curve and corrected for the percentage of carbon dioxide concentration.11
Gut permeability
Permeability testing was performed as described previously.12 Values were normalized by dividing the urinary sugar concentration by the hours of collections. Recovery of sucrose was based on the cumulative overnight collection and the other sugars were based on cumulative 24-hour collection.
Psychological Questionnaires
The Behavior Assessment System for Children-2 measures child emotional and behavior problems and competence (Supplementary Table 2).13 The Children’s Somatization Inventory self-reports physical symptoms experienced in the previous 2 weeks.14 The Adult Responses to Children’s Symptoms assesses parental responses to children’s abdominal pain complaints.15 The Pain Response Inventory measures responses to recurrent pain.16 The Pediatric Quality of Life Generic Core Scales overall score assesses health-related quality of life of children.17 The Symptom Checklist-90R was used to measure parental somatic focus.18
Gastrointestinal microbiome composition
Bacterial DNA was extracted using the MOBIO PowerSoil DNA Isolation kit with the Human Microbiome Project modifications.19 16S ribosomal RNA gene amplicons (V3–V5) were generated and sequenced on the 454 platform (Life Technologies, Branford, CT). QIIME (version 1.7.0) was used to quality filter reads, cluster sequences into operational taxonomic units (OTUs), and evaluate bacterial community composition and diversity metrics.20 Sequences were quality-filtered to remove those containing primer or barcode mismatches, average quality scores less than 20, ambiguous base calls, homopolymers greater than 6, and/or less than 200 base pairs in length. OTUs were generated using an open reference approach using the UCLUST algorithm, a 97% similarity threshold, and the Greengenes reference database (version 12.10).21,22 Potentially chimeric sequences were identified using ChimeraSlayer and those identified among the de novo OTUs were excluded.23 Taxonomic identities were assigned using the Ribosomal Database Project classifier algorithm, the Greengenes reference database, and an 80% confidence threshold. Given variation in sequencing depth, all libraries were subsampled randomly to a depth of 3000 sequences before downstream analysis. Community diversity was characterized using the Shannon and inverse Simpson metrics, and differences in relative abundances were evaluated using Mann–Whitney U tests with Benjamini–Hochberg multiple testing corrections.
Food record
As part of the stool microbiome assessment, a food record was kept for the first 3 days of the baseline period and the last 3 days of the elimination diet to assess compliance with the diet.24
Power Analysis
We calculated the number of subjects needed to detect differences between groups in the mean number of pain episodes. By using data from studies in children and adults, we used an effect size of 0.5.4,25,26 We anticipated being able to recruit a sample size of 64 per group.
Statistics
The study was based on an intent-to-treat basis, meaning every effort was made to obtain follow-up data on all subjects regardless of how much/little of the intervention they received. All subjects who provided follow-up data were included in the analysis. IBM SPSS version 22 (Armonk, NY) was used for statistical evaluation. Data are presented as means ± SEM unless otherwise noted.
The primary outcome measures were the number of pain episodes, the mean pain severity, and the percentage of stools that were normal. Analysis of covariance compared the mean change from baseline with follow-up between groups while controlling for baseline value of the outcome variable. No formal adjustment was made for the 3 outcomes.
To determine if treatment with fiber affected the area under the curve breath hydrogen/methane production or the percentage recovery of sucrose, sucralose, and the lactulose/mannitol ratio, analysis of covariance was used. Linear regression assessed the moderator effect of psychological factors on pain outcomes. The interaction between treatment group and psychological factor tested for a moderating effect.
Results
Subjects
Of the 168 children enrolled and 103 randomized to fiber or placebo (Supplementary Figure 2), 15 dropped out and contact with 3 families was lost. Ten of 15 dropped out before receiving allocation of treatment. Because the study was double blind, treatment assignment did not influence their decision to drop out of the study. Five children in the fiber group and 3 children in the placebo group dropped out after starting treatment. One child in the fiber group was excluded from analysis because the family admitted at study end that the child previously underwent a cholecystectomy at an outside institution.
Children in the fiber group consumed a median of 98% (range, 18%–100%), and in the placebo group a median of 100% (range, 38%–100%) of the treatment dispensed (P = .57). In a random sample of subjects (n = 16), 45% of children were incorrect as to which group they were assigned.
There were no significant differences between groups in demographic and clinical characteristics (Table 1). There were no adverse events reported related to fiber or placebo administration.
Table 1.
