Abstract
Objective
Several case reports of Wernicke’s Encephalopathy in AN due to thiamine deficiency have described mammillary body (MB) injury, but systematic studies are lacking. Here we evaluated whether underweight and weight-restored individuals with AN demonstrate evidence of abnormal MB morphology, via retrospective examination of a previously collected data set.
Method
Using standard-resolution T1-weighted magnetic resonance imaging at 3 Tesla, we measured MB volume and fornix area in a cross-sectional study of 12 underweight AN, 20 weight-restored AN, and 30 age- and sex-matched healthy comparisons. Due to the small size of these structures, a manual tracing approach was necessary to obtain accurate measurements. A blinded expert rater manually traced MB and fornix structures in each participant.
Results
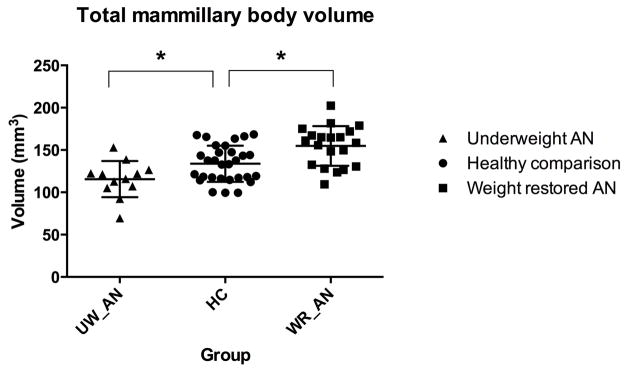
We observed significantly smaller MB volumes in the underweight AN group. However, the weight-restored AN group exhibited significantly larger MB volumes. The right fornix was smaller in the weight-restored AN group only.
Discussion
These findings suggest the possibility that MB volume and fornix area could represent potential biomarkers of acute weight loss and restoration, respectively. Verification of this finding through prospective studies evaluating MB morphology, cognition, and thiamine levels longitudinally across individual illness trajectories might be warranted.
INTRODUCTION
Converging evidence suggests that the prolonged food restriction and emaciation occurring in anorexia nervosa (AN) are associated with cerebral atrophy (1–3). For example, global reductions of cortical gray matter (4), subcortical white matter (5, 6) and corresponding ventricular enlargements (7) have been observed consistently in the underweight state. Regional cortical volume reductions have also been reported, though to a lesser extent. For instance, underweight individuals with AN have demonstrated regional volume decreases in hippocampal (8, 9), anterior cingulate (10), cerebellar, occipital and somatosensory (11) gray matter. Regional increases in brain volumes have also been described, for example, in the orbitofrontal and insular cortices (12). These volumetric differences have been associated with clinically relevant features such as a negative correlation between orbitofrontal gray matter volume and ratings of sweet taste pleasantness (12). In other studies volumetric gray matter changes have not correlated with clinical measures (8, 13), suggesting that additional delineation of illness cerebropathology is needed.
Focal abnormalities in several white matter tracts have also been reported in AN. These have most commonly focused on the fornix, a fiber bundle that has been shown to play key roles in regulating feeding and drinking patterns, memory, anxiety expression, and reward sensitivity in animals (14–16). Since these processes are also frequently disturbed in AN, several studies have examined fornix morphology in humans. Using diffusion tensor imaging, Kazlouski et al (17) identified reduced fractional anisotropy (FA) in the bilateral fimbria-fornix, fronto-occipital fasciculus, and the posterior cingulum in adults with AN. Frank et al (18) subsequently described reduced fornix FA and increased fornix apparent diffusivity, a measure of water diffusion that can reflect cellular damage(19), in a sample of adolescents with AN. More recently, Via et al (20) also observed reduced fornix FA and increased mean diffusivity in the fornix in underweight AN.
Nutritional deficiencies could be a potential source for some of the clinical symptoms and brain abnormalities observed in underweight AN, particularly of essential micronutrients. Although some individuals prone to AN may preemptively take multivitamin supplementation during self-starvation(21), many studies continue to identify micronutrient abnormalities in eating disorder populations. For instance, individuals with AN have reported reduced vitamin intake(22, 23), and exhibited serum deficiencies of several essential vitamins in the underweight state including thiamine (vitamin B1)(22, 24) and vitamin D (25).
Thiamine deficiency can induce a neuropsychiatric syndrome called Wernicke’s Encephalopathy (WE), which is characterized by a classical triad of symptoms that includes ataxia, ocular nystagmus and a confusional state sometimes characterized by memory loss, confabulation or psychosis. In severe cases, WE can result in permanent cognitive dysfunction or fatality. The most common neuropathological finding in WE is damage to the mammillary bodies (MB)(26) and fornix fibers (27). While the most common causes of WE neuropathology are from chronic malnutrition in the setting of alcohol dependence (28), some studies have demonstrated evidence of MB injury due to non-alcoholic starvation (29, 30). Furthermore, numerous case reports (including in recent years), have documented instances of WE and mammillary body damage due to AN (31–34), or a combination of anorexia and alcohol abuse (35). These reports could provide a potential link between thiamine deficiency and some of the subcortical neuropathological changes observed in neuroimaging studies of AN.
The complete triad of WE symptoms is rarely observed, and often only in the most severe cases. WE more commonly presents with early symptoms that are nonspecific, such as fatigue, abdominal discomfort, and headache, leading to the suggestion that WE is prone to under-diagnosis by clinicians(26). The fact that these early WE symptoms are highly overlapping with those frequently endorsed by AN patients raises the possibility that a continuum of neuropathology related to thiamine deficiency may also exist in AN. It also begs the question of whether such symptoms could reflect thiamine deficiency, at least in some cases.
At present no studies at the group level have evaluated MB or fornix pathology in AN. To address this gap in the literature, in the current study we evaluated whether underweight and weight-restored individuals with AN demonstrated evidence of structural differences in the MB and fornix compared with healthy individuals, using standard-resolution T1-weighted MRI. We hypothesized that MB volume and fornix area would be abnormally decreased in the underweight AN group, but would not be different in the weight restored AN group. The fornix hypothesis was derived from diffusion tensor imaging studies in AN and altered fornix pathology in WE.
METHODS AND MATERIALS
Participants
Twelve underweight females with current AN (mean age 19.4 years, mean illness duration 5.4 years), 20 weight-restored AN (18 female, mean age 22.4 years, mean illness duration 6.6 years), and 30 age- equivalent healthy comparisons (HC) were studied. AN participants were diagnosed during a clinical evaluation by a psychiatrist or psychologist with eating disorders expertise, and were considered eligible if they met DSM-IV criteria for AN (excluding the requirement of amenorrhea). Participants were categorized as underweight if they demonstrated a BMI < 18.5 during measurement on the day of the study, which is consistent with the minimum BMI threshold for mild AN under DSM-5. Participants were categorized as weight-restored if they demonstrated a BMI ≥18.5 on the day of the study. AN participants were recruited from the UCLA inpatient Eating Disorders Program, UCLA partial hospitalization program, community outpatient eating disorders centers, and via advertisements. All participants were screened for current major psychiatric illnesses including AN using the Mini International Neuropsychiatric Interview (36), and were specifically excluded if they endorsed any substance dependence (including alcohol dependence). To obtain a sample representative of the clinical population, AN participants were not excluded if they met criteria for comorbid dysthymia, major depressive disorder, panic disorder, generalized anxiety disorder, or social anxiety disorder. However, patients were excluded if they met criteria for a psychotic disorder, bipolar disorder or body dysmorphic disorder. To maximize sample size, Selective Serotonin Reuptake Inhibitor (SSRI) monotherapy was allowed provided the dose was stable for >4 weeks. No other medications were allowed. Exclusion criteria for all participants included suicidal ideation, pregnancy, claustrophobia, or presence of a metal (e.g. braces, permanent retainer) or implanted devices that may induce MRI artifacts precluding visualization of the mammillary bodies.
The protocol was approved by the Institutional Review Board at UCLA. All participants gave written and informed consent before the study.
Magnetic resonance imaging
Whole-brain images were acquired from all participants using a 3 Tesla MRI scanner (Siemens Magnetom Allegra-Trio, Erlangen, Germany), while participants lay supine. Head movement was minimized with foam padding on both sides of the head and by securing tape to the forehead. One T1-weighted magnetization prepared rapid gradient-echo (MP-RAGE) sequence was collected to provide a standard-resolution structural anatomical image of the entire brain (field of view= 250 × 250 mm, flip angle= 9°, slice thickness= 1 mm, TR= 1900 ms, TE= 2.26, voxel size= 1 mm3).
Data analysis
All standard-resolution T1-weighted brain images of AN and comparison participants were visually assessed for any major brain pathology such as cystic lesions, infarcts or other lesions, and for the presence of any mass lesion near the hypothalamus that might alter mammillary body or fornix morphology. These images were also visually examined to ensure the absence of motion artifacts or ghosting due to retainers or dental braces. One healthy control brain was excluded due to significant ghosting artifact, leaving 30 complete healthy comparison brain volumes. Image processing was conducted using the statistical parametric mapping package SPM5 (Wellcome Department of Cognitive Neurology, UK; http://www.fil.ion.ucl.ac.uk/spm/), and Matlab-based (The MathWorks Inc, Natick, MA) custom software.
Total intracranial volume calculation
Standard-resolution T1-weighted images were reoriented (without warping) into a common space. These images were segmented into gray matter, white matter, and cerebrospinal fluid (CSF), resulting in different tissue probability maps using the unified segmentation approach (37) implemented in SPM 5 [Gaussian per class = (3, 2, 2, 5); bias full-width-at- half-maximum = 70 mm cutoff; sampling distance = 2]. Voxels with a probability value ≥0.5 in gray, white, and CSF probability maps were counted, and whole brain gray matter, white matter, and CSF volumes were calculated by multiplying the number of voxels by the volume of each voxel. Total intracranial volume (TIV) was calculated as the sum of gray, white, and CSF volumes.
Mammillary body volume quantification
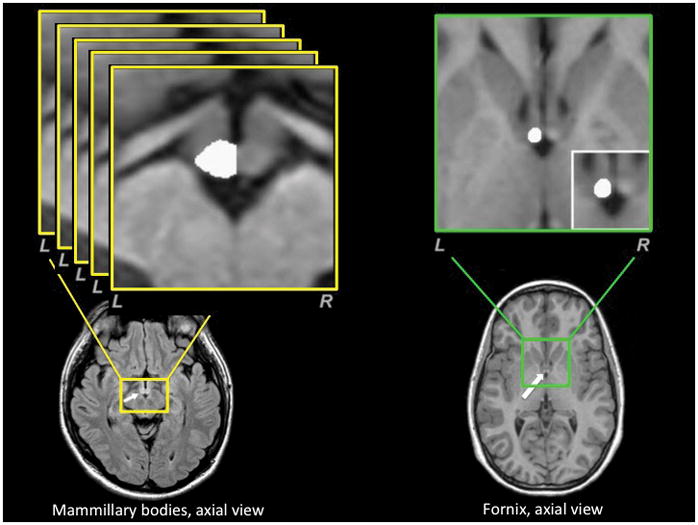
Using the reoriented T1-weighted image volumes, brain sections containing both mammillary bodies were oversampled to a resolution of 0.2×0.2×0.2 mm3. A single investigator, blinded to subject group assignment, performed manual tracing using MRIcron (http://www.sph.sc.edu/comd/rorden/mricron/install.html). Mammillary bodies were distinguished from surrounding tissue by its lighter hue, and traced after determining the brain midline from coronal and axial views (figure 1). Medial borders were defined by tracing tissue in the sagittal plane to the left or right of the midline. Using a central slice in the coronal plane, we determined and connected the superior and inferior mammillary notches that defined superior and lateral boundaries of the structure, respectively. Body outlines were traced in the sagittal plane using the central coronal slice as a guideline, and continued through successive sagittal slices laterally from the medial slice. Lateral boundaries were defined by disappearance of the central coronal slice. Trace edges were smoothed, and voxels filled where appropriate while moving from anterior to posterior limits in the coronal plane; actions were repeated for the axial view, if necessary. We repeated the procedure for the other mammillary body. Traced voxels in the left and right mammillary bodies were counted, and volumes calculated by multiplying the number of traced voxels by the volume of each voxel. Bilateral mammillary body volumes were determined by summing left and right mammillary body volumes (see(38, 39) for previous demonstrations of this method in other clinical samples).