Subject Demographics and IBS Subtypes
| Fiber (n = 37) | Placebo (n = 47) | P value | |
|---|---|---|---|
| Demographics | |||
| Age, y | 13.1 ± 0.4a | 13.5 ± 0.4 | .50 |
| Female | 19 (51%)b | 29 (62%) | .38 |
| Race | .26 | ||
| White | 26 (70%) | 25 (53%) | |
| Black | 4 (11%) | 7 (15%) | |
| Asian | 0 | 2 (4%) | |
| Hispanic | 6 (16%) | 13 (28%) | |
| Insurance type | .22 | ||
| PPO/HMO | 33 (89%) | 34 (73%) | |
| Medicaid/CHIP | 4 (11%) | 10 (21%) | |
| Other | 0 | 2 (4%) | |
| None | 0 | 1 (2%) | |
| IBS subtype | .32 | ||
| Constipation | 18 | 23 | |
| Unsubtyped | 18 | 18 | |
| Mixed | 0 | 2 | |
| Diarrhea | 1 | 4 |
CHIP, children’s health insurance plan; HMO, health maintenance organization; PPO, preferred provider organization.
Means ± SEM.
Percentage.
Pain
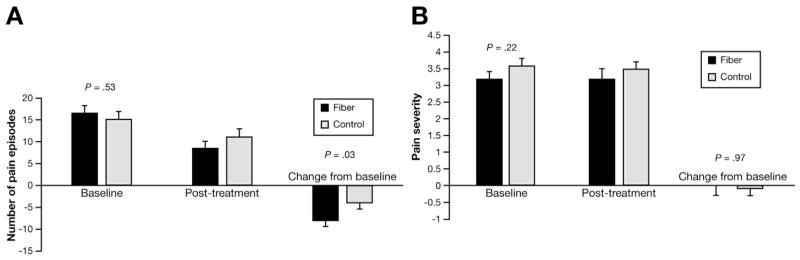
The number of pain episodes over the 2-week baseline period did not differ between groups (Figure 1). Controlling for the baseline value, after treatment there was a significant reduction in pain frequency in the fiber group compared with that in the placebo group (P = .03). Neither baseline pain severity nor change in pain severity differed between groups.
Figure 1.
Abdominal pain symptoms. The reduction in (A) pain frequency but not (B) severity was significantly different between groups. The same Figure legends are used for Figures 2–4.
Stooling
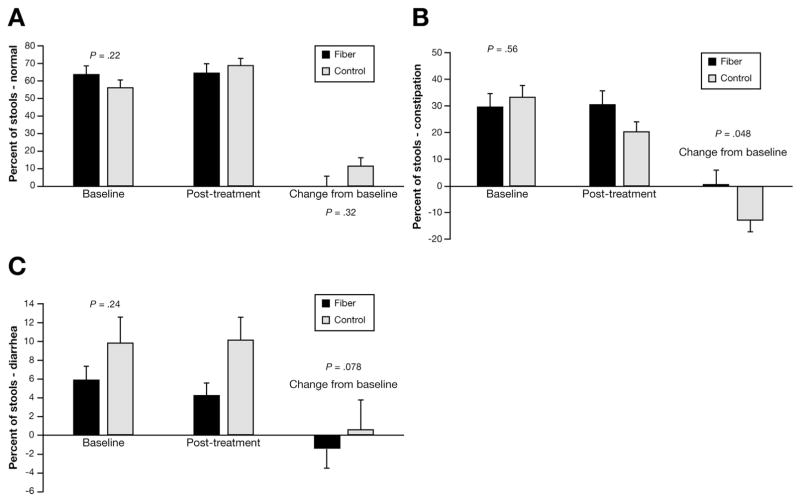
The percentage of stools that were normal during the baseline period was similar between groups, as was the change after treatment controlling for baseline (Figure 2). The percentage of stools rated as constipation (Bristol 1 or 2) at baseline was similar between the groups, but the change from baseline was greater in the fiber group (P = .048). The percentage of stools rated diarrheal (Bristol 6 or 7) was similar at baseline and, controlling for baseline, there was a trend (P = .078) for the fiber group to have a lower percentage of diarrheal stools after treatment.
Figure 2.
Stool characteristics. (B) There was a lower percentage of stools classified as constipation in the placebo group.