Figure 1.
Fornix area quantification
The fornix is a small fiber bundle with a complex three-dimensional course. We chose to measure the cross-sectional area of this structure at a point where it could be most confidently visualized and measured: namely, at the point before it enters the septum parallel to the anterior commissure. Reoriented T1-weighted images were oversampled (0.2×0.2×0.2 mm) in brain areas immediately dorsal to the septum containing the columns of the fornix. Left and right fornix fibers were visualized using a coronal view and marked before entering the septum parallel to the anterior commissure. These tags provided boundaries for the fibers in the medial-lateral direction. Caudal-rostral borders were visualized using a sagittal view and marked for both fornix columns. These tags provided boundaries for the fibers in the anterior-posterior direction. Both tags were used to define the medial-lateral and anterior-posterior extent of the left and right fornix, separately. Using an axial view, fornix cross-sectional areas were outlined and calculated by multiplying the voxel count by the voxel area, for both the left and right fornix separately (figure 1). Total fornix cross-sectional area was determined by summing left and right fornix areas.
Intra-tracer reliabilities
To assess the reliability of the manual tracing measurements of the mammillary bodies, a subset of the original sample (24%) was retraced and intra-tracer reliabilities were calculated using intraclass correlation coefficients (two way, mixed model, absolute agreement).
Statistical analysis
Statistical analysis of demographic data and mammillary body volumes and fornix cross-sectional areas were performed using SPSS v. 22.0 (IBM Corp, Armonk, NY). Demographic data and clinical characteristics were compared using independent samples t-tests separately for the 1) underweight AN versus comparisons and 2) weight restored AN versus comparisons. Mammillary body volume and fornix cross-sectional area differences were evaluated with independent samples t-tests separately for the 1) underweight AN versus comparisons and 2) weight restored AN versus comparisons. Analysis of covariance (ANCOVA), controlling for head size (covariate; TIV) was conducted separately for the 1) underweight AN versus comparisons and 2) weight restored AN versus comparisons. We purposefully did not enter all of the groups into single ANCOVA, as such a test would be inappropriate for testing our second hypothesis that the weight restored group would show normal MB volumes. Since the samples did not differ significantly in mean age or sex proportion, these variables were not included. Body mass index was also not included as a covariate, since this unit is a fundamental biomarker of AN and could therefore partial out effects of interest related to the illness.
RESULTS
Demographics and clinical variables
The groups did not differ with respect to age or sex (table 1). Both groups exhibited significantly lower BMI than the healthy comparisons (p < 0.01), with the underweight AN group showing BMIs in the abnormally low range, and the weight restored group showing BMIs in the normal range. Both groups exhibited significantly higher levels of depression and anxiety with respect to healthy comparisons (p < 0.01). Eating disorder severity was in the moderate range for both groups, as evidenced by Eating Disorder Examination global score.
Table 1.
Demographic and clinical characteristics, mammillary body volumes, fornix area and intracranial volumes for the underweight AN, weight-restored AN and healthy comparison groups
| Underweight AN (UW_AN) | Healthy comparison (HC) | Weight-restored AN (WR_AN) | UW_AN vs. HC | WR_AN vs. HC | |||
|---|---|---|---|---|---|---|---|
| t40/χ2 | p | t48/χ2 | P | ||||
| Age (years) | 19.4+/− 7.3 | 22.5 +/− 4.8 | 22.4+/− 3.9 | −1.61 | .12 | −0.08 | .94 |
| Sex | 12 F | 24 F, 6 M | 18 F, 2 M | 2.8 | .10 | 0.89 | .89 |
| Body Mass Index (BMI) | 16.2 +/− 2.0** | 22.6 +/− 3.4 | 20.2 +/− 1.6** | −6.44 | < .001 | −3.34 | .002 |
| Education (years) | 11.3 +/− 3.3** | 14.3 +/− 2.9 | 14.5 +/− 2.9 | −2.95 | .007 | 0.22 | .82 |
| HAM-A | 7.2 +/− 5.6** | 2.0 +/− 1.9 | 8.6 +/− 7.2** | 4.50 | < .001 | 4.00 | < .001 |
| MADRS | 12.3 +/− 9.5** | 0.87 +/− 1.3 | 12.7 +/− 9.8** | 4.13 | .002 | 5.33 | < .001 |
| EDE – global score | 3.1 +/− 1.4 | - | 3.2 +/− 1.3 | ||||
| AN restricting subtype | 100% | - | 95% | ||||
| Illness duration (years) | 5.4 +/− 6.6 | - | 6.6 +/− 5.0 | ||||
| Lowest BMI | 14.2 +/− 2.0 | - | 15.8 +/− 1.7 | ||||
| % medicated | 25 (n=3) | - | 0 | ||||
| Total MB volume (mm3) | 115.6 +/− 21.4* | 133.8 +/− 21.4 | 154.8 +/− 23.5** | −2.49 | .017 | 3.27 | .002 |
| Left MB | 57.0 +/− 11.4* | 65.5 +/− 11.6 | 75.1 +/− 10.7** | −2.16 | .036 | 2.97 | .005 |
| Right MB | 58.6 +/− 14.7* | 68.3 +/− 13.1 | 79.7 +/− 16.3** | −2.08 | .044 | 2.73 | .009 |
| Total fornix area (mm2) | 12.5 +/− 3.3 | 12.9 +/− 3.1 | 12.0 +/−2.4 | −.41 | .68 | −1.15 | .26 |
| Left fornix | 6.5 +/− 1.1 | 6.0 +/− 2.5 | 6.2 +/− 1.5 | −.10 | .92 | −.81 | .42 |
| Right fornix | 6.6 +/− 1.7 | 6.8 +/− 1.8 | 5.8 +/− 1.1* | −1.10 | .28 | −2.1 | .044 |
| Total intracranial volume (L) | 1.591 +/− 0.128 | 1.730 +/− .315 | 1.593 +/− 0.153 | −1.48 | .15 | −1.80 | .08 |
| Gray matter | 0.717 +/− 0.091 | 0.779 +/− 0.127 | 0.727 +/− 0.063 | −1.54 | .13 | −1.70 | .10 |
| White matter | 0.434 +/− .047 | 0.490 +/− 0.100 | 0.451 +/− .039 | −1.85 | .07 | −1.66 | .10 |
| CSF | 0.443 +/− 0.060 | 0.458 +/− 0.129 | 0.415 +/− 0.091 | −.39 | .70 | −1.29 | .20 |
Means +/− standard deviation. MADRS: Montgomery-Asberg Depression Rating Scale. HAM-A: Hamilton Anxiety Rating Scale. EDE: Eating Disorder Examination Scale. MB: mammillary body.
p < .05,
p < .001.
Mammillary body volume
Total MB volumes were lower in the underweight AN group compared with healthy comparisons (p = 0.017; table 1, figure 1). Left and right MB volumes were also significantly smaller in the underweight AN group (left: p = .036, right: p = 0.044). Significant disparities in total MB volume remained between groups when evaluated with ANCOVA using total intracranial volume as a covariate (p < 0.048), but not when assessed individually (left: p = 0.08, right: p = 0.10).
In contrast, we observed increased total MB volumes in the weight-restored AN group (p = .002; table 1, figure 1). Left and right MB volumes were also significantly larger in the weight-restored AN group (left: p = 0.005, right: p = 0.009). Disparities in total MB volume remained when evaluated with ANCOVA using total intracranial volume as a covariate (p < 0.001), and also when MB volumes were assessed individually (left: p = 0.002, right: 0.003). Intra-tracer reliabilities were excellent for the total (r = .97, p < .001), left (r = .92, p < .001) and right (r = .98, p < .001) volumes.
Fornix area
The underweight AN group did not show significant between group differences in total fornix area (p = 0.68), or for the left (p = 0.92) and the right (p = 0.28) fornix. The weight-restored AN group showed a significant difference in the right fornix (p = 0.044), indicating a smaller right fornix area. There was no significant difference in the left fornix (p = 0.42) or total fornix area (p = 0.26).
DISCUSSION
This study represents the first systematic group-level demonstration of abnormal mammillary body volume and fornix cross-sectional area in AN. Consistent with extant case reports, we observed reduced total mammillary body volume in the underweight AN group. Surprisingly, we observed increased total mammillary body volume in the weight-restored AN group. We did not find any differences in fornix area in the underweight AN group, but observed decreased right fornix area in the weight-restored group.
Reduced mammillary body volume in underweight AN is consistent with previous reports of generalized cerebral atrophy in AN(1–4), and with prior case reports of acute WE in AN (31–33, 35). It seems possible that decrements in mammillary body volume in underweight AN could reflect generalized atrophy due to overall reductions in energy availability, might reflect a specific sensitivity to thiamine deficiency, or alternatively, pathology due to overlapping nutritional deficiencies. We cannot substantiate or differentiate these possibilities from the current study, as this was a retrospectively evaluated data set, and we did not measure serum thiamine or other vitamin levels. Therefore, while we interpret the current results as preliminary evidence of a potentially interesting biomarker effect, verification through further testing will be required to confirm the finding.
The increased mammillary body volume in weight-restored AN was unanticipated, but is not unprecedented. Increased gray matter volumes have been previously observed in weight-restored AN in cortical regions such as the insula and orbitofrontal cortex (12), using manual tracing methods similar to those employed in the current study, as well as using automated methods. Furthermore, some global volumetric changes also appear to be reversible (4, 40–42), indicating that the brain responds dynamically to changes in caloric and nutrient availability. For example, recent studies have shown that cortical thickness can rapidly improve during weight restoration in AN independently of illness duration, hydration status, or symptom improvement (43) (for a review, see (44). Despite such malleability, the presence of residual cognitive deficits (45), and other persisting volumetric reductions following treatment and weight restoration (12, 46) indicates that some forms neuroanatomical pathology can linger. The significance of enlarged MB volumes in weight-restored states is unclear. While we might speculate that this could reflect morphological reorganization following injury to this neural structure, we are unable to determine this possibility from the current study, as we did not employ a longitudinal design. We also did not utilize other measures of cerebral pathology, such as fractional anisotropy and diffusivity. Replication of the current findings in larger samples, in studies utilizing a longitudinal pre- and post-treatment design, and incorporating detailed assessments of clinical characteristics and cognitive functions related to MB (e.g. measures of memory) would provide key clarification of these possibilities. Controlling for potential acute influences on brain volume, such as dehydration(47) or excessive exercise(48) will also be important(49). The current study did not assess these factors or other acute indices of dehydration (e.g. urine specific gravity, as in(42)).