Breath Testing
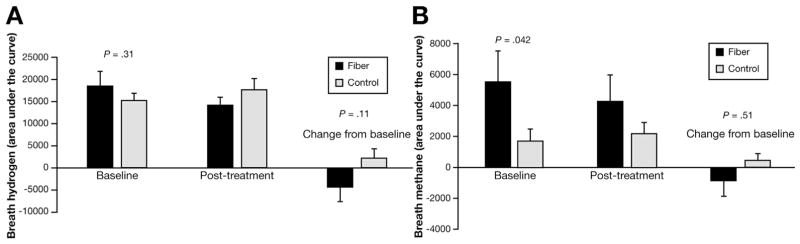
Breath testing was completed in 31 of 37 and 42 of 47 children in the fiber and placebo groups, respectively (Figure 3). Baseline hydrogen production was similar between groups. The change from baseline after treatment tended to be less in the fiber group (P = .11).
Figure 3.
Breath hydrogen and methane production. There were no differences between groups in (A) hydrogen or (B) methane area under the curve.
Baseline methane production was greater in the fiber group (P = .042). However, controlling for baseline value, after treatment there was no difference between groups in methane production. There was no association between hydrogen or methane production and abdominal pain symptoms (data not shown).
Gastrointestinal Permeability
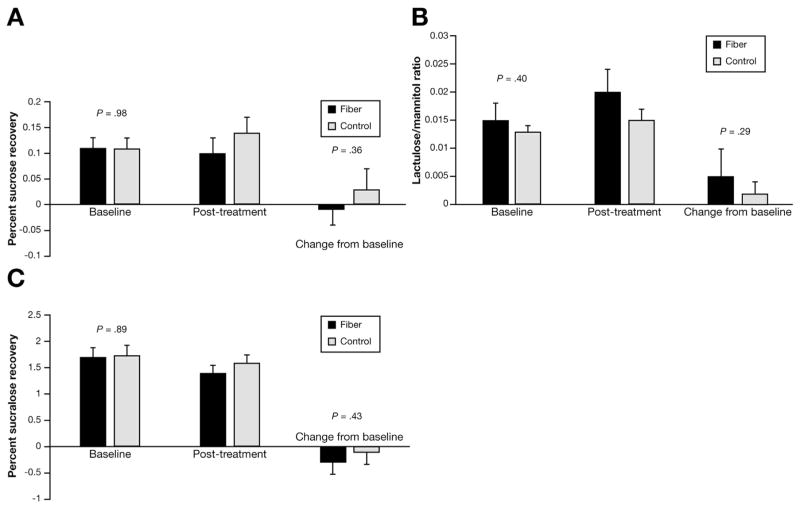
Permeability testing was completed in 33 of 37 and 43 of 47 children in the fiber and placebo groups, respectively (Figure 4). Baseline sucrose recovery, lactulose/mannitol ratio, and sucralose recovery were similar between groups and, controlling for baseline values, there were no differences between groups as a function of treatment.
Figure 4.
Gut permeability. Permeability was similar between groups.
Psychological Measures
There were no differences in psychological measures between groups for either the children or parents at baseline (Supplementary Table 2). Scores for depression and anxiety were in the normal range. The use of psychological measures as a covariate did not affect the primary outcomes of the study.
Gastrointestinal Microbiome Composition
Fifteen children in the fiber group and 18 children in the placebo group had stool microbiome composition measured (Supplementary Figure 3). Ages of the children did not differ between groups (fiber group, 13.5 ± 0.7 y; placebo group 12.5 ± 0.7 y).
There were trends toward an increased relative abundance of bacteria belonging to the phylum Bacteroidetes and a decreased relative abundance of Firmicutes in the fiber vs the placebo group at baseline (q = 0.068 in both cases, Mann–Whitney test with FDR correction). Similar trends were observed at the class level with the fiber subjects marginally enriched in bacteria belonging to the class Bacteroidia and the placebo group in bacteria belonging to the class Clostridia (q = 0.094 for both). Differences at finer levels of the taxonomic hierarchy (family down to OTU) were not observed.
Controlling for baseline, gastrointestinal microbiome composition did not change in either group as a function of treatment. This was true for both the phylum and class levels, as well as at finer levels of taxonomic detail (data not shown). Baseline differences between the groups were not accounted for by differences in dietary intake.
Discussion
Children treated with psyllium experienced a significantly greater reduction in the number of abdominal pain episodes compared with children treated with placebo taking into account baseline pain frequency (Figure 1A). Abdominal pain intensity was unaffected. Fiber treatment did not alter the percentage of stools rated as normal. Psychological measures of the child or parent did not appear to influence pain outcomes. Fiber treatment appeared to potentially reduce breath hydrogen production but did not affect gut permeability. Finally, stool microbiome composition did not appear to be altered in a consistent manner by psyllium fiber treatment.