Since the current study was cross-sectional, we also cannot discount an alternate interpretation: that individuals born with larger MB may be better predisposed for weight restoration recovery from AN, or perhaps buffered somehow from the effects of acute illness. The MB constitutes an integral component of the Papez circuit (50). Accordingly, it has been implicated in homeostatic regulation of hypothalamic and hippocampal processing, and associated emotional experiences, learning, and memory. While speculative, it may be possible that individuals with larger MB might be more capable of integrating emotional experience with learning and memory, which could possibly enable them to respond more adaptively to psychotherapeutic processes (and hence weight restoration). Or at least, to have the cognitive and emotional resources to do so on their own.
Despite the cross-sectional comparison of these data sets, we feel it is important to emphasize that the observed MB volume differences do not appear to be related to global atrophy (in the case of underweight AN) or global cerebral enlargement (in the case of weight restored AN). This is because there were no volume differences in GM, WM, or CSF between either AN group and the healthy comparisons. Also, the fornix was not smaller in the underweight sample while the MBs were smaller, providing further evidence suggesting that the underweight state does not necessarily induce global atrophy. However, both AN groups had volumes that in some cases bordered on significant (WM for underweight, TIV for weight restored; Table 1). At this point it is also important to mention that most findings on WM volumes in AN come from studies with small sample sizes, which suggest mixed findings, and limited evidence for regional differences. Castro-Fornieles et al (4) did not see any white matter volume differences at acute inpatient admission (12 AN vs. 9 HC) or at 7 month follow up. Katzman et al (5) observed WM volume increases in 6 weight restored AN patients. Swayze et al (6) found lower WM volume during inpatient admission vs. increased WM volume after weight normalization (13 patients). Meta-analyses of studies using pooled data have been conducted in the past several years. These suggested that there are global reductions in GM and WM, and increased CSF (Titova et al (1), n=228 patients; Van den Eynde et al, (2), n=236 patients). Despite a substantial overlap of the studies that were reviewed, each meta-analysis reached somewhat different conclusions about WM differences: global reductions in the former, and inconclusive evidence in the latter. Larger prospective studies are desperately needed in this area, and would provide some much needed progress in understanding the underlying cerebral pathology of AN.
Another point worth discussing is the potential difference in lowest BMI reported between the underweight and weight-restored AN groups (14.2±2.0 vs.15.8±1.7, respectively). This might raise the possibility that there were less severely affected patients in the restored group, and that a milder illness phenotype would explain the larger MB volumes in this group. However, this logic would seem to be contradicted by an opposing qualitative observation that the weight-restored group had longer illness durations. Furthermore, we did not perform any comparisons of brain volume or clinical demographics between the underweight and weight restored as the reference of interest in this cross-sectional study was healthy comparisons. Longitudinal comparisons would help to address this possibility.
Although there is some convergence in the DTI literature suggestive of fornix pathology in underweight AN (51), this region is particularly prone to artifacts (52) which provided some justification for the targeted approach to manually evaluating fornix area. Our observation of reduced right fornix area in weight-restored AN provides some suggestive macroscopic evidence of the relevance of this region that could potentially corroborate with the microstructural differences observed in diffusion tensor imaging studies (17, 18, 20). However, the unilateral finding and absence of corroboration with clinical symptoms in this study limit further speculation as to the clinical relevance of this finding. It is also possible that the lack of differences in fornix area in the underweight AN group, while unpredicted, could be explained by a small sample size. Or, alternatively, it could due to the incomplete measurement of this structure in the current study. A high-resolution volumetric study, or a study employing multiple morphological measurements of the fornix (e.g., multiple cross sections) would help to address this limitation.
One noteworthy aspect of the current study relates to the fact that our MRI measurements of MB volumes for the healthy participants are in good agreement with large studies using MRI measurement as well as the gold standard method of post mortem evaluation. For example, in a normative study directly evaluating MB volumes, Sheedy et al(53) reported unilateral MB volumes of 63.4 ± 19.8 mm3 in a postmortem sample of 2048 neurologically healthy individuals.1 To compare the volume differences between these two methods, we calculated error scores for the healthy samples using the formula: (normative study mean – current study mean)/normative study mean × 100%. This yielded a combined measurement error of less than 5.5% for the healthy total MB (3.3% for the left MB and 7.7% for the right MB). Given the small size of this structure and difficulty tracing it using automated methods, we interpret this as evidence positively supporting the validity of the manual tracing approach.
Limitations and future directions
1) The sample sizes of the AN subgroups were not large by current normative standards. This is a general limitation common to many AN studies, and may be in part influenced by a low prevalence rate in the population. Thus while our sample sizes are equivalent to, and in several cases larger than many of the prior studies cited here, replication is warranted to confirm the finding. 2) We did not ascertain cellular estimates of neuropathology in the current study. Such measures could be obtained via diffusion tensor imaging (e.g., fractional anisotropy, diffusivity). Other measures, such as positron emission tomography or magnetic resonance spectroscopy would allow assessments of receptor density and glucose metabolism and metabolite levels. Unfortunately such approaches are currently unfeasible due to the poorer spatial resolution of these methods (compared with MRI), and the small size of the MB and fornix. 3) We did not assess or control for several acute factors that could potentially affect cerebral volume, such as the duration of the underweight or weight restored state, hydration status, or the refractory period associated with acute exercise. We recommend that future studies incorporate such measures prospectively. 4) We were unable to characterize nutritional intake patterns, hydration status, or serum vitamin levels (e.g., thiamine), as this study was conducted retrospectively on a pre-existing neuroimaging data set. Such measurements at the time of scanning would have been helpful in discerning whether the observed differences relate to a thiamine or other vitamin deficiency, and we recommend that future studies incorporate such measures prospectively. 5) We did not obtain intratracer reliability estimates for the fornix measurements. 6) Three of the underweight patients were taking serotonergic medications. This could have affected the observed group results. 7) Participant recruitment for the current study was heterogenous, including individuals from different treatment centers, acuity levels, and stages of recovery. This clinical variability might have increased the variability of biomarkers measured in this study. Replicating this finding in a more homogenous sample would provide evidence against this notion. 8) We did not utilize a longitudinal design. This would substantially improve the ability to make causal inferences about the relationships between changes in body weight and changes in mammillary body size. It would especially allow for predictive analyses of the relationship between changes in volume and illness trajectory. We highly recommended this approach for future studies in this area.
Conclusions
In summary, this is the first study to systematically evaluate mammillary body morphology in AN. Our results suggest that in AN the underweight state may be associated with reductions in mammillary body volume, and that weight restoration may be conversely associated with increases in mammillary body volume and a reduced fornix area. Further study appears warranted to determine whether these limbic regions respond dynamically to changes in weight and illness state in AN, and to identify whether such changes are related to nutrient intake, clinical characteristics, or therapeutic outcomes.
Figure 2.
Acknowledgments
We would like to thank Courtney Sheen for assistance with study coordination, Alex Zai, Saranya Akumalla and Sarah Madsen for assistance with data preparation. For assistance with participant recruitment we would like to thank Dawn Theodore and the entire Eating Disorder Centers of California staff, Jennifer Henretty and the entire Center for Discovery staff, and the entire staff at the UCLA Inpatient Eating Disorders Program and UCLA Partial Hospitalization Program.
Support:
This research was supported by NIMH grant number 3R01MH093535-02S2 (JF), and by NIH R01HL113251, R01NR013625, R01NR015038, and R01NR014669 (RK). Dr. Strober received support from the Resnick Endowed Chair in Eating Disorders. Dr. Khalsa received support from the David Wilder Trust, the William K. Warren Foundation, and a NARSAD Young Investigator Award.
Footnotes
In this normative study only unilateral MB volumes, left or right, were selected for measurement based on perceived ease of assessment for each case by the experimenter. This is the typical approach employed by most investigators using postmortem methodology (C. Harper, personal communication).
Contributors:
SSK and RK designed the study. JDF collected the data. SSK, RK and VP analyzed the data. SSK principally wrote the paper with the help of RK, MS and JDF.
Disclosure of Conflicts:
All authors do not have any conflicts to disclose.
Results presented at the International Conference on Eating Disorders Conference, Boston, MA, USA, April 2015.
References
- 1.Titova OE, Hjorth OC, Schioth HB, Brooks SJ. Anorexia nervosa is linked to reduced brain structure in reward and somatosensory regions: a meta-analysis of VBM studies. BMC psychiatry. 2013;13(110):1–11. doi: 10.1186/1471-244X-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eynde F, Suda M, Broadbent H, Guillaume S, Eynde M, Steiger H, et al. Structural Magnetic Resonance Imaging in Eating Disorders: A Systematic Review of Voxel-Based Morphometry Studies. European Eating Disorders Review. 2012;20(2):94–105. doi: 10.1002/erv.1163. [DOI] [PubMed] [Google Scholar]
- 3.Fonville L, Giampietro V, Williams SCR, Simmons A, Tchanturia K. Alterations in brain structure in adults with anorexia nervosa and the impact of illness duration. Psychological Medicine. 2013;44(09):1965–75. doi: 10.1017/S0033291713002389. [DOI] [PubMed] [Google Scholar]
- 4.Castro-Fornieles J, Bargalló N, Lázaro L, Andrés S, Falcon C, Plana MT, Junqué C. A cross-sectional and follow-up voxel-based morphometric MRI study in adolescent anorexia nervosa. Journal of Psychiatric Research. 2009;43(3):331–40. doi: 10.1016/j.jpsychires.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Katzman DK, Lambe EK, Mikulis DJ, Ridgley JN, Goldbloom DS, Zipursky RB. Cerebral gray matter and white matter volume deficits in adolescent girls with anorexia nervosa. J Pediatr. 1996;129(6):794–803. doi: 10.1016/s0022-3476(96)70021-5. [DOI] [PubMed] [Google Scholar]
- 6.Swayze VW, Andersen AE, Andreasen NC, Arndt S, Sato Y, Ziebell S. Brain tissue volume segmentation in patients with anorexia nervosa before and after weight normalization. International Journal of Eating Disorders. 2003;33(1):33–44. doi: 10.1002/eat.10111. [DOI] [PubMed] [Google Scholar]
- 7.Chui HT, Christensen BK, Zipursky RB, Richards BA, Hanratty MK, Kabani NJ, et al. Cognitive Function and Brain Structure in Females With a History of Adolescent-Onset Anorexia Nervosa. Pediatrics. 2008;122(2):e426–e37. doi: 10.1542/peds.2008-0170. [DOI] [PubMed] [Google Scholar]
- 8.Connan F, Murphy F, Connor SEJ, Rich P, Murphy T, Bara-Carill N, et al. Hippocampal volume and cognitive function in anorexia nervosa. Psychiatry Research: Neuroimaging. 2006;146(2):117–25. doi: 10.1016/j.pscychresns.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Giordano GD, Renzetti P, Parodi RC, Foppiani L, Zandrino F, Giordano G, Sardanelli F. Volume measurement with magnetic resonance imaging of hippocampus-amygdala formation in patients with anorexia nervosa. J Endocrinol Invest. 2001;24(7):510–4. doi: 10.1007/BF03343884. [DOI] [PubMed] [Google Scholar]