In the one previous psyllium fiber treatment study of which we are aware, Christensen27 studied 40 children (ages, 3–15 y) discharged from the hospital with a diagnosis of recurrent abdominal pain. They were randomized to receive a cracker with a psyllium fiber content of 2% (placebo) or 66% (treatment) for 7 weeks.27 In contrast to our results, the mean number of pain episodes during treatment did not differ. In addition to differences in the patient population (recurrent abdominal pain vs IBS), interpretation of the previous study was complicated by issues such as unclear randomization, unknown number of subjects per group, and to which groups excluded subjects belonged.27,28
Fiber treatment did not change the percentage of stools rated normal on the Bristol stool scale (Figure 2A). Children in the placebo group had a significant reduction in the percentage of stools rated as constipation (Figure 2B). The reasons for this are not clear, but it is possible that psyllium caused the stools to be bulkier and, as such, rated as constipation. Given psyllium’s water-holding abilities, it is understandable why fewer stools were rated as diarrheal in the fiber group (Figure 2C). Psyllium comprises 3 fractions, with the gel-forming component making up the largest proportion (55%) and responsible for its water holding, and, thus, laxation abilities.29
There was a trend for less breath hydrogen production in the fiber vs placebo groups (Figure 3A). Although perhaps counterintuitive, of the 3 fractions in psyllium, only fraction C (<15% of the total) is fermented rapidly and would contribute significantly to hydrogen production.29 The other 2 gel-like fractions provide steric hindrance, minimizing bacterial interaction/ degradation with the fermentable fraction (McRorie JW, personal communication, 2015). In a study of healthy adults (n = 7), breath hydrogen production was similar between psyllium and placebo groups and rectal hydrogen excretion was less after psyllium compared with placebo.30
Along these same lines, we did not find that psyllium had an effect on the relative abundance of any taxonomic group in the gut community profiles of subjects in the fiber group. Individual microbial community profiles were altered after psyllium supplementation for some subjects, but these shifts (generally resulting in increased relative abundances of bacteria belonging to the class Clostridia) (Supplementary Figure 3) did not occur in a consistent manner among subjects, suggesting a variable and/or individualized response. Our data fit with those from a study of healthy women (n = 11) showing no prebiotic effect of psyllium on Bifidobacterium species except perhaps in those study subjects who harbored low numbers at the outset of the study.31 The effects of psyllium on other members of the gut microbial community were not evaluated.31
Previous studies have shown that some patients with IBS have increased gut permeability, which previous studies have suggested can be decreased by fiber treatment, likely through binding bile acids.32–34 However, we were not able to detect a difference in gut permeability as a function of fiber supplementation.
Multiple lines of research have suggested a role for psychosocial factors in IBS and other functional abdominal pain disorders. The impact of child emotional/ temperamental state and child/parent coping style, somatic focus, and parent response to illness are not unique to IBS, but common both conceptually and empirically to other specific and general child chronic pain experiences.35,36 The vast proportion of the research in this area has been correlational in nature, therefore the direction of causation or exacerbation, if any, is not known. This study afforded us the opportunity to test whether baseline psychological characteristics of the child or parent, which have been found to be related to pain experience, produced any differential impact on abdominal pain outcome (Supplementary Table 2). Somewhat to our surprise, none of the psychological variables were related to the main outcomes. However, it is possible that lack of a relationship also could be owing to a lack of power to detect an interaction.
It has been suggested, although no data were provided, that maltodextrin may affect the response to placebo in IBS.37 However, this is unlikely because we have shown previously that maltodextrin is well digested even in preterm and young infants.38,39
There were a number of strengths to our study. We used a strict, non–cross-over, double-blind design. Subjects were screened carefully before enrollment and those whose pain appeared to be related solely to poorly absorbable dietary carbohydrates were excluded. Uniquely, we included psychological measures as potential mediators of treatment response. Limitations are that with a larger sample size, we might have been able to detect significant differences between fiber and placebo groups in breath hydrogen production. We used 3 outcomes—pain frequency, severity, and stooling pattern—because these are the main symptoms of IBS. However, the use of 3 primary outcomes, strictly speaking, diminishes the impact of the finding of reduced abdominal pain frequency (the P = .03 value does not account for multiple testing for 3 outcomes). However, it should be pointed out that abdominal pain is the most disruptive IBS symptom in children and adults.40,41
In conclusion, the results of our study suggest that administration of psyllium fiber to children with IBS results in a decrease in the number of abdominal pain episodes. Further studies are needed to investigate the potential mechanism whereby psyllium decreases abdominal pain frequency in children with IBS.
Supplementary Material
Acknowledgments
The authors are grateful to Konsyl Pharmaceuticals (Easton, MD) for providing the psyllium, and to Grain Processing Corporation (Muscatine, IA) for providing the maltodextrin.
Funding
This study was supported in part by R01 NR05337 and R01 NR05337-S1 (stool microbiome analysis) from the National Institutes of Health, the Daffy’s Foundation, the USDA/ARS under Cooperative Agreement 6250-51000-043, and P30 DK56338, which funds the Texas Medical Center Digestive Disease Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work is a publication of the USDA/ARS Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, and Texas Children’s Hospital. The contents do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Abbreviations used in this paper
- IBS
irritable bowel syndrome
- OTU
operational taxonomic unit
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2016.03.045.
References
- 1.Saps M, Seshadri R, Sztainberg M, et al. A prospective school-based study of abdominal pain and other common somatic complaints in children. J Pediatr. 2009;154:322–326. doi: 10.1016/j.jpeds.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 2.Mobley AR, Jones JM, Rodriguez J, et al. Identifying practical solutions to meet America’s fiber needs: proceedings from the Food & Fiber Summit. Nutrients. 2014;6:2540–2551. doi: 10.3390/nu6072540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moayyedi P, Quigley EM, Lacy BE, et al. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1367–1374. doi: 10.1038/ajg.2014.195. [DOI] [PubMed] [Google Scholar]
- 4.Ruepert L, Quartero AO, de Wit NJ, et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;8:CD003460. doi: 10.1002/14651858.CD003460.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czyzewski DI, Lane MM, Weidler EM, et al. The interpretation of Rome III criteria and method of assessment affect the irritable bowel syndrome classification of children. Aliment Pharmacol Ther. 2011;33:403–411. doi: 10.1111/j.1365-2036.2010.04535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) and nonallergic food intolerance: FODMAPs or food chemicals? Therap Adv Gastroenterol. 2012;5:261–268. doi: 10.1177/1756283X11436241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemens R, Kranz S, Mobley AR, et al. Filling America’s fiber intake gap: summary of a roundtable to probe realistic solutions with a focus on grain-based foods. J Nutr. 2012;142:1390S–1401S. doi: 10.3945/jn.112.160176. [DOI] [PubMed] [Google Scholar]
- 8.Von Baeyer CL, Spagrud LJ, McCormick JC, et al. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity. Pain. 2009;143:223–227. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 10.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 11.Niu HC, Schoeller DA, Klein PD. Improved gas chromatographic quantitation of breath hydrogen by normalization to respiratory carbon dioxide. J Lab Clin Med. 1979;94:755–763. [PubMed] [Google Scholar]
- 12.McOmber ME, Ou CN, Shulman RJ. Effects of timing, sex, and age on site-specific gastrointestinal permeability testing in children and adults. J Pediatr Gastroenterol Nutr. 2010;50:269–275. doi: 10.1097/MPG.0b013e3181aa3aa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds CR, Kamphaus RW. Behavior assessment system for children. Circle Pines, MN: American Guidance Service, Inc; 2004. [Google Scholar]
- 14.Walker LS, Garber J, Greene JW. Somatization symptoms in pediatric abdominal pain patients: relation to chronicity of abdominal pain and parent somatization. J Abnorm Child Psychol. 1991;19:379–394. doi: 10.1007/BF00919084. [DOI] [PubMed] [Google Scholar]
- 15.Van Slyke DA, Walker LS. Mothers’ responses to children’s pain. Clin J Pain. 2006;22:387–391. doi: 10.1097/01.ajp.0000205257.80044.01. [DOI] [PubMed] [Google Scholar]
- 16.Walker LS, Smith CA, Garber J, et al. Testing a model of pain appraisal and coping in children with chronic abdominal pain. Health Psychol. 2005;24:364–374. doi: 10.1037/0278-6133.24.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varni JW, Bendo CB, Denham J, et al. PedsQL Gastrointestinal Symptoms Scales and Gastrointestinal Worry Scales in pediatric patients with functional and organic gastrointestinal diseases in comparison to healthy controls. Qual Life Res. 2015;24:363–378. doi: 10.1007/s11136-014-0781-x. [DOI] [PubMed] [Google Scholar]