- 10.Joos A, Klöppel S, Hartmann A, Glauche V, Tüscher O, Perlov E, et al. Voxel-based morphometry in eating disorders: Correlation of psychopathology with grey matter volume. Psychiatry Research: Neuroimaging. 2010;182(2):146–51. doi: 10.1016/j.pscychresns.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Amianto F, Caroppo P, D’Agata F, Spalatro A, Lavagnino L, Caglio M, et al. Brain volumetric abnormalities in patients with anorexia and bulimia nervosa: A Voxel-based morphometry study. Psychiatry Research: Neuroimaging. 2013;213(3):210–6. doi: 10.1016/j.pscychresns.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Frank GK, Shott ME, Hagman JO, Mittal VA. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. American Journal of Psychiatry. 2013;(170):1152–60. doi: 10.1176/appi.ajp.2013.12101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambe EK, Katzman DK, Mikulis DJ, Kennedy SH, Zipursky RB. Cerebral gray matter volume deficits after weight recovery from anorexia nervosa. JAMA Psychiatry. 1997;54:537–42. doi: 10.1001/archpsyc.1997.01830180055006. [DOI] [PubMed] [Google Scholar]
- 14.Osborne B, Dodek AB. Disrupted patterns of consummatory behavior in rats with fornix transections. Behavioral and neural biology. 1986;45(2):212–22. doi: 10.1016/s0163-1047(86)90783-1. [DOI] [PubMed] [Google Scholar]
- 15.Cassel JC, Duconseille E, Jeltsch H, Will B. The fimbria-fornix/cingular bundle pathways: a review of neurochemical and behavioural approaches using lesions and transplantation techniques. Prog Neurobiol. 1997;51(6):663–716. doi: 10.1016/s0301-0082(97)00009-9. [DOI] [PubMed] [Google Scholar]
- 16.Degroot A, Treit D. Anxiety is functionally segregated within the septo-hippocampal system. Brain Research. 2004;1001(1–2):60–71. doi: 10.1016/j.brainres.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 17.Kazlouski D, Rollin MDH, Tregellas J, Shott ME, Jappe LM, Hagman JO, et al. Altered fimbria-fornix white matter integrity in anorexia nervosa predicts harm avoidance. Psychiatry Research: Neuroimaging. 2011;192(2):109–16. doi: 10.1016/j.pscychresns.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank GK, Shott ME, Hagman JO, Yang TT. Localized Brain Volume and White Matter Integrity Alterations in Adolescent Anorexia Nervosa. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52(10):1066–75. e5. doi: 10.1016/j.jaac.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nature Reviews Neuroscience. 2003;4(6):469–80. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- 20.Via E, Zalesky A, Sánchez I, Forcano L, Harrison B, Pujol J, et al. Disruption of brain white matter microstructure in women with anorexia nervosa. Journal of Psychiatry & Neuroscience. 2014;39(6):367–75. doi: 10.1503/jpn.130135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braisted JR, Mellin L, Gong EJ, Irwin CE., Jr The adolescent ballet dancer. Nutritional practices and characteristics associated with anorexia nervosa. Journal of adolescent health care : official publication of the Society for Adolescent Medicine. 1985;6(5):365–71. doi: 10.1016/s0197-0070(85)80004-8. [DOI] [PubMed] [Google Scholar]
- 22.Philipp E, Karl-Martin P, Seidl M, Tuschl RJ, Fichter MM, Eckert M, Wolfram G. Vitamin status in patients with anorexia nervosa and bulimia nervosa. International Journal of Eating Disorders. 1989;8(2):209–18. [Google Scholar]
- 23.Thibault L, Roberge AG. The nutritional status of subjects with anorexia nervosa. International journal for vitamin and nutrition research Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung Journal international de vitaminologie et de nutrition. 1987;57(4):447–52. [PubMed] [Google Scholar]
- 24.Winston AP, Jamieson Cp, Madira W, Gatward NM, Palmer RL. Prevalence of thiamin deficiency in anorexia nervosa. The International journal of eating disorders. 2000;28(4):451–4. doi: 10.1002/1098-108x(200012)28:4<451::aid-eat14>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 25.Modan-Moses D, Levy-Shraga Y, Pinhas-Hamiel O, Kochavi B, Enoch-Levy A, Vered I, Stein D. High prevalence of vitamin D deficiency and insufficiency in adolescent inpatients diagnosed with eating disorders. International Journal of Eating Disorders. 2014;48(6):607–14. doi: 10.1002/eat.22347. [DOI] [PubMed] [Google Scholar]
- 26.Sechi G, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. The Lancet Neurology. 2007;6(5):442–55. doi: 10.1016/S1474-4422(07)70104-7. [DOI] [PubMed] [Google Scholar]
- 27.Thomas AG, Koumellis P, Dineen RA. The fornix in health and disease: an imaging review. Radiographics. 2011;(31):1107–21. doi: 10.1148/rg.314105729. [DOI] [PubMed] [Google Scholar]
- 28.Kril JJ, Harper CG. Neuroanatomy and neuropathology associated with Korsakoff’s syndrome. Neuropsychology review. 2012;22(2):72–80. doi: 10.1007/s11065-012-9195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogershok PR, Rahman A, Nestor S, Brick J. Wernicke encephalopathy in nonalcoholic patients. American Journal of the Medical Science. 2002;323(2):107–11. doi: 10.1097/00000441-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Zuccoli G, Gallucci M, Capellades J, Regnicolo L, Tumiati B, Giadas TC, et al. Wernicke Encephalopathy: MR Findings at Clinical Presentation in Twenty-Six Alcoholic and Nonalcoholic Patients. American Journal of Neuroradiology. 2007;28(7):1328–31. doi: 10.3174/ajnr.A0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Handler CE, Perkin GD. Anorexia nervosa and Wernicke’s encephalopathy: an underdiagnosed association. Lancet. 1982;2(8301):771–2. doi: 10.1016/s0140-6736(82)90955-2. [DOI] [PubMed] [Google Scholar]