- 18.Derogatis LR, Lipman RS, Covi L. SLC-90: an outpatient psychiatric rating scale–preliminary report. Psychopharmacol Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- 19.Aagaard K, Petrosino J, Keitel W, et al. The Human Microbiome Project strategy for comprehensive sampling of the human microbiome and why it matters. FASEB J. 2013;27:1012–1022. doi: 10.1096/fj.12-220806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 22.DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas BJ, Gevers D, Earl AM, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson MJ, Moore CE, Tsai CM, et al. Child and parent perceived food-induced gastrointestinal symptoms and quality of life in children with functional gastrointestinal disorders. J Acad Nutr Diet. 2014;114:403–413. doi: 10.1016/j.jand.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldman W, McGrath P, Hodgson C, et al. The use of dietary fiber in the management of simple, childhood, idiopathic, recurrent abdominal pain. Results in a prospective, double-blind, randomized, controlled trial. Am J Dis Child. 1985;139:1216–1218. doi: 10.1001/archpedi.1985.02140140050025. [DOI] [PubMed] [Google Scholar]
- 26.Ford AC, Talley NJ, Spiegel BM, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313. doi: 10.1136/bmj.a2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen MF. Recurrent abdominal pain and dietary fiber. Am J Dis Child. 1986;140:738–739. doi: 10.1001/archpedi.1986.02140220020009. [DOI] [PubMed] [Google Scholar]
- 28.Huertas-Ceballos AA, Logan S, Bennett C, et al. Dietary interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database Syst Rev. 2014;2:CD003019. doi: 10.1002/14651858.CD003019.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Marlett JA, Fischer MH. The active fraction of psyllium seed husk. Proc Nutr Soc. 2003;62:207–209. doi: 10.1079/pns2002201. [DOI] [PubMed] [Google Scholar]
- 30.Marteau P, Flourie B, Cherbut C, et al. Digestibility and bulking effect of ispaghula husks in healthy humans. Gut. 1994;35:1747–1752. doi: 10.1136/gut.35.12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elli M, Cattivelli D, Soldi S, et al. Evaluation of prebiotic potential of refined psyllium (Plantago ovata) fiber in healthy women. J Clin Gastroenterol. 2008;42(Suppl 3):S174–S176. doi: 10.1097/MCG.0b013e31817f183a. [DOI] [PubMed] [Google Scholar]
- 32.Shulman RJ, Eakin MN, Czyzewski DI, et al. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J Pediatr. 2008;153:646–650. doi: 10.1016/j.jpeds.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41–46. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim H, Bartley GE, Young SA, et al. Altered hepatic gene expression profiles associated with improved fatty liver, insulin resistance, and intestinal permeability after hydroxypropyl methylcellulose (HPMC) supplementation in diet-induced obese mice. J Agric Food Chem. 2013;61:6404–6411. doi: 10.1021/jf400545w. [DOI] [PubMed] [Google Scholar]
- 35.van Tilburg MA, Levy RL, Walker LS, et al. Psychosocial mechanisms for the transmission of somatic symptoms from parents to children. World J Gastroenterol. 2015;21:5532–5541. doi: 10.3748/wjg.v21.i18.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fales JL, Essner BS, Harris MA, et al. When helping hurts: miscarried helping in families of youth with chronic pain. J Pediatr Psychol. 2014;39:427–437. doi: 10.1093/jpepsy/jsu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao SS, Yu S, Fedewa A. Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment Pharmacol Ther. 2015;41:1256–1270. doi: 10.1111/apt.13167. [DOI] [PubMed] [Google Scholar]
- 38.Shulman RJ, Kerzner B, Sloan HR, et al. Absorption and oxidation of glucose polymers of different lengths in young infants. Pediatr Res. 1986;20:740–743. doi: 10.1203/00006450-198608000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Shulman RJ, Feste A, Ou C. Absorption of lactose, glucose polymers, or combination in premature infants. J Pediatr. 1995;127:626–631. doi: 10.1016/s0022-3476(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 40.Varni JW, Shulman RJ, Self MM, et al. Symptom profiles in patients with irritable bowel syndrome or functional abdominal pain compared with healthy controls. J Pediatr Gastroenterol Nutr. 2015;61:323–329. doi: 10.1097/MPG.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 41.Cain KC, Headstrom P, Jarrett ME, et al. Abdominal pain impacts quality of life in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:124–132. doi: 10.1111/j.1572-0241.2006.00404.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.