- 32.Peters TE, Parvin M, Petersen C, Faircloth VC, Levine RL. A Case Report of Wernicke’s Encephalopathy in a Pediatric Patient with Anorexia Nervosa - Restricting Type. Journal of Adolescent Health. 2007;40(4):376–83. doi: 10.1016/j.jadohealth.2006.11.140. [DOI] [PubMed] [Google Scholar]
- 33.Renthal W, Marin-Valencia I, Evans PA. Thiamine Deficiency Secondary to Anorexia Nervosa: An Uncommon Cause of Peripheral Neuropathy and Wernicke Encephalopathy in Adolescence. Pediatric Neurology. 2014;51(1):100–3. doi: 10.1016/j.pediatrneurol.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 34.Peters TE, Parvin M, Petersen C, Faircloth VC, Levine RL. A case report of Wernicke’s encephalopathy in a pediatric patient with anorexia nervosa--restricting type. J Adolesc Health. 2007;40(4):376–83. doi: 10.1016/j.jadohealth.2006.11.140. [DOI] [PubMed] [Google Scholar]
- 35.Saad L, Silva LFAL, Banzato CEM, Dantas CR, Garcia C. Anorexia nervosa and Wernicke-Korsakoff syndrome: a case report. Journal of Medical Case Reports. 2010;4(1):217. doi: 10.1186/1752-1947-4-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- 37.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Kumar R, Birrer BV, Macey PM, Woo MA, Gupta RK, Yan-Go FL, Harper RM. Reduced mammillary body volume in patients with obstructive sleep apnea. Neuroscience Letters. 2008;438(3):330–4. doi: 10.1016/j.neulet.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 39.Kumar R, Woo MA, Birrer BV, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Mammillary bodies and fornix fibers are injured in heart failure. Neurobiology of Disease. 2009;33(2):236–42. doi: 10.1016/j.nbd.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner A, Greer P, Bailer UF, Frank GK, Henry SE, Putnam K, et al. Normal Brain Tissue Volumes after Long-Term Recovery in Anorexia and Bulimia Nervosa. Biological Psychiatry. 2006;59(3):291–3. doi: 10.1016/j.biopsych.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 41.Roberto CA, Mayer LE, Brickman AM, Barnes A, Muraskin J, Yeung LK, et al. Brain tissue volume changes following weight gain in adults with anorexia nervosa. International Journal of Eating Disorders. 2011;44(5):406–11. doi: 10.1002/eat.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.King JA, Geisler D, Ritschel F, Boehm I, Seidel M, Roschinski B, et al. Global Cortical Thinning in Acute Anorexia Nervosa Normalizes Following Long-Term Weight Restoration. Biological Psychiatry. 2015;77(7):624–32. doi: 10.1016/j.biopsych.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Bernardoni F, King JA, Geisler D, Stein E, Jaite C, Natsch D, et al. Weight restoration therapy rapidly reverses cortical thinning in anorexia nervosa: A longitudinal study. Neuroimage. 2016;130:214–22. doi: 10.1016/j.neuroimage.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Seitz J, Buhren K, von Polier GG, Heussen N, Herpertz-Dahlmann B, Konrad K. Morphological changes in the brain of acutely ill and weight-recovered patients with anorexia nervosa. A meta-analysis and qualitative review. Zeitschrift fur Kinder- und Jugendpsychiatrie und Psychotherapie. 2014;42(1):7–17. doi: 10.1024/1422-4917/a000265. quiz -8. [DOI] [PubMed] [Google Scholar]
- 45.Green MW, Elliman NA, Wakeling A, Rogers PJ. Cognitive functioning, weight change and therapy in anorexia nervosa. J Psychiatr Res. 1996;30(5):401–10. doi: 10.1016/0022-3956(96)00026-x. [DOI] [PubMed] [Google Scholar]
- 46.Katzman DK, RBZ, Lambe EK, Mikulis DJ. A longitudinal magnetic resonance imaging study of brain changes in adolescents with anorexia nervosa. JAMA Pediatrics. 1997;151:793–97. doi: 10.1001/archpedi.1997.02170450043006. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura K, Brown RA, Araujo D, Narayanan S, Arnold DL. Correlation between brain volume change and T2 relaxation time induced by dehydration and rehydration: implications for monitoring atrophy in clinical studies. Neuroimage Clin. 2014;23(6):166–70. doi: 10.1016/j.nicl.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freund W, Faust S, Gaser C, Gron G, Birklein F, Wunderlich AP, et al. Regionally accentuated reversible brain grey matter reduction in ultra marathon runners detected by voxel-based morphometry. BMC Sports Sci Med Rehabil. 2014;6(1):4. doi: 10.1186/2052-1847-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frank GK. What Causes Eating Disorders, and What Do They Cause? Biol Psychiatry. 2015;77(7):602–3. doi: 10.1016/j.biopsych.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Papez JW. A proposed mechanism of emotion. 1937. J Neuropsychiatry Clin Neurosci. 1995;7(1):103–12. doi: 10.1176/jnp.7.1.103. [DOI] [PubMed] [Google Scholar]
- 51.Frank GK. Advances from neuroimaging studies in eating disorders. CNS Spectr. 2015;20(4):391–400. doi: 10.1017/S1092852915000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR in biomedicine. 2010;23(7):803–20. doi: 10.1002/nbm.1543. [DOI] [PubMed] [Google Scholar]
- 53.Sheedy D, Lara A, Garrick T, Harper C. Size of mamillary bodies in health and disease: useful measurements in neuroradiological diagnosis of Wernicke’s encephalopathy. Alcoholism, clinical and experimental research. 1999;23(10):1624–8. [PMC free article] [PubMed] [Google Scholar]